Transition to Advanced Heart Failure: From Identification to Improving Prognosis
Abstract
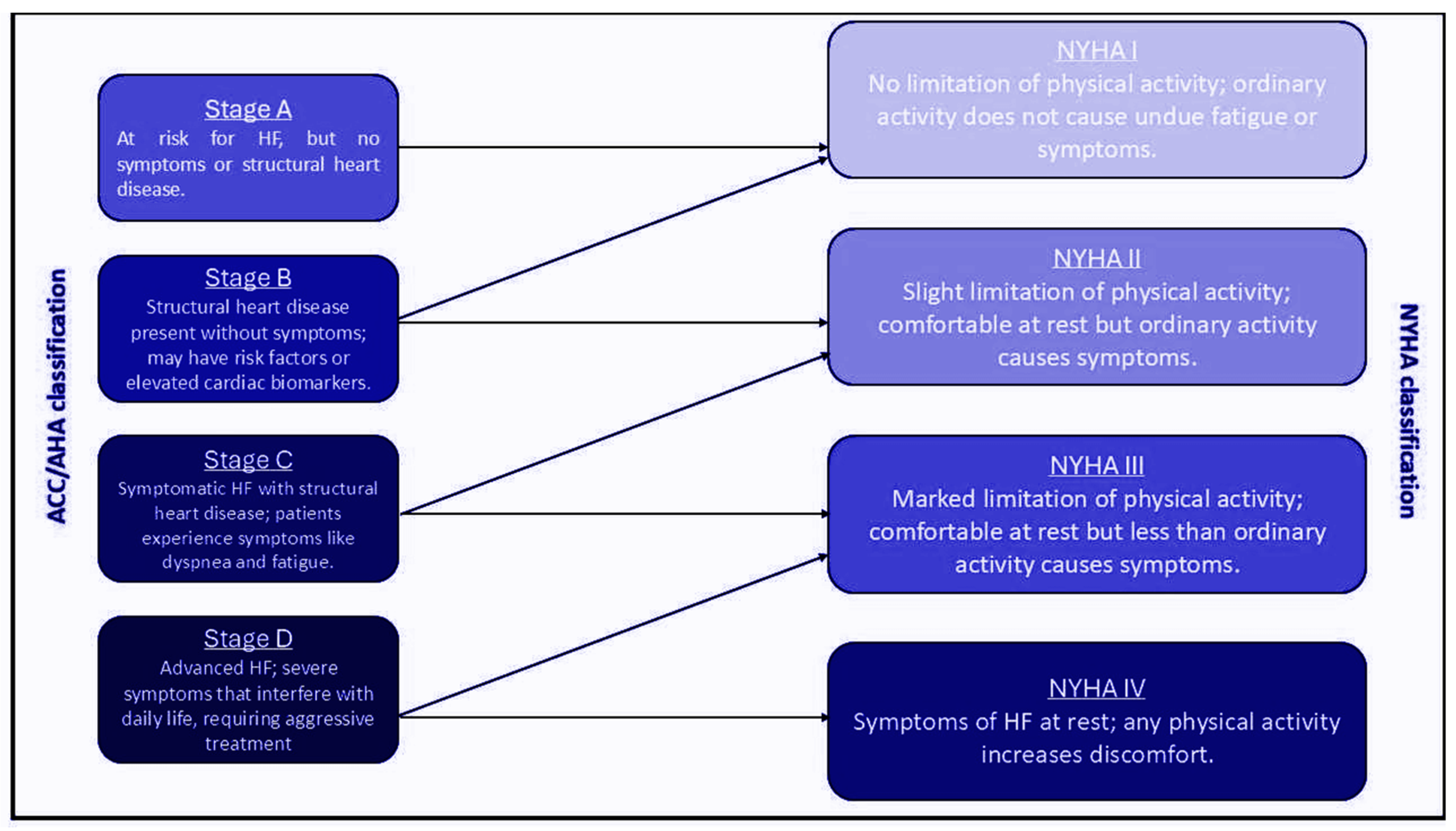
1. Definition of Advanced Heart Failure
1.1. Clinical Criteria
1.1.1. NYHA Class III-IV Symptoms
1.1.2. Dependence on Intravenous Inotropic Support or Recurrent Hospitalizations
1.1.3. Refractory Symptoms Despite Optimal GDMT
1.2. Epidemiology
1.3. Pathophysiology
| Mechanism | Description |
|---|---|
| Neurohormonal activation | Activation of RAAS, SNS [18,19] |
| Cardiac remodeling | Systolic/diastolic dysfunction and fibrosis [18,21] |
| Inflammation | Elevation of IL-6 and TNFα levels [18,23] |
| Oxidative stress | Mitochondrial dysfunction [20,23] |
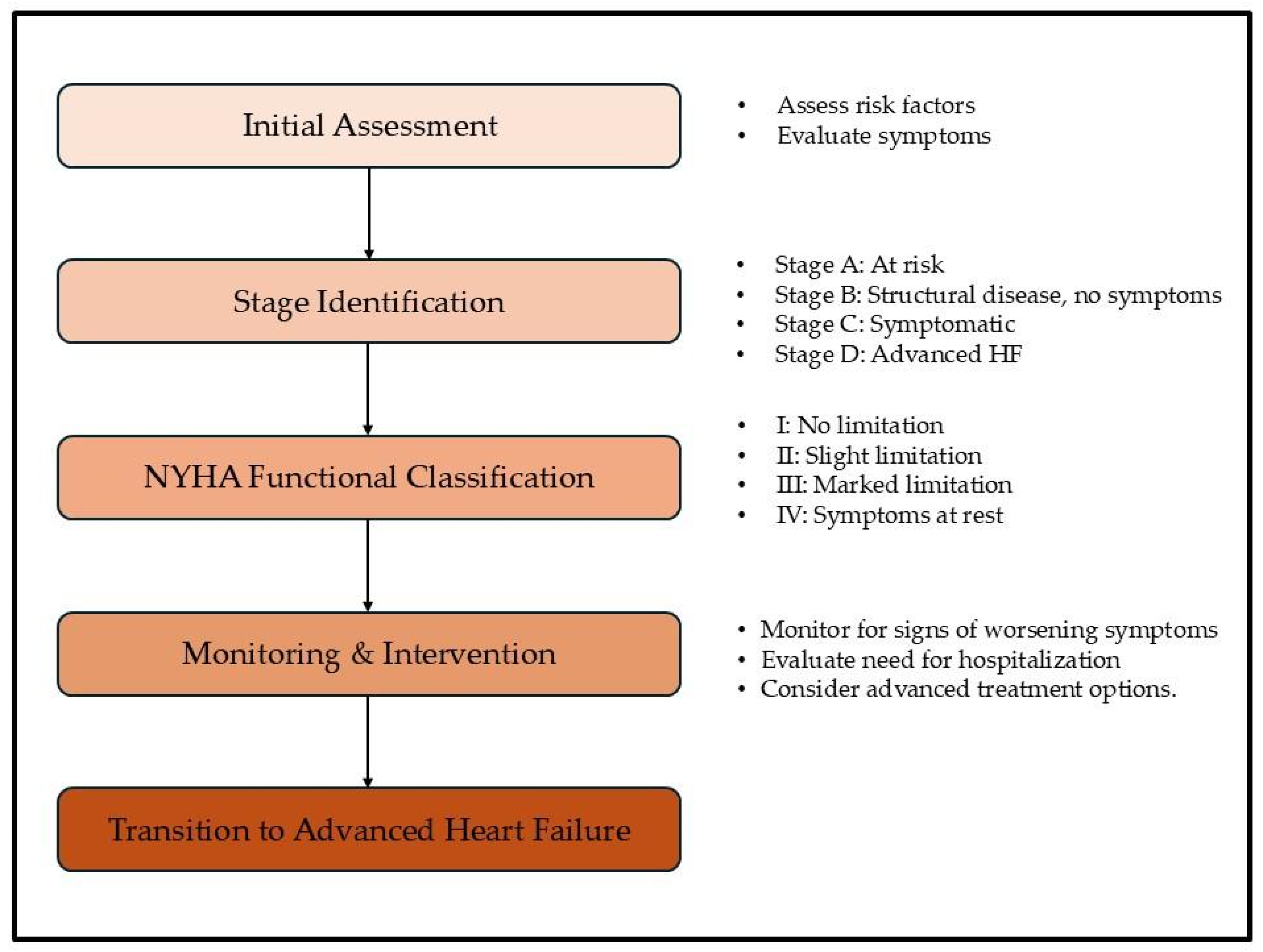
2. Transitioning to Advanced Heart Failure
3. Therapeutic Approaches in Advanced Heart Failure
3.1. Pharmacological Therapies
3.2. Device-Based Interventions
3.3. AF Ablation and Pacing Strategies in HF
3.4. Mechanical Circulatory Support
3.5. Heart Transplantation
3.6. Palliative Care
4. Future Perspectives
4.1. Novel Pharmacological and Device Therapies
4.2. AI and Machine Learning in HF Management
4.3. Advances in Remote Monitoring and Wearable Devices
4.4. Personalized Medicine
4.5. Preventive Strategies
5. Limitations in Advanced Heart Failure
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garascia, A.; Palazzini, M.; Tedeschi, A.; Sacco, A.; Oliva, F.; Gentile, P. Advanced heart failure: From definitions to therapeutic options. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C283–C291. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.-H.; Kang, S.-M. Advanced heart failure: A contemporary approach. Korean J. Intern. Med. 2023, 38, 471–483. [Google Scholar] [CrossRef]
- Farooqui, N.; Killian, J.M.; Smith, J.; Redfield, M.M.; Dunlay, S.M. Advanced Heart Failure Characteristics and Outcomes in Women and Men. J. Am. Heart Assoc. 2024, 13, e033374. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.C.; Ewald, G.A.; Allen, L.A.; Butler, J.; Westlake Canary, C.A.; Colvin-Adams, M.; Dickinson, M.G.; Levy, P.; Stough, W.G.; Sweitzer, N.K.; et al. Advanced (stage D) heart failure: A statement from the Heart Failure Society of America Guidelines Committee. J. Card. Fail. 2015, 21, 519–534. [Google Scholar] [CrossRef]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Crespo-Leiro, M.G.; Barge-Caballero, E. Advanced Heart Failure: Definition, Epidemiology, and Clinical Course. Heart Fail. Clin. 2021, 17, 533–545. [Google Scholar] [CrossRef]
- Bjork, J.B.; Alton, K.K.; Georgiopoulou, V.V.; Butler, J.; Kalogeropoulos, A.P. Defining Advanced Heart Failure: A Systematic Review of Criteria Used in Clinical Trials. J. Card. Fail. 2016, 22, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.P.; Stewart, G.C. Advanced Heart Failure: Prevalence, Natural History, and Prognosis. Heart Fail. Clin. 2016, 12, 323–333. [Google Scholar] [CrossRef]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Beghini, A.; Sammartino, A.M.; Papp, Z.; von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 update in heart failure. ESC Heart Fail. 2024, 12, 8–42. [Google Scholar] [CrossRef]
- AbouEzzeddine, O.F.; Redfield, M.M. Who has advanced heart failure?: Definition and epidemiology. Congest. Heart Fail. 2011, 17, 160–168. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Mills, R.M.; Wynne, J. Characteristics of “Stage D” heart failure: Insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). Am. Heart J. 2008, 155, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Briancon, S.; Juilliere, Y.; Mertes, P.-M.; Villemot, J.-P.; Alla, F.; Virion, J.-M. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: The EPICAL Study. J. Am. Coll. Cardiol. 1999, 33, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Samman Tahhan, A.; Vaduganathan, M.; Greene, S.J.; Alrohaibani, A.; Anker, S.D.; Vardeny, O.; Fonarow, G.C.; Butler, J. Trends in prevalence of comorbidities in heart failure clinical trials. Eur. J. Heart Fail. 2020, 22, 1032–1042. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Ponikowski, P.; Jankowska, E.A. Pathogenesis and clinical presentation of acute heart failure. Rev. Esp. Cardiol. 2015, 68, 331–337. [Google Scholar] [CrossRef]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef]
- Mentz, R.J.; O’Connor, C.M. Pathophysiology and clinical evaluation of acute heart failure. Nat. Rev. Cardiol. 2015, 13, 28–35. [Google Scholar] [CrossRef]
- Palumbo, P.; Cannizzaro, E.; Palumbo, M.M.; Di Cesare, A.; Bruno, F.; Acanfora, C.; Arceri, A.; Evangelista, L.; Arrigoni, F.; Grassi, F.; et al. Heart Failure and Cardiomyopathies: CT and MR from Basics to Advanced Imaging. Diagnostics 2022, 12, 2298. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef]
- Falco, L.; Martucci, M.L.; Valente, F.; Verrengia, M.; Pacileo, G.; Masarone, D. Pathophysiology-Based Management of Acute Heart Failure. Clin. Pract. 2023, 13, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Cotter, G.; Milo, O.; Davison, B. The pathophysiology of AHF—New insights from recent studies of novel diuretics and vascular modulating therapies. World J. Cardiovasc. Dis. 2013, 3, 133–145. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Samman-Tahhan, A.; Hedley, J.S.; McCue, A.A.; Bjork, J.B.; Markham, D.W.; Bhatt, K.N.; Georgiopoulou, V.V.; Smith, A.L.; Butler, J. Progression to Stage D Heart Failure Among Outpatients With Stage C Heart Failure and Reduced Ejection Fraction. JACC Heart Fail. 2017, 5, 528–537. [Google Scholar] [CrossRef]
- Bozkurt, B. Treatment of Advanced (Stage D) Heart Failure in the New Era. JACC Heart Fail. 2023, 11, 258–260. [Google Scholar] [CrossRef]
- Greene, S.J.; Bauersachs, J.; Brugts, J.J.; Ezekowitz, J.A.; Lam, C.S.P.; Lund, L.H.; Ponikowski, P.; Voors, A.A.; Zannad, F.; Zieroth, S.; et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 413–424. [Google Scholar] [CrossRef]
- Greene, S.J.; Fonarow, G.C.; Butler, J. Risk Profiles in Heart Failure: Baseline, Residual, Worsening, and Advanced Heart Failure Risk. Circ. Heart Fail. 2020, 13, e007132. [Google Scholar] [CrossRef]
- Allen, L.A.; Gheorghiade, M.; Reid, K.J.; Dunlay, S.M.; Chan, P.S.; Hauptman, P.J.; Zannad, F.; Konstam, M.A.; Spertus, J.A. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 389–398. [Google Scholar] [CrossRef]
- Baumwol, J. “I Need Help”—A mnemonic to aid timely referral in advanced heart failure. J. Heart Lung Transplant. 2017, 36, 593–594. [Google Scholar] [CrossRef]
- Agdamag, A.C.; Van Iterson, E.H.; Tang, W.H.W.; Finet, J.E. Prognostic Role of Metabolic Exercise Testing in Heart Failure. J. Clin. Med. 2023, 12, 4438. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef] [PubMed]
- de Groote, P.; Dagorn, J.; Soudan, B.; Lamblin, N.; McFadden, E.; Bauters, C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J. Am. Coll. Cardiol. 2004, 43, 1584–1589. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Whellan, D.J.; Lee, K.L.; Keteyian, S.J.; Cooper, L.S.; Ellis, S.J.; Leifer, E.S.; Kraus, W.E.; Kitzman, D.W.; Blumenthal, J.A.; et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009, 301, 1439–1450. [Google Scholar] [CrossRef]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- Smith, E.J.; Gartman, E.J. The Clinical Utility of Cardiopulmonary Exercise Testing. Rhode Isl. Med. J. 2021, 104, 14–19. [Google Scholar]
- Nadruz, W.; West, E.; Sengeløv, M.; Santos, M.; Groarke, J.D.; Forman, D.E.; Claggett, B.; Skali, H.; Shah, A.M. Prognostic Value of Cardiopulmonary Exercise Testing in Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e006000. [Google Scholar] [CrossRef]
- Ho, J.E.; Zern, E.K.; Wooster, L.; Bailey, C.S.; Cunningham, T.; Eisman, A.S.; Hardin, K.M.; Zampierollo, G.A.; Jarolim, P.; Pappagianopoulos, P.P.; et al. Differential Clinical Profiles, Exercise Responses, and Outcomes Associated With Existing HFpEF Definitions. Circulation 2019, 140, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.; Pereira, N.L. Right heart catheterization and risk stratification in advanced heart failure. Curr. Heart Fail. Rep. 2006, 3, 143–152. [Google Scholar] [CrossRef]
- Bellettini, M.; Frea, S.; Pidello, S.; Boffini, M.; Boretto, P.; Gallone, G.; Bongiovanni, F.; Masetti, M.; Sabatino, M.; Raineri, C.; et al. Pretransplant Right Ventricular Dysfunction Is Associated With Increased Mortality After Heart Transplantation: A Hard Inheritance to Overcome. J. Card. Fail. 2021, 28, 259–269. [Google Scholar] [CrossRef]
- Drazner, M.H.; Velez-Martinez, M.; Ayers, C.R.; Reimold, S.C.; Thibodeau, J.T.; Mishkin, J.D.; Mammen, P.P.; Markham, D.W.; Patel, C.B. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure: Insights from the ESCAPE trial. Circ. Heart Fail. 2013, 6, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Bartolome, S.; Denton, C.P.; Gatzoulis, M.A.; Gu, S.; Khanna, D.; Badesch, D.; Montani, D. Definition, classification and diagnosis of pulmonary hypertension. Eur. Respir. J. 2024, 64, 2401324. [Google Scholar] [CrossRef]
- Cyrille-Superville, N.; Rao, S.D.; Feliberti, J.P.; Patel, P.A.; Swayampakala, K.; Sinha, S.S.; Jeng, E.I.; Goswami, R.M.; Snipelisky, D.F.; Carroll, A.M.; et al. PREDICT HF: Risk stratification in advanced heart failure using novel hemodynamic parameters. Clin. Cardiol. 2024, 47, e24277. [Google Scholar] [CrossRef] [PubMed]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Samman-Tahhan, A.; Hedley, J.S.; McCue, A.A.; Bjork, J.B.; Georgiopoulou, V.V.; Morris, A.A.; Butler, J.; Kalogeropoulos, A.P. INTERMACS Profiles and Outcomes Among Non-Inotrope-Dependent Outpatients With Heart Failure and Reduced Ejection Fraction. JACC Heart Fail. 2018, 6, 743–753. [Google Scholar] [CrossRef]
- Kottam, A.; Hanneman, K.; Schenone, A.; Daubert, M.A.; Sidhu, G.D.; Gropler, R.J.; Garcia, M.J.; American Heart Association Council on Cardiovascular Radiology and Intervention. State-of-the-Art Imaging of Infiltrative Cardiomyopathies: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Imaging 2023, 16, e000081. [Google Scholar] [CrossRef]
- Călburean, P.-A.; Lupu, S.; Huțanu, A.; Oprica, M.; Opriș, D.R.; Stan, A.; Scurtu, A.-C.; Aniței, D.; Harpa, M.; Brînzaniuc, K.; et al. Natriuretic peptides and soluble ST2 improves echocardiographic diagnosis of elevated left ventricular filling pressures. Sci. Rep. 2024, 14, 22171. [Google Scholar] [CrossRef]
- Bejar, D.; Colombo, P.C.; Latif, F.; Yuzefpolskaya, M. Infiltrative Cardiomyopathies. Clin. Med. Insights Cardiol. 2015, 9 (Suppl. S2), 29–38. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef]
- Hulten, E.; Aslam, S.; Osborne, M.; Abbasi, S.; Bittencourt, M.S.; Blankstein, R. Cardiac sarcoidosis—State of the art review. Cardiovasc. Diagn. Ther. 2016, 6, 50–63. [Google Scholar]
- Kouranos, V.; Sharma, R. Cardiac sarcoidosis: State-of-the-art review. Heart 2021, 107, 1591–1599. [Google Scholar] [CrossRef]
- Azevedo, O.; Gago, M.F.; Miltenberger-Miltenyi, G.; Sousa, N.; Cunha, D. Fabry Disease Therapy: State-of-the-Art and Current Challenges. Int. J. Mol. Sci. 2020, 22, 206. [Google Scholar] [CrossRef]
- McCafferty, E.H.; Scott, L.J. Migalastat: A Review in Fabry Disease. Drugs 2019, 79, 543–554, Erratum in Drugs 2019, 79, 1363. [Google Scholar] [CrossRef] [PubMed]
- Sabinot, A.; Ghetti, G.; Pradelli, L.; Bellucci, S.; Lausi, A.; Palladini, G. State-of-the-art review on AL amyloidosis in Western Countries: Epidemiology, health economics, risk assessment and therapeutic management of a rare disease. Blood Rev. 2023, 59, 101040. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S. Cardiac amyloidosis: State-of-the-art review. J. Geriatr. Cardiol. 2023, 20, 361–375. [Google Scholar] [CrossRef]
- Allen, L.A.; Stevenson, L.W.; Grady, K.L.; Goldstein, N.E.; Matlock, D.D.; Arnold, R.M.; Cook, N.R.; Felker, G.M.; Francis, G.S.; Hauptman, P.J.; et al. Decision making in advanced heart failure: A scientific statement from the American Heart Association. Circulation 2012, 125, 1928–1952. [Google Scholar] [CrossRef] [PubMed]
- Bistola, V.; Arfaras-Melainis, A.; Polyzogopoulou, E.; Ikonomidis, I.; Parissis, J. Inotropes in Acute Heart Failure: From Guidelines to Practical Use: Therapeutic Options and Clinical Practice. Card. Fail. Rev. 2019, 5, 133–139. [Google Scholar] [CrossRef]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef]
- Rogers, J.G.; Butler, J.; Lansman, S.L.; Gass, A.; Portner, P.M.; Pasque, M.K.; Pierson, R.N., 3rd; INTrEPID Investigators. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: Results of the INTrEPID Trial. J. Am. Coll. Cardiol. 2007, 50, 741–747. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar]
- Al-Mohammad, A. Hydralazine and nitrates in the treatment of heart failure with reduced ejection fraction. ESC Heart Fail. 2019, 6, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.; Hsiao, C.; Shiau, J.; Chiou, K. Hydralazine combined with conventional therapy improved outcomes in severe systolic dysfunction and mitral regurgitation. ESC Heart Fail. 2023, 11, 198–208. [Google Scholar] [CrossRef]
- Sapna, F.; Raveena, F.; Chandio, M.; Bai, K.; Sayyar, M.; Varrassi, G.; Khatri, M.; Kumar, S.; Mohamad, T. Advancements in Heart Failure Management: A Comprehensive Narrative Review of Emerging Therapies. Cureus 2023, 15, e46486. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.J.S.; Anker, S.D.; Baumbach, A.; Alfieri, O.; von Bardeleben, R.S.; Bauersachs, J.; Bax, J.J.; Boveda, S.; Čelutkienė, J.; Cleland, J.G.; et al. The management of secondary mitral regurgitation in patients with heart failure: A joint position statement from the Heart Failure Association (HFA), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), and European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur. Heart J. 2021, 42, 1254–1269. [Google Scholar]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Al-Khatib, S.M.; Ezekowitz, J.A.; Cooper, L.B.; Fordyce, C.B.; Felker, G.M.; Bardy, G.H.; Poole, J.E.; Bigger, J.T.; Buxton, A.E.; et al. Implantable cardioverter-defibrillators in heart failure patients with reduced ejection fraction and diabetes. Eur. J. Heart Fail. 2018, 20, 1031–1038. [Google Scholar] [CrossRef]
- Barakat, A.F.; Mahmoud, A.N.; Elgendy, I.Y. Primary prevention implantable cardioverter defibrillator in patients with reduced ejection fraction: For ischemic or non-ischemic cardiomyopathy or both? J. Thorac. Dis. 2017, 9, 2749–2751. [Google Scholar] [CrossRef]
- Jaffe, L.M.; Morin, D.P. Cardiac resynchronization therapy: History, present status, and future directions. Ochsner. J. 2014, 14, 596–607. [Google Scholar]
- Kong, N.W.; Upadhyay, G.A. Cardiac resynchronization considerations in left bundle branch block. Front. Physiol. 2022, 13, 962042. [Google Scholar] [CrossRef]
- Fumagalli, S.; Pieragnoli, P.; Ricciardi, G.; Mascia, G.; Mascia, F.; Michelotti, F.; Mascioli, G.; Beltrami, M.; Padeletti, M.; Nesti, M.; et al. Cardiac resynchronization therapy improves functional status and cognition. Int. J. Cardiol. 2016, 219, 212–217. [Google Scholar] [CrossRef]
- Pipilas, D.C.; Hanley, A.; Singh, J.P.; Mela, T. Cardiac Contractility Modulation for Heart Failure: Current and Future Directions. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2 Part B, 101176. [Google Scholar] [CrossRef]
- Masarone, D.; Kittleson, M.M.; D’onofrio, A.; Falco, L.; Fumarulo, I.; Massetti, M.; Crea, F.; Aspromonte, N.; Pacileo, G. Basic science of cardiac contractility modulation therapy: Molecular and electrophysiological mechanisms. Heart Rhythm 2023, 21, 82–88. [Google Scholar] [CrossRef]
- Kuschyk, J.; Kloppe, A.; Schmidt-Schweda, S.; Bonnemeier, H.; Rousso, B.; Röger, S. Cardiac Contractility Modulation: A Technical Guide for Device Implantation. Rev. Cardiovasc. Med. 2017, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Muñoz, D.; Crespo-Leiro, M.G.; Fernández Lozano, I.; Zamorano Gómez, J.L.; Peinado Peinado, R.; Manzano Espinosa, L.; de Juan Bagudá, J.; Marco Del Castillo, Á.; Arribas Ynsaurriaga, F.; Salguero Bodes, R. Conduction system pacing and atrioventricular node ablation in heart failure: The PACE-FIB study design. ESC Heart Fail. 2023, 10, 3700–3709. [Google Scholar] [CrossRef]
- Ivanovski, M.; Mrak, M.; Mežnar, A.Z.; Žižek, D. Biventricular versus Conduction System Pacing after Atrioventricular Node Ablation in Heart Failure Patients with Atrial Fibrillation. J. Cardiovasc. Dev. Dis. 2022, 9, 209. [Google Scholar] [CrossRef]
- Rali, A.S.; Inampudi, C.; Zalawadiya, S.; Shah, A.; Teuteberg, J.J.; Stewart, G.C.; Cantor, R.S.; Deng, L.; Jacobs, J.P.; Kirklin, J.K.; et al. Changing Strategy Between Bridge to Transplant and Destination LVAD Therapy After the First 3 Months: Analysis of the STS-INTERMACS Database. J. Card. Fail. 2023, 30, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Weiss, E.S.; Schaffer, J.M.; Patel, N.D.; Ullrich, S.L.; Russell, S.D.; Shah, A.S.; Conte, J.V. Quality of life and functional status in patients surviving 12 months after left ventricular assist device implantation. J. Heart Lung Transplant. 2010, 29, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; MacIver, J.; Foroutan, F.; Alba, C.; Billia, F.; Rao, V. Long-term use of left ventricular assist devices: A report on clinical outcomes. Can. J. Surg. 2017, 60, 236–246. [Google Scholar] [CrossRef]
- Kilic, A.; Acker, M.A.; Atluri, P. Dealing with surgical left ventricular assist device complications. J. Thorac. Dis. 2015, 7, 2158–2164. [Google Scholar]
- Westerdahl, D.E.; Kobashigawa, J.A. Heart Transplantation for Advanced Heart Failure. Cardiac Intensive Care 2019, 504–524.e2. [Google Scholar]
- Weill, D. Lung transplantation: Indications and contraindications. J. Thorac. Dis. 2018, 10, 4574–4587. [Google Scholar] [CrossRef] [PubMed]
- Barghash, M.H.; Pinney, S.P. Heart Retransplantation: Candidacy, Outcomes, and Management. Curr. Transplant. Rep. 2019, 7, 12–17. [Google Scholar] [CrossRef]
- Nelson, J.; Alvey, N.; Bowman, L.; Schulte, J.; Segovia, M.C.; McDermott, J.; Te, H.S.; Kapila, N.; Levine, D.J.; Gottlieb, R.L.; et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: Endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacotherapy 2022, 42, 599–633. [Google Scholar] [CrossRef]
- Shah, M.R.; Starling, R.C.; Schwartz Longacre, L.; Mehra, M.R.; Working Group Participants. Heart transplantation research in the next decade—A goal to achieving evidence-based outcomes: National Heart, Lung, And Blood Institute Working Group. J. Am. Coll. Cardiol. 2012, 59, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.E.; Goodlin, S.J. End-of-Life Decisions and Palliative Care in Advanced Heart Failure. Can. J. Cardiol. 2016, 32, 1148–1156. [Google Scholar] [CrossRef]
- Ogundunmade, B.G.; John, D.O.; Chigbo, N.N. Ensuring quality of life in palliative care physiotherapy in developing countries. Front. Rehabil. Sci. 2024, 5, 1331885. [Google Scholar] [CrossRef]
- Lee, J.-H.; Hwang, K.-K. End-of-Life Care for End-stage Heart Failure Patients. Korean Circ. J. 2022, 52, 659–679. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Huang, X.; Wu, C.; Pórszász, R. Omecamtiv Mecarbil in the treatment of heart failure: The past, the present, and the future. Front. Cardiovasc. Med. 2024, 11, 1337154. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, J.R.; Felker, G.M.; McMurray, J.J.V.; Ponikowski, P.; Metra, M.; Filippatos, G.S.; Ezekowitz, J.A.; Dickstein, K.; Cleland, J.G.F.; Kim, J.B.; et al. Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure: The ATOMIC-AHF Study. J. Am. Coll. Cardiol. 2016, 67, 1444–1455. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Felker, G.M.; McMurray, J.J.; Solomon, S.D.; Adams, K.F., Jr.; Cleland, J.G.; Ezekowitz, J.A.; Goudev, A.; Macdonald, P.; Metra, M.; et al. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): A phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet 2016, 388, 2895–2903. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Diaz, R.; Felker, G.M.; McMurray, J.J.V.; Metra, M.; Solomon, S.D.; Adams, K.F.; Anand, I.; Arias-Mendoza, A.; Biering-Sørensen, T.; et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N. Engl. J. Med. 2021, 384, 105–116. [Google Scholar] [CrossRef]
- Lewis, G.D.; Voors, A.A.; Cohen-Solal, A.; Metra, M.; Whellan, D.J.; Ezekowitz, J.A.; Böhm, M.; Teerlink, J.R.; Docherty, K.F.; Lopes, R.D.; et al. Effect of Omecamtiv Mecarbil on Exercise Capacity in Chronic Heart Failure With Reduced Ejection Fraction: The METEORIC-HF Randomized Clinical Trial. JAMA 2022, 328, 259–269. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlávek, J.; Böhm, M.; Chiang, C.-E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients with Heart Failure with and Without Diabetes. JAMA 2020, 323, 1353–1368. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Vaduganathan, M.; Claggett, B.L.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Baseline Characteristics of Patients with HF With Mildly Reduced and Preserved Ejection Fraction: DELIVER Trial. JACC Heart Fail. 2022, 10, 184–197. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Saia, F.; Loforte, A.; Pacini, D. Innovative transcatheter procedures for the treatment of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 292–300. [Google Scholar] [CrossRef]
- Schuler, A.; Nyman, C.; Wollborn, J.; Sheu, R. Illuminating the Significance of the Clinical Trial to Evaluate Cardiovascular Outcomes In Patients Treated With the Tricuspid Valve Repair System Pivotal Study. J. Cardiothorac. Vasc. Anesth. 2023, 37, 2173–2175. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Hahn, R.T.; Eleid, M.F.; Kipperman, R.; Smith, R.; Lim, D.S.; Gray, W.A.; Narang, A.; Pislaru, S.V.; Koulogiannis, K.; et al. Feasibility Study of the Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2021, 77, 345–356. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Edwards EVOQUE Transcatheter Tricuspid Valve Replacement: Pivotal Clinical Investigation of Safety and Clinical Efficacy Using a Novel Device. ClinicalTrials.gov Identifier: NCT04482062. Available online: https://clinicaltrials.gov/ct2/show/NCT04482062 (accessed on 27 January 2022).
- Navia, J.L.; Kapadia, S.; Elgharably, H.; Harb, S.C.; Krishnaswamy, A.; Unai, S.; Mick, S.; Rodriguez, L.; Hammer, D.; Gillinov, A.M.; et al. First-in-Human Implantations of the NaviGate Bioprosthesis in a Severely Dilated Tricuspid Annulus and in a Failed Tricuspid Annuloplasty Ring. Circ. Cardiovasc. Interv. 2017, 10, e005840. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. The Early Feasibility Study of the Transcatheter Tricuspid Valve Replacement System Transfemoral System. ClinicalTrials.gov Identifier: NCT04433065. Available online: https://clinicaltrials.gov/ct2/show/NCT04433065 (accessed on 27 January 2022).
- Lu, F.L.; Ma, Y.; An, Z.; Cai, C.L.; Li, B.L.; Song, Z.G.; Han, L.; Wang, J.; Qiao, F.; Xu, Z.Y. First-in-Man Experience of Transcatheter Tricuspid Valve Replacement with LuX-Valve in High-Risk Tricuspid Regurgitation Patients. JACC Cardiovasc. Interv. 2020, 13, 1614–1616. [Google Scholar] [CrossRef]
- Nietlispach, F. Tricinch for TR: Technique and outcomes. Presented at the Transcatheter Valve Therapies, Chicago, IL, USA, 14–17 June 2017. [Google Scholar]
- ClinicalTrials.gov. Safety and Performance of the Trialign Percutaneous Tricuspid Valve Annuloplasty System (PTVAS) for Symptomatic Chronic Functional Tricuspid Regurgitation. ClinicalTrials.gov Identifier: NCT03225612. Available online: https://clinicaltrials.gov/ct2/show/NCT03225612 (accessed on 27 January 2022).
- Greenbaum, A. Transcatheter tricuspid valve repair: Available techniques and patient candidates criteria. Presented at the Cardiovascular Research Technologies, Washington, DC, USA, 3–6 March 2018. [Google Scholar]
- Al Hazzouri, A.; Attieh, P.; Sleiman, C.; Hamdan, R.; Ghadieh, H.E.; Harbieh, B. Left Ventricular Assist Device in Advanced Refractory Heart Failure: A Comprehensive Review of Patient Selection, Surgical Approaches, Complications and Future Perspectives. Diagnostics 2024, 14, 2480. [Google Scholar] [CrossRef]
- Dual, S.A.; Cowger, J.; Roche, E.; Nayak, A. The Future of Durable Mechanical Circulatory Support: Emerging Technological Innovations and Considerations to Enable Evolution of the Field. J. Card. Fail. 2024, 30, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Pumpinheart. Available online: https://pumpinheart.com/ (accessed on 21 December 2023).
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Taherdoost, H.; Ghofrani, A. AI’s role in revolutionizing personalized medicine by reshaping pharmacogenomics and drug therapy. Intell. Pharm. 2024, 2, 643–650. [Google Scholar] [CrossRef]
- Bhaltadak, V.; Ghewade, B.; Yelne, S. A Comprehensive Review on Advancements in Wearable Technologies: Revolutionizing Cardiovascular Medicine. Cureus 2024, 16, e61312. [Google Scholar] [CrossRef]
- Yogev, D.; Goldberg, T.; Arami, A.; Tejman-Yarden, S.; Winkler, T.E.; Maoz, B.M. Current state of the art and future directions for implantable sensors in medical technology: Clinical needs and engineering challenges. APL Bioeng. 2023, 7, 031506. [Google Scholar] [CrossRef]
- Lee, D.S.M.; Cardone, K.M.; Zhang, D.Y.; Tsao, N.L.; Abramowitz, S.; Sharma, P.; DePaolo, J.S.; Conery, M.; Aragam, K.G.; Biddinger, K.; et al. Common- and rare-variant genetic architecture of heart failure across the allele frequency spectrum. medRxiv [Preprint] 2024. [Google Scholar] [CrossRef]
- Patel, K.K.; Venkatesan, C.; Abdelhalim, H.; Zeeshan, S.; Arima, Y.; Linna-Kuosmanen, S.; Ahmed, Z. Genomic approaches to identify and investigate genes associated with atrial fibrillation and heart failure susceptibility. Hum. Genom. 2023, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; D’ambrosio, M.F.; Correale, M.; Brunetti, N.D.; Santacroce, R.; Iacoviello, M.; Margaglione, M. The Role of Genetics in the Management of Heart Failure Patients. Int. J. Mol. Sci. 2023, 24, 15221. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Ayyadurai, P.; Saad, M.; Kosmas, C.E.; Dogar, M.U.; Patel, U.; Vittorio, T.J. Galactin-3 and soluble ST2 as complementary tools to cardiac MRI for sudden cardiac death risk stratification in heart failure: A review. JRSM Cardiovasc. Dis. 2020, 9, 2048004020957840. [Google Scholar] [CrossRef]
- Boehm, K.M.; Khosravi, P.; Vanguri, R.; Gao, J.; Shah, S.P. Harnessing multimodal data integration to advance precision oncology. Nat. Rev. Cancer 2021, 22, 114–126. [Google Scholar] [CrossRef]
- Sokos, G.; Kido, K.; Panjrath, G.; Benton, E.; Page, R., 2nd; Patel, J.; Smith, P.J.; Korous, S.; Guglin, M. Multidisciplinary Care in Heart Failure Services. J. Card. Fail. 2023, 29, 943–958. [Google Scholar] [CrossRef]
- Shrivastava, A.; Haase, T.; Zeller, T.; Schulte, C. Biomarkers for Heart Failure Prognosis: Proteins, Genetic Scores and Non-coding RNAs. Front. Cardiovasc. Med. 2020, 7, 601364. [Google Scholar] [CrossRef]
- Mazimba, S. Toward equitable utilization of durable left ventricular assist device therapy in advanced heart failure—Raising the veil of health disparities. J. Card. Surg. 2022, 37, 3595–3597. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef]
- Marvasti, T.B.; Gao, Y.; Murray, K.R.; Hershman, S.; McIntosh, C.; Moayedi, Y. Unlocking Tomorrow’s Health Care: Expanding the Clinical Scope of Wearables by Applying Artificial Intelligence. Can. J. Cardiol. 2024, 40, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Abul-Husn, N.S.; Kenny, E.E. Personalized Medicine and the Power of Electronic Health Records. Cell 2019, 177, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Endalamaw, A.; Khatri, R.B.; Erku, D.; Zewdie, A.; Wolka, E.; Nigatu, F.; Assefa, Y. Barriers and strategies for primary health care workforce development: Synthesis of evidence. BMC Prim. Care 2024, 25, 99. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value/Percentage |
|---|---|
| Percentage of AHF among patients with HF | 5–10% [10,15] |
| Annual mortality rate | 20–50% [16] |
| Hospitalizations | 80% of AHF patients are hospitalized per year [16] |
| Incidence in patients > 65 years old | 10% [10] |
| Diabetes mellitus | 40% [16] |
| Chronic kidney disease | 30–50% [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepetes, N.-I.; Kourek, C.; Papamichail, A.; Xanthopoulos, A.; Kostakou, P.; Paraskevaidis, I.; Briasoulis, A. Transition to Advanced Heart Failure: From Identification to Improving Prognosis. J. Cardiovasc. Dev. Dis. 2025, 12, 104. https://doi.org/10.3390/jcdd12030104
Tepetes N-I, Kourek C, Papamichail A, Xanthopoulos A, Kostakou P, Paraskevaidis I, Briasoulis A. Transition to Advanced Heart Failure: From Identification to Improving Prognosis. Journal of Cardiovascular Development and Disease. 2025; 12(3):104. https://doi.org/10.3390/jcdd12030104
Chicago/Turabian StyleTepetes, Nikolaos-Iason, Christos Kourek, Adamantia Papamichail, Andrew Xanthopoulos, Peggy Kostakou, Ioannis Paraskevaidis, and Alexandros Briasoulis. 2025. "Transition to Advanced Heart Failure: From Identification to Improving Prognosis" Journal of Cardiovascular Development and Disease 12, no. 3: 104. https://doi.org/10.3390/jcdd12030104
APA StyleTepetes, N.-I., Kourek, C., Papamichail, A., Xanthopoulos, A., Kostakou, P., Paraskevaidis, I., & Briasoulis, A. (2025). Transition to Advanced Heart Failure: From Identification to Improving Prognosis. Journal of Cardiovascular Development and Disease, 12(3), 104. https://doi.org/10.3390/jcdd12030104












