Spinal Cord Stimulation for Intractable Chronic Limb Ischemia: A Narrative Review
Abstract
:1. Introduction
2. Methods
2.1. Mechanisms of Action of SCS
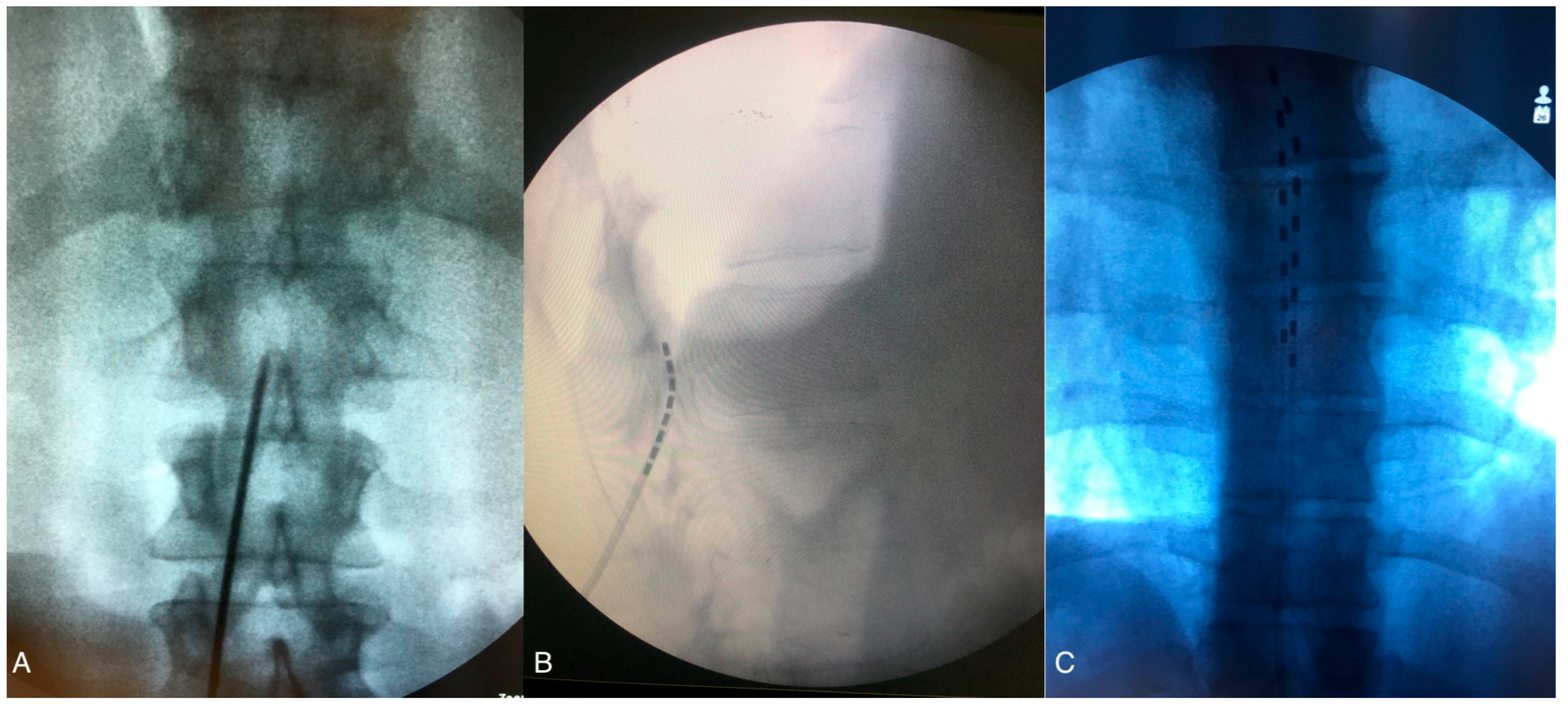
2.2. SCS Surgical
2.3. Patient Selection: Inclusion and Exclusion Criteria for Spinal Cord Stimulation in CLI Patients
2.4. Spinal Cord Stimulation and CLI
2.5. Novel Neurostimulation Modalities and Waveforms in Chronic Pain
2.6. Novel Neurostimulation Modalities and CLI
2.7. Exploring the Necessity of SCS Trials in the Treatment of CLI Critical Limb Ischemia
3. Complications
4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CLI | critical limb ischemia |
| CLTI | chronic limb-threatening ischemia |
| SCS | spinal cord stimulation |
| DTM | differential target multiplexed |
| FBSS | failed back surgery syndrome |
| PAD | peripheral artery disease |
| TcPO2 | transcutaneous oxygen tension |
References
- Dormandy, J.A.; Rutherford, R.B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J. Vasc. Surg. 2000, 31 Pt 2, S1–S296. [Google Scholar]
- Powell, J.T.; Edwards, R.J.; Worrell, P.C.; Franks, P.J.; Greenhalgh, R.M.; Poulter, N.R. Risk factors associated with the development of peripheral arterial disease in smokers: A case-control study. Atherosclerosis 1997, 129, 41–48. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef]
- Novo, S. Classification, epidemiology, risk factors, and natural history of peripheral arterial disease. Diabetes Obes. Metab. 2002, 4 (Suppl. 2), S1–S6. [Google Scholar] [CrossRef]
- Abu Dabrh, A.M.; Steffen, M.W.; Undavalli, C.; Asi, N.; Wang, Z.; Elamin, M.B.; Conte, M.S.; Murad, M.H. The natural history of untreated severe or critical limb ischemia. J. Vasc. Surg. 2015, 62, 1642–1651.e3. [Google Scholar] [CrossRef]
- Amann, W.; Berg, P.; Gersbach, P.; Gamain, J.; Raphael, J.; Ubbink, D. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: Results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS). Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2003, 26, 280–286. [Google Scholar] [CrossRef]
- Piedade, G.S.; Vesper, J.; Reichstein, D.; Dauphin, A.K.; Damirchi, S. Spinal cord stimulation in non-reconstructable critical limb ischemia: A retrospective study of 71 cases. Acta Neurochir. (Wien) 2023, 165, 967–973. [Google Scholar] [CrossRef]
- Joosten, E.A.; Franken, G. Spinal cord stimulation in chronic neuropathic pain: Mechanisms of action, new locations, new paradigms. Pain 2020, 161, S104–S113. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Kaufman, S.; Alpaugh, E.S.; Lawson, J.; Apgar, T.L.; Imani, F.; Khademi, S.H.; Cornett, E.M.; Kaye, A.D. Burst Spinal Cord Stimulation in the Management of Chronic Pain: Current Perspectives. Anesth. Pain Med. 2022, 12, e126416. [Google Scholar] [CrossRef]
- Ayoo, K.; Mikhaeil, J.; Huang, A.; Wąsowicz, M. The opioid crisis in North America: Facts and future lessons for Europe. Anaesthesiol. Intensive Ther. 2020, 52, 139–147. [Google Scholar] [CrossRef]
- Spicarova, D.; Nerandzic, V.; Palecek, J. Update on the Role of Spinal Cord TRPV1 Receptors in Pain Modulation. Physiol. Res. 2014, 63, S225–S236. [Google Scholar] [CrossRef]
- Naoum, J.J.; Arbid, E.J. Spinal Cord Stimulation for Chronic Limb Ischemia. Methodist DeBakey Cardiovasc. J. 2013, 9, 99–102. [Google Scholar] [CrossRef]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation J. Int. Neuromodulation Soc. 2014, 17, 515–550; discussion 550. [Google Scholar] [CrossRef]
- Reig, E.; Abejón, D.; del Pozo, C.; Wojcikiewicz, R. Spinal cord stimulation in peripheral vascular disease: A retrospective analysis of 95 cases. Pain Pract. Off. J. World Inst. Pain 2001, 1, 324–331. [Google Scholar] [CrossRef]
- Pedrini, L.; Magnoni, F. Spinal cord stimulation for lower limb ischemic pain treatment. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 495–500. [Google Scholar] [CrossRef]
- Cohen, S.P.; Huang, J.H.Y.; Brummett, C. Facet joint pain—Advances in patient selection and treatment. Nat. Rev. Rheumatol. 2013, 9, 101–116. [Google Scholar] [CrossRef]
- Wu, M.; Linderoth, B.; Foreman, R.D. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: A review of experimental studies. Auton Neurosci. Basic Clin. 2008, 138, 9–23. [Google Scholar] [CrossRef]
- Tanaka, S.; Komori, N.; Barron, K.W.; Chandler, M.J.; Linderoth, B.; Foreman, R.D. Mechanisms of sustained cutaneous vasodilation induced by spinal cord stimulation. Auton Neurosci. Basic Clin. 2004, 114, 55–60. [Google Scholar] [CrossRef]
- Wahezi, S.E.; Caparo, M.A.; Malhotra, R.; Sundaram, L.; Batti, K.; Ejindu, P.; Veeramachaneni, R.; Anitescu, M.; Hunter, C.W.; Naeimi, T.; et al. Current Waveforms in Spinal Cord Stimulation and Their Impact on the Future of Neuromodulation: A Scoping Review. Neuromodulation J. Int. Neuromodulation Soc. 2024, 27, 47–58. [Google Scholar] [CrossRef]
- Deogaonkar, M.; Zibly, Z.; Slavin, K.V. Spinal cord stimulation for the treatment of vascular pathology. Neurosurg. Clin. N. Am. 2014, 25, 25–31. [Google Scholar] [CrossRef]
- Klinkova, A.; Kamenskaya, O.; Ashurkov, A.; Murtazin, V.; Orlov, K.; Lomivorotov, V.V.; Karaskov, A. The Clinical Outcomes in Patients with Critical Limb Ischemia One Year after Spinal Cord Stimulation. Ann. Vasc. Surg. 2020, 62, 356–364. [Google Scholar] [CrossRef]
- Horsch, S.; Claeys, L. Epidural spinal cord stimulation in the treatment of severe peripheral arterial occlusive disease. Ann. Vasc. Surg. 1994, 8, 468–474. [Google Scholar] [CrossRef]
- Ubbink, D.T.; Vermeulen, H. Spinal Cord Stimulation for Critical Leg Ischemia: A Review of Effectiveness and Optimal Patient Selection. J. Pain Symptom Manag. 2006, 31, S30–S35. [Google Scholar] [CrossRef]
- Goudman, L.; Rigoard, P.; Billot, M.; Duarte, R.V.; Eldabe, S.; Moens, M. Patient Selection for Spinal Cord Stimulation in Treatment of Pain: Sequential Decision-Making Model—A Narrative Review. J. Pain Res. 2022, 15, 1163–1171. [Google Scholar] [CrossRef]
- Kumar, K.; Rizvi, S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. Malden Mass. 2013, 14, 1631–1649. [Google Scholar] [CrossRef]
- Eldabe, S.; Buchser, E.; Duarte, R.V. Complications of Spinal Cord Stimulation and Peripheral Nerve Stimulation Techniques: A Review of the Literature. Pain Med. Malden Mass. 2016, 17, 325–336. [Google Scholar] [CrossRef]
- Kilchukov, M.; Kiselev, R.; Gorbatykh, A.; Klinkova, A.; Murtazin, V.; Kamenskaya, O.; Orlov, K. High-Frequency versus Low-Frequency Spinal Cord Stimulation in Treatment of Chronic Limb-Threatening Ischemia: Short-Term Results of a Randomized Trial. Ster. Funct. Neurosurg. 2023, 101, 1–11. [Google Scholar] [CrossRef]
- Ouerchefani, N.; Desgranges, P.; Goldberg, E. Spinal cord stimulation in non-reconstructable critical limb threatening ischemia (CLTI): A single-site long-term review of 10-year experience. In Proceedings of the 16th World Congress of the International Neuromodulation Society INS, Vancouver, Canada, 11–16 May 2024. [Google Scholar]
- Cook, A.W.; Oygar, A.; Baggenstos, P.; Pacheco, S.; Kleriga, E. Vascular disease of extremities. Electric stimulation of spinal cord and posterior roots. N. Y. State J. Med. 1976, 76, 366–368. [Google Scholar]
- Van Damme, H.; Suy, R.; Brande, P.V.D.; De Vleeshauwer, P.; Demelenne, J.; Haxhe, J.P. Spinal cord stimulation. New regulation of national health insurance. Acta Chir. Belg. 2008, 108, 139–141. [Google Scholar] [CrossRef]
- Augustinsson, L.E.; Carlsson, C.A.; Holm, J.; Jivegård, L. Epidural electrical stimulation in severe limb ischemia. Pain relief, increased blood flow, and a possible limb-saving effect. Ann. Surg. 1985, 202, 104–110. [Google Scholar] [CrossRef]
- Klomp, H.M.; Spincemaille, G.H.; Steyerberg, E.W.; Habbema, J.D.; van Urk, H. Spinal-cord stimulation in critical limb ischaemia: A randomised trial. ESES Study Group. Lancet 1999, 353, 1040–1044. [Google Scholar] [CrossRef]
- Spincemaille, G.H.; Klomp, H.M.; Steyerberg, E.W.; Habbema, J.D. Pain and quality of life in patients with critical limb ischaemia: Results of a randomized controlled multicentre study on the effect of spinal cord stimulation. ESES study group. Eur. J. Pain 2000, 4, 173–184. [Google Scholar] [CrossRef]
- Ubbink, D.T.; Vermeulen, H. Spinal cord stimulation for patients with chronic critical leg ischaemia who cannot have blood vessel surgery. Cochrane Database Syst. Rev. 2013, 2013, CD004001. [Google Scholar] [CrossRef]
- Liu, J.T.; Su, C.H.; Chen, S.Y.; Liew, S.J.; Chang, C.S. Spinal Cord Stimulation Improves the Microvascular Perfusion Insufficiency Caused by Critical Limb Ischemia. Neuromodulation 2018, 21, 489–494. [Google Scholar] [CrossRef]
- Tshomba, Y.; Psacharopulo, D.; Frezza, S.; Marone, E.M.; Astore, D.; Chiesa, R. Predictors of improved quality of life and claudication in patients undergoing spinal cord stimulation for critical lower limb ischemia. Ann. Vasc. Surg. 2014, 28, 628–632. [Google Scholar] [CrossRef]
- Cucuruz, B.; Kopp, R.; Hampe-Hecht, H.; Andercou, O.; Schierling, W.; Pfister, K.; Koller, M.; Noppeney, T. Treatment of end-stage peripheral artery disease by neuromodulation. Clin. Hemorheol. Microcirc. 2022, 81, 315–324. [Google Scholar] [CrossRef]
- Cyrek, A.E.; Henn, N.; Meinhardt, F.; Lainka, M.; Pacha, A.; Paul, A.; Koch, D. Improving Limb Salvage for Chronic Limb-Threatening Ischemia with Spinal Cord Stimulation: A Retrospective Analysis. Vasc. Endovasc. Surg. 2021, 55, 367–373. [Google Scholar] [CrossRef]
- Cui, J.G.; O’Connor, W.T.; Ungerstedt, U.; Linderoth, B.; Meyerson, B.A. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain 1997, 73, 87–95. [Google Scholar] [CrossRef]
- Vallejo, R.; Kelley, C.A.; Gupta, A.; Smith, W.J.; Vallejo, A.; Cedeño, D.L. Modulation of neuroglial interactions using differential target multiplexed spinal cord stimulation in an animal model of neuropathic pain. Mol. Pain 2020, 16, 1744806920918057. [Google Scholar] [CrossRef]
- Cedeño, D.L.; Kelley, C.A.; Chakravarthy, K.; Vallejo, R. Modulation of Glia-Mediated Processes by Spinal Cord Stimulation in Animal Models of Neuropathic Pain. Front. Pain Res. Lausanne Switz. 2021, 2, 702906. [Google Scholar] [CrossRef]
- Fishman, M.; Cordner, H.; Justiz, R.; Provenzano, D.; Merrell, C.; Shah, B.; Naranjo, J.; Kim, P.; Calodney, A.; Carlson, J.; et al. Twelve-Month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Pract. 2021, 21, 912–923. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S. Burst and Tonic Spinal Cord Stimulation: Different and Common Brain Mechanisms. Neuromodulation J. Int. Neuromodulation Soc. 2016, 19, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Schu, S.; Slotty, P.J.; Bara, G.; von Knop, M.; Edgar, D.; Vesper, J. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation J. Int. Neuromodulation Soc. 2014, 17, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Brown, L.L.; Yearwood, T.L.; et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Bae, C.; Lee, D.; Kagan, Z.; Bradley, K.; Chung, J.M.; La, J.-H. Low-intensity, Kilohertz Frequency Spinal Cord Stimulation Differently Affects Excitatory and Inhibitory Neurons in the Rodent Superficial Dorsal Horn. Neuroscience 2020, 428, 132–139. [Google Scholar] [CrossRef]
- Metzger, C.S.; Hammond, M.B.; Paz-Solis, J.F.; Newton, W.J.; Thomson, S.J.; Pei, Y.; Jain, R.; Moffitt, M.; Annecchino, L.; Doan, Q. A novel fast-acting sub-perception spinal cord stimulation therapy enables rapid onset of analgesia in patients with chronic pain. Expert Rev. Med. Devices 2021, 18, 299–306. [Google Scholar] [CrossRef]
- Asimakidou, E.; Matis, G.K. Spinal cord stimulation in the treatment of peripheral vascular disease: A systematic review—Revival of a promising therapeutic option? Br. J. Neurosurg. 2022, 36, 555–563. [Google Scholar] [CrossRef]
- Ueno, K.; Tachibana, K.; Masunaga, N.; Shinoda, Y.; Minamisaka, T.; Inui, H.; Amiya, R.; Inoue, S.; Murakami, A.; Hoshida, S. Clinical outcomes of spinal cord stimulation in patients with intractable leg pain in Japan. Pain Pract. Off. J. World Inst. Pain 2024, 24, 826–831. [Google Scholar] [CrossRef]
- Kretzschmar, M.; Okaro, U.; Schwarz, M.; Reining, M.; Lesser, T. Spinal Neuromodulation for Peripheral Arterial Disease of Lower Extremities: A Ten-Year Retrospective Analysis. Neuromodulation J. Int. Neuromodulation Soc. 2023, in press. [Google Scholar] [CrossRef]
- De Caridi, G.; Massara, M.; David, A.; Giardina, M.; La Spada, M.; Stilo, F.; Spinelli, F.; Grande, R.; Butrico, L.; de Franciscis, S.; et al. Spinal cord stimulation to achieve wound healing in a primary lower limb critical ischaemia referral centre. Int. Wound J. 2016, 13, 220–225. [Google Scholar] [CrossRef]
- Duarte, R.V.; Thomson, S. Trial Versus No Trial of Spinal Cord Stimulation for Chronic Neuropathic Pain: Cost Analysis in United Kingdom National Health Service. Neuromodulation 2019, 22, 208–214. [Google Scholar] [CrossRef]
- Chadwick, R.; McNaughton, R.; Eldabe, S.; Baranidharan, G.; Bell, J.; Brookes, M.; Duarte, R.V.; Earle, J.; Gulve, A.; Houten, R.; et al. To Trial or Not to Trial Before Spinal Cord Stimulation for Chronic Neuropathic Pain: The Patients’ View From the TRIAL-STIM Randomized Controlled Trial. Neuromodulation 2021, 24, 459–470. [Google Scholar] [CrossRef]
- Cameron, T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: A 20-year literature review. J Neurosurg. 2004, 100, 254–267. [Google Scholar] [CrossRef]
- Spincemaille, G.H.; de Vet, H.C.; Ubbink, D.T.; Jacobs, M.J. The results of spinal cord stimulation in critical limb ischaemia: A review. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2001, 21, 99–105. [Google Scholar] [CrossRef]
- Traeger, A.C.; Gilbert, S.E.; Harris, I.A.; Maher, C.G. Spinal cord stimulation for low back pain. Cochrane Database Syst. Rev. 2023, 2023, CD014789. [Google Scholar] [CrossRef]
- Narouze, S.; Benzon, H.T.; Provenzano, D.A.; Buvanendran, A.; De Andres, J.; Deer, T.R.; Rauck, R.; Huntoon, M.A. Interventional Spine and Pain Procedures in Patients on Antiplatelet and Anticoagulant Medications. Reg. Anesth. Pain Med. 2015, 40, 182–212. [Google Scholar] [CrossRef]

| Perfusion Index | Effect of SCS | Description | References |
|---|---|---|---|
| Transcutaneous Oxygen Pressure (TcPO2) | Increase in TcPO2 Levels | SCS has been shown to significantly increase TcPO2 levels in CLI patients, indicating improved microcirculation and oxygenation. Higher baseline TcPO2 and increases post-SCS correlate with better limb salvage outcomes. | Horsch and Claeys [22] (1996); Amann et al. [6] (1984); Kumar et al. [25] (1997) |
| Ankle-Brachial Index (ABI) | Variable Improvements | While SCS may improve ABI in some patients, results are inconsistent. Improvements are more likely in patients with initially higher TcPO2 levels. | Horsch and Claeys [22] (1996); Ubbink et al. [23] (1999) |
| Skin Perfusion Pressure (SPP) | Enhancement | SCS enhances SPP by promoting vasodilation and increasing blood flow to ischemic regions, potentially improving wound healing. | Eldabe et al. [26] (2010); Klinkova et al. [21] (2015) |
| Capillary Blood Flow | Increased Flow | SCS may improve capillary blood flow by reducing vasoconstriction and stimulating vasodilator release, contributing to pain relief and ulcer healing. | Kilchukov et al. [27] (2022) |
| Resting TcPO2 and Orthostatic TcPO2 | Significant Rise in Orthostatic TcPO2 | SCS often leads to a marked increase in orthostatic TcPO2, which is associated with positive clinical outcomes and reduced amputation risk. | Reig et al. [14] (1991); Ouerchefani et al. [28] (2024) |
| References | N° pts | Study Characteristics | Disease Type | SCS Device | Follow Up | Pain Relief | Limb Salvage | Complications |
|---|---|---|---|---|---|---|---|---|
| De Caridi [51] (2016) | 34 | Observational Study | PAD; CLI | Nevro Senza; Medtronic; St. Jude Medical | 12 mths | From 57% to 100% of pain relief | 4 cases of limb amputation | 1 infection 2 removals for wound dehiscence |
| Kilchukov [26] (2023) | 50 | RCT Randomized Clinical Trial | CLTI | Boston Scientific, Precision spectra: 31 cases; Abbot, Proclaim XR: 7 cases; Medtronic, Restore Sensor SureScan MRI: 1 case; Stimwave, Freedom 8A: 11 cases | 12 mths | Preop HF-SCS: 7.8 LF-SCS:8.1 Postop HF-SCS 2.8 LF-SCS 3.3 | 98% (1 limb amputation with LF-SCS) | 1 infection (LF-SCS) 1 lead migration (LF-SCS) |
| Kretzman [50] (2023) | 49 | Retrospective Study | PAD | St. Jude Medical/Abbott | 48 mths | Preop: 7.7 Postop:0.40 | 89.8% (5 cases of limb amputation) | 9 lead replacement |
| Ueno [49] (2024) | 20 | Retrospective Study | CLTI, SALI | Medtronic Intellis: 18 LF, 1 HF, 1 DTM | 17 ± 14 mths | Preop: 10 Postop: 4 | 100% | 1 infection |
| Ouerchefani [28] (2024) | 53 | Observational Case Series Study | CLTI | Boston Scientific 32 LF; 20 FAST | 24 mths | Preop: 9.4 Postop: 3.7 | 85% (5 limb amputations, Fontaine stage IV) | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazzeri, R.; Castrucci, T.; Leoni, M.L.G.; Mercieri, M.; Occhigrossi, F. Spinal Cord Stimulation for Intractable Chronic Limb Ischemia: A Narrative Review. J. Cardiovasc. Dev. Dis. 2024, 11, 260. https://doi.org/10.3390/jcdd11090260
Gazzeri R, Castrucci T, Leoni MLG, Mercieri M, Occhigrossi F. Spinal Cord Stimulation for Intractable Chronic Limb Ischemia: A Narrative Review. Journal of Cardiovascular Development and Disease. 2024; 11(9):260. https://doi.org/10.3390/jcdd11090260
Chicago/Turabian StyleGazzeri, Roberto, Tommaso Castrucci, Matteo Luigi Giuseppe Leoni, Marco Mercieri, and Felice Occhigrossi. 2024. "Spinal Cord Stimulation for Intractable Chronic Limb Ischemia: A Narrative Review" Journal of Cardiovascular Development and Disease 11, no. 9: 260. https://doi.org/10.3390/jcdd11090260





