Prevalence and Correlates of Dilated and Non-Dilated Left Ventricular Cardiomyopathy in Transfusion-Dependent Thalassemia: Data from a National, Multicenter, Observational Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CMR Protocol
2.3. Laboratory Investigation
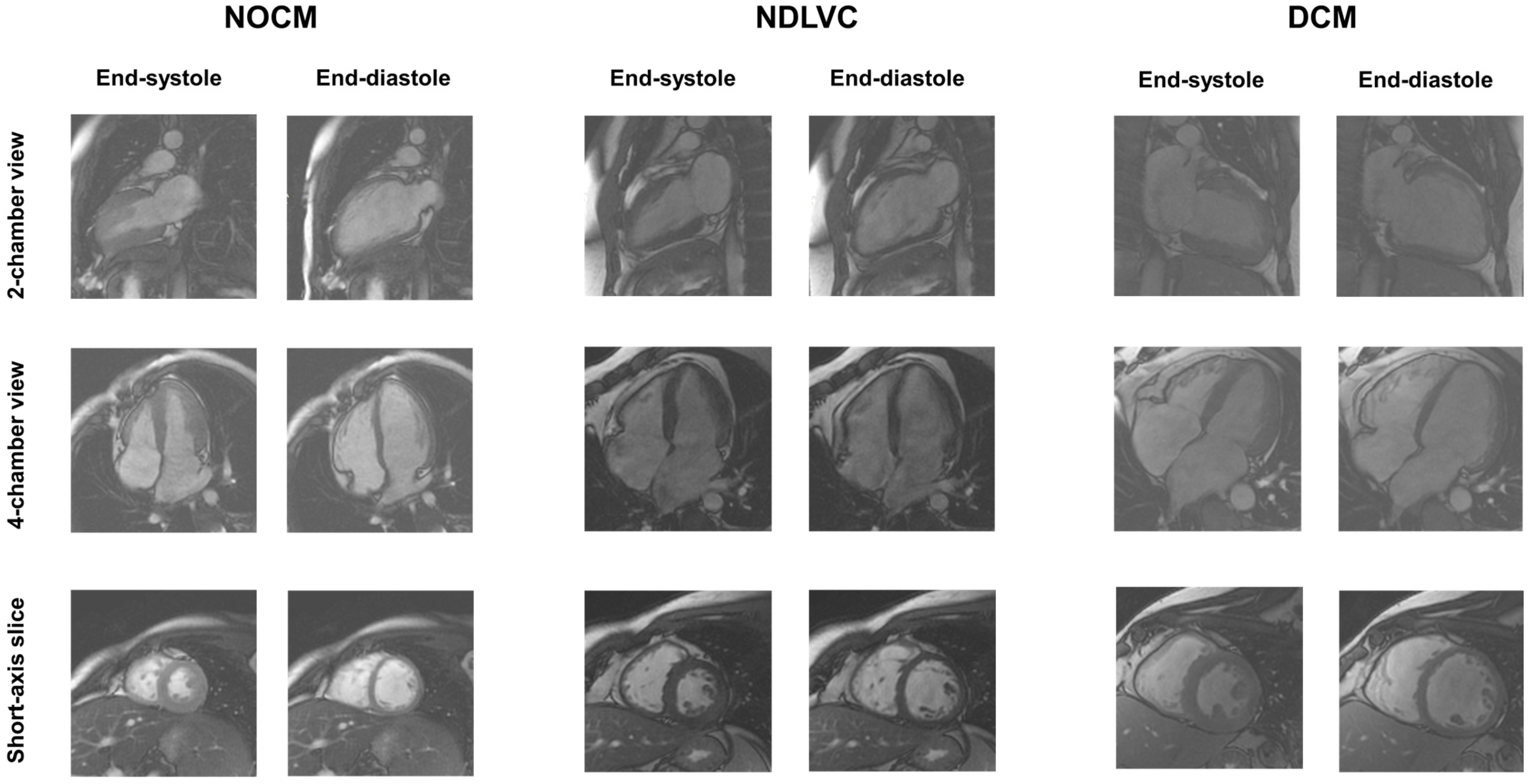
2.4. Diagnostic Criteria
- -
- DCM: presence of LV dilatation and systolic dysfunction, unexplained by congenital, valvular, hypertensive, or coronary heart diseases. Non-ischemic LGE may be absent or present. Right ventricular dilatation and dysfunction may be present but were not included in the diagnosis.
- -
- NDLVC: presence of non-ischemic LGE, fatty replacement, or global LV systolic dysfunction in the absence of LV dilatation.
- -
- NOCM (no overt cardiomyopathy): absence of LV systolic dysfunction, LGE, and fatty replacement areas.
2.5. Clinical Follow-Up and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Data
3.2. Demographic and Clinical Characteristics of TDT Patients Stratified Based on Cardiac Phenotype
3.3. Myocardial Iron Overload and Cardiac Phenotype
3.4. CMR Findings and Cardiac Phenotype
3.5. Outcome Analysis
3.6. Stratification Based on MIO
3.7. Chelation Treatment
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kattamis, A.; Forni, G.L.; Aydinok, Y.; Viprakasit, V. Changing patterns in the epidemiology of β-thalassemia. Eur. J. Haematol. 2020, 105, 692–703. [Google Scholar] [CrossRef]
- Tuo, Y.; Li, Y.; Li, Y.; Ma, J.; Yang, X.; Wu, S.; Jin, J.; He, Z. Global, regional, and national burden of thalassemia, 1990–2021: A systematic analysis for the global burden of disease study 2021. eClinicalMedicine 2024, 72, 102619. [Google Scholar] [CrossRef]
- Rund, D.; Rachmilewitz, E. Beta-thalassemia. N. Engl. J. Med. 2005, 353, 1135–1146. [Google Scholar] [CrossRef]
- Cao, A.; Galanello, R. Beta-thalassemia. Genet. Med. 2010, 12, 61–76. [Google Scholar] [CrossRef]
- Galanello, R.; Origa, R. Beta-thalassemia. Orphanet J. Rare Dis. 2010, 5, 11. [Google Scholar] [CrossRef]
- Weatherall, D.J. The definition and epidemiology of non-transfusion-dependent thalassemia. Blood Rev. 2012, 26 (Suppl. S1), S3–S6. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Cohen, A.; Porter, J.; Taher, A.; Viprakasit, V. Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT) [Internet], 3rd ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2014. [Google Scholar]
- Farmakis, D.; Porter, J.; Taher, A.; Domenica Cappellini, M.; Angastiniotis, M.; Eleftheriou, A. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. Hemasphere 2022, 6, e732. [Google Scholar] [CrossRef]
- Andrews, P.A. Disorders of iron metabolism. N. Engl. J. Med. 2000, 342, 1293; author reply 1294. [Google Scholar]
- Ozment, C.P.; Turi, J.L. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim. Biophys. Acta 2009, 1790, 694–701. [Google Scholar] [CrossRef]
- Shander, A.; Cappellini, M.D.; Goodnough, L.T. Iron overload and toxicity: The hidden risk of multiple blood transfusions. Vox Sang. 2009, 97, 185–197. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Taher, A.T.; Saliba, A.N. Iron overload in thalassemia: Different organs at different rates. Hematology 2017, 2017, 265–271. [Google Scholar] [CrossRef]
- Pinto, V.M.; Forni, G.L. Management of Iron Overload in Beta-Thalassemia Patients: Clinical Practice Update Based on Case Series. Int. J. Mol. Sci. 2020, 21, 8771. [Google Scholar] [CrossRef]
- Aydınok, Y.; Oymak, Y.; Atabay, B.; Aydoğan, G.; Yeşilipek, A.; Ünal, S.; Kılınç, Y.; Oflaz, B.; Akın, M.; Vergin, C.; et al. A National Registry of Thalassemia in Turkey: Demographic and Disease Characteristics of Patients, Achievements, and Challenges in Prevention. Turk. J. Haematol. 2018, 35, 12–18. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Cecinati, V.; Maggio, A.; Sorrentino, F.; Filosa, A.; Rosso, R.; et al. National networking in rare diseases and reduction of cardiac burden in thalassemia major. Eur. Heart J. 2022, 43, 2482–2492. [Google Scholar] [CrossRef]
- Akiki, N.; Hodroj, M.H.; Bou-Fakhredin, R.; Matli, K.; Taher, A.T. Cardiovascular Complications in β-Thalassemia: Getting to the Heart of It. Thalass. Rep. 2023, 13, 38–50. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Rugolotto, S.; De Stefano, P.; Zhao, H.; Cappellini, M.D.; Del Vecchio, G.C.; Romeo, M.A.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004, 89, 1187–1193. [Google Scholar]
- Modell, B.; Khan, M.; Darlison, M.; Westwood, M.A.; Ingram, D.; Pennell, D.J. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 42. [Google Scholar] [CrossRef]
- Argyropoulou, M.I.; Kiortsis, D.N.; Astrakas, L.; Metafratzi, Z.; Chalissos, N.; Efremidis, S.C. Liver, bone marrow, pancreas and pituitary gland iron overload in young and adult thalassemic patients: A T2 relaxometry study. Eur. Radiol. 2007, 17, 3025–3030. [Google Scholar] [CrossRef]
- Meloni, A.; Positano, V.; Ruffo, G.B.; Spasiano, A.; D’Ascola, D.G.; Peluso, A.; Keilberg, P.; Restaino, G.; Valeri, G.; Renne, S.; et al. Improvement of heart iron with preserved patterns of iron store by CMR-guided chelation therapy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 325–334. [Google Scholar] [CrossRef]
- Tanner, M.A.; Galanello, R.; Dessi, C.; Smith, G.C.; Westwood, M.A.; Agus, A.; Roughton, M.; Assomull, R.; Nair, S.V.; Walker, J.M.; et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation 2007, 115, 1876–1884. [Google Scholar] [CrossRef]
- Pennell, D.J.; Berdoukas, V.; Karagiorga, M.; Ladis, V.; Piga, A.; Aessopos, A.; Gotsis, E.D.; Tanner, M.A.; Smith, G.C.; Westwood, M.A.; et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood 2006, 107, 3738–3744. [Google Scholar] [CrossRef]
- Pennell, D.J.; Porter, J.B.; Piga, A.; Lai, Y.; El-Beshlawy, A.; Belhoul, K.M.; Elalfy, M.; Yesilipek, A.; Kilinc, Y.; Lawniczek, T.; et al. A 1-year randomized controlled trial of deferasirox vs deferoxamine for myocardial iron removal in beta-thalassemia major (CORDELIA). Blood 2014, 123, 1447–1454. [Google Scholar] [CrossRef]
- Pennell, D.J.; Udelson, J.E.; Arai, A.E.; Bozkurt, B.; Cohen, A.R.; Galanello, R.; Hoffman, T.M.; Kiernan, M.S.; Lerakis, S.; Piga, A.; et al. Cardiovascular function and treatment in beta-thalassemia major: A consensus statement from the American Heart Association. Circulation 2013, 128, 281–308. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Borsellino, Z.; Cuccia, L.; Borgna-Pignatti, C.; Maggio, A.; Restaino, G.; Gagliardotto, F.; Caruso, V.; Spasiano, A.; et al. Myocardial fibrosis by late gadolinium enhancement cardiac magnetic resonance and hepatitis C virus infection in thalassemia major patients. J. Cardiovasc. Med. 2015, 16, 689–695. [Google Scholar] [CrossRef]
- Assomull, R.G.; Prasad, S.K.; Lyne, J.; Smith, G.; Burman, E.D.; Khan, M.; Sheppard, M.N.; Poole-Wilson, P.A.; Pennell, D.J. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1977–1985. [Google Scholar] [CrossRef]
- Wood, J.C. Cardiac complications in thalassemia major. Hemoglobin 2009, 33 (Suppl. S1), S81–S86. [Google Scholar] [CrossRef]
- Kremastinos, D.T.; Farmakis, D.; Aessopos, A.; Hahalis, G.; Hamodraka, E.; Tsiapras, D.; Keren, A. Beta-thalassemia cardiomyopathy: History, present considerations, and future perspectives. Circ. Heart Fail. 2010, 3, 451–458. [Google Scholar] [CrossRef]
- Barbero, U.; Ajassa, M.; Gaglioti, C.M.; Piga, A.; Ferrero, G.B.; Longo, F. The Influence of Cardiovascular Risk Factors and Hypogonadism on Cardiac Outcomes in an Aging Population of Beta-Thalassemia Patients. J. Cardiovasc. Dev. Dis. 2021, 9, 3. [Google Scholar] [CrossRef]
- Dahiya, A.; Vollbon, W.; Jellis, C.; Prior, D.; Wahi, S.; Marwick, T. Echocardiographic assessment of raised pulmonary vascular resistance: Application to diagnosis and follow-up of pulmonary hypertension. Heart 2010, 96, 2005–2009. [Google Scholar] [CrossRef]
- Cho, I.J.; Mun, Y.C.; Kwon, K.H.; Shin, G.J. Effect of anemia correction on left ventricular structure and filling pressure in anemic patients without overt heart disease. Korean J. Intern. Med. 2014, 29, 445–453. [Google Scholar] [CrossRef]
- Hegde, N.; Rich, M.W.; Gayomali, C. The cardiomyopathy of iron deficiency. Tex. Heart Inst. J. 2006, 33, 340–344. [Google Scholar]
- Grothues, F.; Moon, J.C.; Bellenger, N.G.; Smith, G.S.; Klein, H.U.; Pennell, D.J. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am. Heart J. 2004, 147, 218–223. [Google Scholar] [CrossRef]
- Salerno, M.; Sharif, B.; Arheden, H.; Kumar, A.; Axel, L.; Li, D.; Neubauer, S. Recent Advances in Cardiovascular Magnetic Resonance: Techniques and Applications. Circ. Cardiovasc. Imaging 2017, 10, e003951. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Westwood, M.A.; Anderson, L.J.; Maceira, A.M.; Shah, F.T.; Prescott, E.; Porter, J.B.; Wonke, B.; Walker, J.M.; Pennell, D.J. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. J. Magn. Reson. Imaging 2007, 25, 1147–1151. [Google Scholar] [CrossRef]
- Carpenter, J.P.; Alpendurada, F.; Deac, M.; Maceira, A.; Garbowski, M.; Kirk, P.; Walker, J.M.; Porter, J.B.; Shah, F.; Banya, W.; et al. Right ventricular volumes and function in thalassemia major patients in the absence of myocardial iron overload. J. Cardiovasc. Magn. Reson. 2010, 12, 24. [Google Scholar] [CrossRef]
- Meloni, A.; Righi, R.; Missere, M.; Renne, S.; Schicchi, N.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Spasiano, A.; Roberti, M.G.; et al. Biventricular Reference Values by Body Surface Area, Age, and Gender in a Large Cohort of Well-Treated Thalassemia Major Patients Without Heart Damage Using a Multiparametric CMR Approach. J. Magn. Reson. Imaging 2021, 53, 61–70. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Rossi, G.; Midiri, M.; Missere, M.; Valeri, G.; Sorrentino, F.; D’Ascola, D.G.; Spasiano, A.; Filosa, A.; et al. Prediction of cardiac complications for thalassemia major in the widespread cardiac magnetic resonance era: A prospective multicentre study by a multi-parametric approach. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 299–309. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Cecinati, V.; Ricchi, P.; Gerardi, C.; Restaino, G.; Righi, R.; et al. Multi-Parametric Cardiac Magnetic Resonance for Prediction of Heart Failure Death in Thalassemia Major. Diagnostics 2023, 13, 890. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Figliozzi, S.; Sanguineti, F.; Aquaro, G.D.; di Bella, G.; Stamatelopoulos, K.; Chiribiri, A.; Garot, J.; Masci, P.G.; Ismail, T.F. Prognostic Impact of Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance in Myocarditis. Circ. Cardiovasc. Imaging 2021, 14, e011492. [Google Scholar] [CrossRef]
- Aquaro, G.D.; De Gori, C.; Faggioni, L.; Parisella, M.L.; Cioni, D.; Lencioni, R.; Neri, E. Diagnostic and prognostic role of late gadolinium enhancement in cardiomyopathies. Eur. Heart J. Suppl. 2023, 25, C130–C136. [Google Scholar] [CrossRef]
- Papanastasiou, C.A.; Kokkinidis, D.G.; Kampaktsis, P.N.; Bikakis, I.; Cunha, D.K.; Oikonomou, E.K.; Greenwood, J.P.; Garcia, M.J.; Karamitsos, T.D. The Prognostic Role of Late Gadolinium Enhancement in Aortic Stenosis: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2020, 13, 385–392. [Google Scholar] [CrossRef]
- Meier, C.; Eisenblätter, M.; Gielen, S. Myocardial Late Gadolinium Enhancement (LGE) in Cardiac Magnetic Resonance Imaging (CMR)—An Important Risk Marker for Cardiac Disease. J. Cardiovasc. Dev. Dis. 2024, 11, 40. [Google Scholar] [CrossRef]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Ramazzotti, A.; Pepe, A.; Positano, V.; Rossi, G.; De Marchi, D.; Brizi, M.G.; Luciani, A.; Midiri, M.; Sallustio, G.; Valeri, G.; et al. Multicenter validation of the magnetic resonance t2* technique for segmental and global quantification of myocardial iron. J. Magn. Reson. Imaging 2009, 30, 62–68. [Google Scholar] [CrossRef]
- Marsella, M.; Borgna-Pignatti, C.; Meloni, A.; Caldarelli, V.; Dell’Amico, M.C.; Spasiano, A.; Pitrolo, L.; Cracolici, E.; Valeri, G.; Positano, V.; et al. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: A T2* magnetic resonance imaging study. Haematologica 2011, 96, 515–520. [Google Scholar] [CrossRef]
- Reiter, G.; Reiter, U.; Rienmüller, R.; Gagarina, N.; Ryabikin, A. On the value of geometry-based models for left ventricular volumetry in magnetic resonance imaging and electron beam tomography: A Bland-Altman analysis. Eur. J. Radiol. 2004, 52, 110–118. [Google Scholar] [CrossRef]
- Wang, Y.; Moss, J.; Thisted, R. Predictors of body surface area. J. Clin. Anesth. 1992, 4, 4–10. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Todiere, G.; Strata, E.; Barison, A.; Di Bella, G.; Lombardi, M. Usefulness of India ink artifact in steady-state free precession pulse sequences for detection and quantification of intramyocardial fat. J. Magn. Reson. Imaging 2014, 40, 126–132. [Google Scholar] [CrossRef]
- Meloni, A.; Restaino, G.; Borsellino, Z.; Caruso, V.; Spasiano, A.; Zuccarelli, A.; Valeri, G.; Toia, P.; Salvatori, C.; Positano, V.; et al. Different patterns of myocardial iron distribution by whole-heart T2* magnetic resonance as risk markers for heart complications in thalassemia major. Int. J. Cardiol. 2014, 177, 1012–1019. [Google Scholar] [CrossRef]
- Positano, V.; Pepe, A.; Santarelli, M.F.; Scattini, B.; De Marchi, D.; Ramazzotti, A.; Forni, G.; Borgna-Pignatti, C.; Lai, M.E.; Midiri, M.; et al. Standardized T2* map of normal human heart in vivo to correct T2* segmental artefacts. NMR Biomed. 2007, 20, 578–590. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar]
- Meloni, A.; Maggio, A.; Positano, V.; Leto, F.; Angelini, A.; Putti, M.C.; Maresi, E.; Pucci, A.; Basso, C.; Perazzolo Marra, M.; et al. CMR for Myocardial Iron Overload Quantification: Calibration Curve from the MIOT Network. Eur. Radiol. 2020, 29, 2246–2252. [Google Scholar] [CrossRef]
- Satoh, H.; Sano, M.; Suwa, K.; Saitoh, T.; Nobuhara, M.; Saotome, M.; Urushida, T.; Katoh, H.; Hayashi, H. Distribution of late gadolinium enhancement in various types of cardiomyopathies: Significance in differential diagnosis, clinical features and prognosis. World J. Cardiol. 2014, 6, 585–601. [Google Scholar] [CrossRef]
- Positano, V.; Pingitore, A.; Giorgetti, A.; Favilli, B.; Santarelli, M.F.; Landini, L.; Marzullo, P.; Lombardi, M. A fast and effective method to assess myocardial necrosis by means of contrast magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2005, 7, 487–494. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Yaarubi, S.A.; Skordis, N.; Khater, D.; El Kholy, M.; Stoeva, I.; Fiscina, B.; Angastiniotis, M.; et al. The ICET-A Recommendations for the Diagnosis and Management of Disturbances of Glucose Homeostasis in Thalassemia Major Patients. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016058. [Google Scholar] [CrossRef]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Buxton, A.E.; Calkins, H.; Callans, D.J.; DiMarco, J.P.; Fisher, J.D.; Greene, H.L.; Haines, D.E.; Hayes, D.L.; Heidenreich, P.A.; Miller, J.M.; et al. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). Circulation 2006, 114, 2534–2570. [Google Scholar]
- Cogliandro, T.; Derchi, G.; Mancuso, L.; Mayer, M.C.; Pannone, B.; Pepe, A.; Pili, M.; Bina, P.; Cianciulli, P.; De Sanctis, V.; et al. Guideline recommendations for heart complications in thalassemia major. J. Cardiovasc. Med. 2008, 9, 515–525. [Google Scholar] [CrossRef]
- Cheung, Y.F.; Chan, G.C.; Ha, S.Y. Effect of deferasirox (ICL670) on arterial function in patients with beta-thalassaemia major. Br. J. Haematol. 2008, 141, 728–733. [Google Scholar] [CrossRef]
- Gujja, P.; Rosing, D.R.; Tripodi, D.J.; Shizukuda, Y. Iron overload cardiomyopathy: Better understanding of an increasing disorder. J. Am. Coll. Cardiol. 2010, 56, 1001–1012. [Google Scholar] [CrossRef]
- Albakri, A. Iron overload cardiomyopathy: A review of literature on clinical status and meta-analysis of diagnostic and clinical management using iron chelators. Intern. Med. Care 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Macdonald, R.A.; Mallory, G.K. Hemochromatosis and hemosiderosis. Study of 211 autopsied cases. Arch. Intern. Med. 1960, 105, 686–700. [Google Scholar] [CrossRef]
- Buja, L.M.; Roberts, W.C. Iron in the heart. Etiology and clinical significance. Am. J. Med. 1971, 51, 209–221. [Google Scholar] [CrossRef]
- Olson, L.J.; Edwards, W.D.; McCall, J.T.; Ilstrup, D.M.; Gersh, B.J. Cardiac iron deposition in idiopathic hemochromatosis: Histologic and analytic assessment of 14 hearts from autopsy. J. Am. Coll. Cardiol. 1987, 10, 1239–1243. [Google Scholar] [CrossRef]
- McCrohon, J.A.; Moon, J.C.; Prasad, S.K.; McKenna, W.J.; Lorenz, C.H.; Coats, A.J.; Pennell, D.J. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 2003, 108, 54–59. [Google Scholar] [CrossRef]
- Moon, J.C.; Reed, E.; Sheppard, M.N.; Elkington, A.G.; Ho, S.Y.; Burke, M.; Petrou, M.; Pennell, D.J. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2004, 43, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Positano, V.; Capra, M.; Maggio, A.; Lo Pinto, C.; Spasiano, A.; Forni, G.; Derchi, G.; Favilli, B.; Rossi, G.; et al. Myocardial scarring by delayed enhancement cardiovascular magnetic resonance in thalassaemia major. Heart 2009, 95, 1688–1693. [Google Scholar] [CrossRef]
- Meloni, A.; Pepe, A.; Positano, V.; Favilli, B.; Maggio, A.; Capra, M.; Lo Pinto, C.; Gerardi, C.; Santarelli, M.F.; Midiri, M.; et al. Influence of myocardial fibrosis and blood oxygenation on heart T2* values in thalassemia patients. J. Magn. Reson. Imaging 2009, 29, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Pistoia, L.; Ricchi, P.; Longo, F.; Cecinati, V.; Sorrentino, F.; Borsellino, Z.; Bagnato, S.; Rossi, V.; Fina, P.; et al. Magnetic Resonance Evaluation of Tissue Iron Deposition and Cardiac Function in Adult Regularly Transfused Thalassemia Intermedia Compared with Thalassemia Major Patients. J. Clin. Med. 2024, 13, 4791. [Google Scholar] [CrossRef]
- Castrichini, M.; De Luca, A.; De Angelis, G.; Neves, R.; Paldino, A.; Dal Ferro, M.; Barbati, G.; Medo, K.; Barison, A.; Grigoratos, C.; et al. Magnetic Resonance Imaging Characterization and Clinical Outcomes of Dilated and Arrhythmogenic Left Ventricular Cardiomyopathies. J. Am. Coll. Cardiol. 2024, 83, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Eda, Y.; Nabeta, T.; Iikura, S.; Takigami, Y.; Fujita, T.; Iida, Y.; Ikeda, Y.; Ishii, S.; Ako, J. Non-dilated left ventricular cardiomyopathy vs. dilated cardiomyopathy: Clinical background and outcomes. ESC Heart Fail. 2024, 11, 1463–1471. [Google Scholar] [CrossRef]
- Davis, B.A.; Porter, J.B. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood 2000, 95, 1229–1236. [Google Scholar] [CrossRef]
- Anderson, L.J.; Westwood, M.A.; Holden, S.; Davis, B.; Prescott, E.; Wonke, B.; Porter, J.B.; Walker, J.M.; Pennell, D.J. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: A prospective study using T2* cardiovascular magnetic resonance. Br. J. Haematol. 2004, 127, 348–355. [Google Scholar] [CrossRef]
- Tanner, M.A.; Galanello, R.; Dessi, C.; Smith, G.C.; Westwood, M.A.; Agus, A.; Pibiri, M.; Nair, S.V.; Walker, J.M.; Pennell, D.J. Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J. Cardiovasc. Magn. Reson. 2008, 10, 12. [Google Scholar] [CrossRef]
- Corrado, D.; Thiene, G.; Bauce, B.; Calore, C.; Cipriani, A.; De Lazzari, M.; Migliore, F.; Perazzolo Marra, M.; Pilichou, K.; Rigato, I.; et al. The “Padua classification” of cardiomyopathies: Combining pathobiological basis and morpho-functional remodeling. Int. J. Cardiol. 2025, 418, 132571. [Google Scholar] [CrossRef]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.R.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 63, 447–456. [Google Scholar] [CrossRef]
- Chadalavada, S.; Fung, K.; Rauseo, E.; Lee, A.M.; Khanji, M.Y.; Amir-Khalili, A.; Paiva, J.; Naderi, H.; Banik, S.; Chirvasa, M.; et al. Myocardial Strain Measured by Cardiac Magnetic Resonance Predicts Cardiovascular Morbidity and Death. J. Am. Coll. Cardiol. 2024, 84, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Rider, O.; Cvijic, M.; Valkovič, L.; Remme, E.W.; Voigt, J.-U. Myocardial Strain Imaging: Theory, Current Practice, and the Future. JACC Cardiovasc. Imaging 2025, 18, 340–381. [Google Scholar] [CrossRef] [PubMed]
- Premawardhena, A.; De Silva, S.; Rajapaksha, M.; Ratnamalala, V.; Nallarajah, J.; Galappatthy, G. Myocardial infarction in patients with severe beta thalassaemia: A case series. Int. J. Emerg. Med. 2023, 16, 16. [Google Scholar] [CrossRef]
- Hahalis, G.; Zacharioglou, E.; Xanthopoulou, I.; Koniari, I.; Kalogeropoulou, C.; Tsota, I.; Rigopoulou, A.; Diamantopoulos, A.; Gkizas, V.; Davlouros, P.; et al. Coronary atherosclerosis burden is not advanced in patients with β-thalassemia despite premature extracardiac atherosclerosis: A coronary artery calcium score and carotid intima-media thickness study. J. Geriatr. Cardiol. 2016, 13, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Borgna-Pignatti, C.; Cappellini, M.D.; De Stefano, P.; Del Vecchio, G.C.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; Origa, R.; Piga, A.; Romeo, M.A.; et al. Survival and complications in thalassemia. Ann. N. Y. Acad. Sci. 2005, 1054, 40–47. [Google Scholar] [CrossRef]
- Casolo, G.; Minneci, S.; Manta, R.; Sulla, A.; Del Meglio, J.; Rega, L.; Gensini, G. Identification of the ischemic etiology of heart failure by cardiovascular magnetic resonance imaging: Diagnostic accuracy of late gadolinium enhancement. Am. Heart J. 2006, 151, 101–108. [Google Scholar] [CrossRef]
- Valle-Muñoz, A.; Estornell-Erill, J.; Soriano-Navarro, C.J.; Nadal-Barange, M.; Martinez-Alzamora, N.; Pomar-Domingo, F.; Corbí-Pascual, M.; Payá-Serrano, R.; Ridocci-Soriano, F. Late gadolinium enhancement–cardiovascular magnetic resonance identifies coronary artery disease as the aetiology of left ventricular dysfunction in acute new-onset congestive heart failure. Eur. J. Echocardiogr. 2009, 10, 968–974. [Google Scholar] [CrossRef]

| NOCM (n = 294) | NDLVC (n = 109) | DCM (n = 12) | p-Value | |
|---|---|---|---|---|
| Age (years) | 29.18 ± 8.99 | 30.05 ± 8.49 | 33.02 ± 9.01 | 0.236 |
| Females, n (%) | 164 (55.8) | 54 (49.5) | 5 (41.7) | 0.373 |
| Splenectomy, n (%) | 154 (52.4) | 67 (61.5) | 12 (100.0) | 0.002 |
| Age at splenectomy (years) | 11.64 ± 8.63 | 11.06 ± 8.93 | 12.60 ± 6.74 | 0.466 |
| Age at start of regular transfusions (years) | 1.69 ± 1.52 | 1.49 ± 1.18 | 1.50 ± 0.84 | 0.766 |
| Age at start of chelation therapy (years) | 5.36 ± 5.15 | 4.78 ± 4.37 | 4.89 ± 5.51 | 0.467 |
| Chelation therapy, n (%) | 0.517 | |||
| Deferoxamine | 100 (34.0) | 49 (45.0) | 5 (41.7) | |
| Deferiprone | 61 (20.7) | 19 (17.4) | 3 (25.0) | |
| Deferasirox | 62 (21.1) | 16 (14.7) | 1 (8.3) | |
| Combined DFO + DFP | 38 (12.9) | 16 (14.7) | 1 (8.3) | |
| Sequential DFO/DFP | 33 (11.2) | 9 (8.3) | 2 (16.7) | |
| Mean pre-transfusion hemoglobin (g/dL) | 9.69 ± 0.65 | 9.78 ± 1.24 | 9.23 ± 0.67 | 0.139 |
| Mean serum ferritin (ng/mL) | 1639.18 ± 1462.14 | 1747.32 ± 1879.88 | 1428.25 ± 860.56 | 0.985 |
| Past/active HCV infection, n (%) | 191 (65.0) | 75 (68.8) | 9 (75) | 0.623 |
| Diabetes, n (%) | 23/283 (8.1) | 15/105 (14.3) | 1 (8.3) | 0.189 |
| Overweight, n (%) | 49 (16.7) | 16 (14.7) | 3 (25.0) | 0.638 |
| Global heart T2* values (ms) | 29.16 ± 11.78 | 24.83 ± 12.95 | 19.12 ± 13.26 | 0.001 |
| Significant MIO, n (%) | 78 (26.5) | 44 (40.4) | 8 (66.7) | 0.001 |
| N. of segments with T2* < 20 ms | 4.38 ± 5.84 | 6.45 ± 6.80 | 9.92 ± 7.57 | 0.001 |
| LV EDVI (ml/m2) | 82.25 ± 15.59 | 87.44 ± 16.09 | 129.78 ± 11.09 | <0.0001 |
| LV ESVI (ml/m2) | 29.74 ± 7.55 | 36.08 ± 9.94 | 62.88 ± 14.76 | <0.0001 |
| LV SVI (ml/m2) | 52.28 ± 9.66 | 51.65 ± 9.82 | 66.73 ± 8.93 | <0.0001 |
| LV mass index (g/m2) | 57.30± 13.02 | 60.04 ± 12.16 | 75.68 ± 14.82 | <0.0001 |
| LV EF (%) | 64.02 ± 4.53 | 59.30 ± 7.27 | 51.92 ± 7.54 | <0.0001 |
| RV EDVI (ml/m2) | 79.32 ± 16.26 | 83.27 ± 17.32 | 121.95 ± 13.42 | <0.0001 |
| RV ESVI (ml/m2) | 30.02 ± 8.99 | 34.09 ± 9.83 | 58.39 ± 15.48 | <0.0001 |
| RV SVI (ml/m2) | 49.24 ± 10.48 | 48.78 ± 11.83 | 63.56 ± 11.41 | <0.0001 |
| RV EF (%) | 62.34 ± 6.37 | 59.34 ± 6.99 | 52.83 ± 9.21 | <0.0001 |
| Left atrial area index (cm2/m2) | 12.42 ± 2.27 | 13.45 ± 2.75 | 16.33 ± 3.37 | <0.0001 |
| Right atrial area index (cm2/m2) | 11.89 ± 2.11 | 12.90 ± 2.41 | 16.12 ± 2.82 | <0.0001 |
| Replacement myocardial fibrosis, n (%) | 0 (0.0) | 66/100 (66.0) | 4 (33.3) | <0.0001 |
| n (%) in Group | n (%) of Cardiac Events | Univariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | |||
| Age | 1.08 (1.04–1.12) | <0.0001 | ||
| Sex | ||||
| females | 223 (53.7) | 13 (5.8) | Reference | |
| males | 192 (46.3) | 19 (9.9) | 2.12 (1.04–4.30) | 0.038 |
| Splenectomy | ||||
| no | 182 (43.9) | 9 (4.9) | Reference | |
| yes | 233 (56.1) | 23 (9.9) | 2.21 (1.02–4.79) | 0.044 |
| Age at start of regular transfusions | 0.86 (0.58–1.29) | 0.474 | ||
| Age at start of chelation therapy | 1.06 (0.99–1.13) | 0.108 | ||
| Mean pre-transfusion hemoglobin | 0.71 (0.39–1.28) | 0.256 | ||
| Mean serum ferritin | 1.00 (1.00–1.00) | 0.461 | ||
| Past/active HCV infection | ||||
| no | 140 (33.7) | 7 (5.0) | Reference | |
| yes | 275 (9.1) | 25 (9.1) | 1.99 (0.86–4.62) | 0.106 |
| Diabetes mellitus | ||||
| no | 361/400 (90.2) | 23 (6.4) | Reference | |
| yes | 39/400 (9.8) | 8 (20.5) | 3.35 (1.49–7.53) | 0.003 |
| Significant MIO | ||||
| no | 285 (68.7) | 15 (5.3) | Reference | |
| yes | 130 (31.3) | 17 (13.1) | 2.14 (1.07–4.29) | 0.033 |
| Cardiac phenotype | ||||
| NOCM | 294 (70.8) | 11 (3.7) | Reference | |
| NDLVC | 109 (26.3) | 18 (16.5) | 4.26 (2.01–9.04) | <0.0001 |
| DCM | 12 (2.9) | 3 (25.0) | 8.81 (2.41–32.17) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Pistoia, L.; Spasiano, A.; Sorrentino, F.; Messina, G.; Santodirocco, M.; Borsellino, Z.; Cecinati, V.; Positano, V.; Restaino, G.; et al. Prevalence and Correlates of Dilated and Non-Dilated Left Ventricular Cardiomyopathy in Transfusion-Dependent Thalassemia: Data from a National, Multicenter, Observational Registry. J. Cardiovasc. Dev. Dis. 2025, 12, 103. https://doi.org/10.3390/jcdd12030103
Meloni A, Pistoia L, Spasiano A, Sorrentino F, Messina G, Santodirocco M, Borsellino Z, Cecinati V, Positano V, Restaino G, et al. Prevalence and Correlates of Dilated and Non-Dilated Left Ventricular Cardiomyopathy in Transfusion-Dependent Thalassemia: Data from a National, Multicenter, Observational Registry. Journal of Cardiovascular Development and Disease. 2025; 12(3):103. https://doi.org/10.3390/jcdd12030103
Chicago/Turabian StyleMeloni, Antonella, Laura Pistoia, Anna Spasiano, Francesco Sorrentino, Giuseppe Messina, Michele Santodirocco, Zelia Borsellino, Valerio Cecinati, Vincenzo Positano, Gennaro Restaino, and et al. 2025. "Prevalence and Correlates of Dilated and Non-Dilated Left Ventricular Cardiomyopathy in Transfusion-Dependent Thalassemia: Data from a National, Multicenter, Observational Registry" Journal of Cardiovascular Development and Disease 12, no. 3: 103. https://doi.org/10.3390/jcdd12030103
APA StyleMeloni, A., Pistoia, L., Spasiano, A., Sorrentino, F., Messina, G., Santodirocco, M., Borsellino, Z., Cecinati, V., Positano, V., Restaino, G., Schicchi, N., Grassedonio, E., Vallone, A., Emdin, M., Clemente, A., & Barison, A. (2025). Prevalence and Correlates of Dilated and Non-Dilated Left Ventricular Cardiomyopathy in Transfusion-Dependent Thalassemia: Data from a National, Multicenter, Observational Registry. Journal of Cardiovascular Development and Disease, 12(3), 103. https://doi.org/10.3390/jcdd12030103









