Effect of Anisotropic Electrical Conductivity Induced by Fiber Orientation on Ablation Characteristics of Pulsed Field Ablation in Atrial Fibrillation Treatment: A Computational Study
Abstract
1. Introduction
2. Materials and Methods
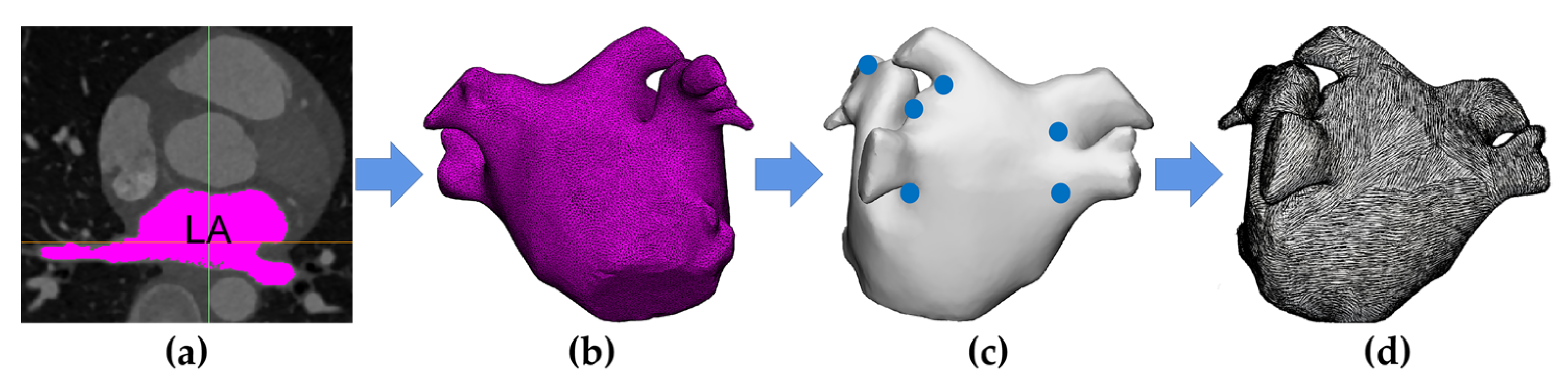
2.1. Model Construction
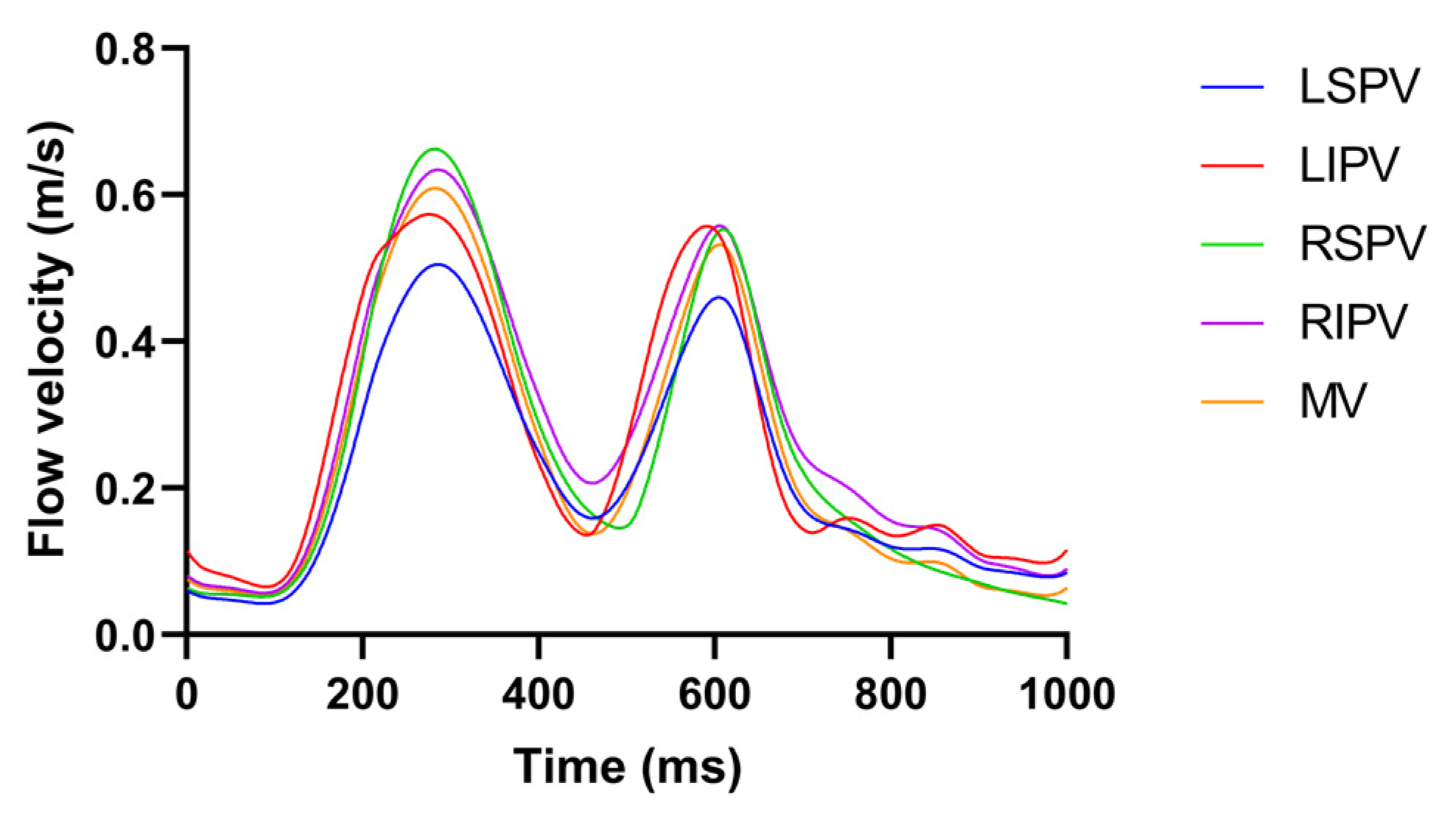
2.2. Pulsatile Blood Flow Profiles
2.3. Computational Study
2.3.1. Governing Equations
- Electrical Equations
- Thermal Equations
- CFD Equations
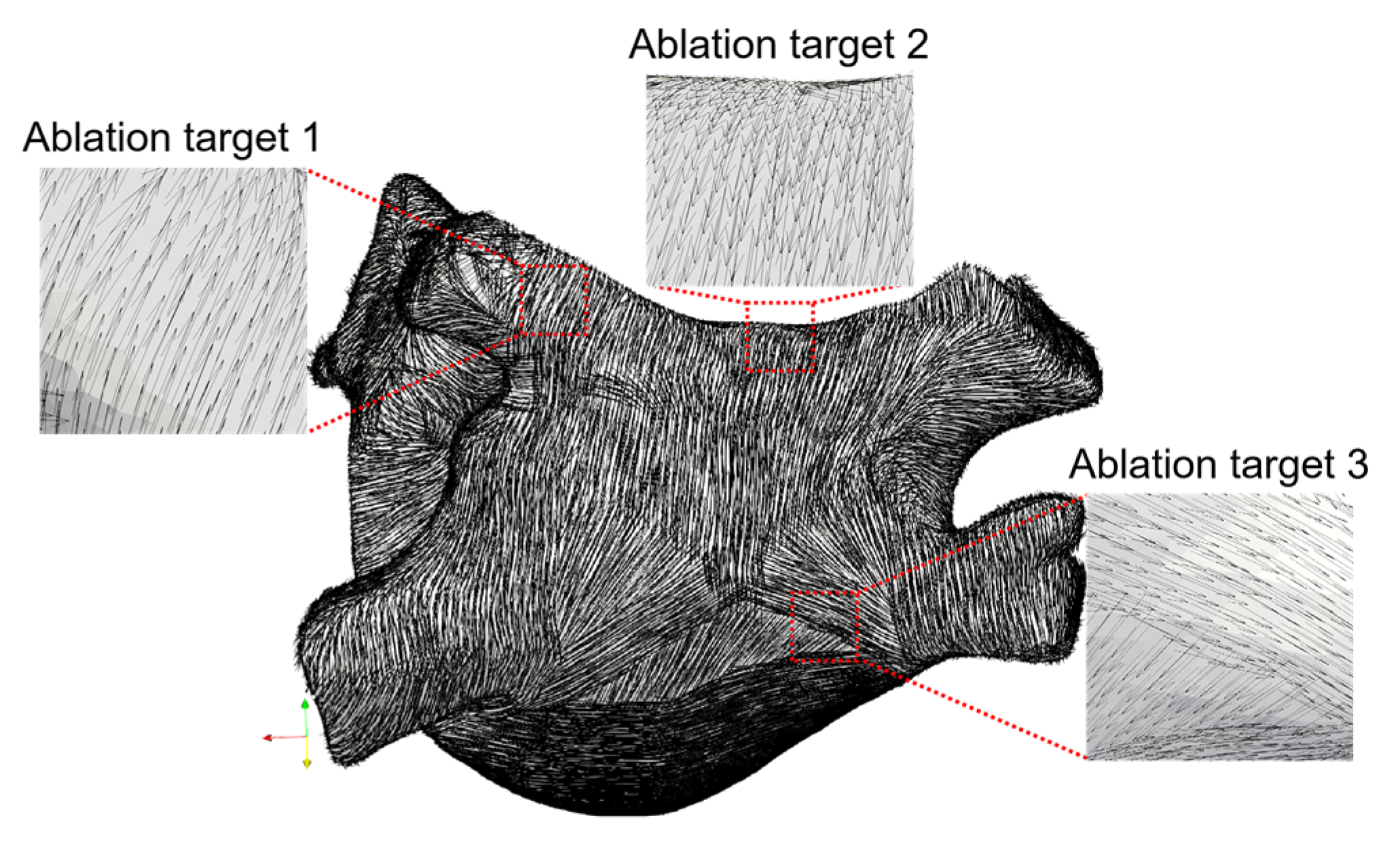
2.3.2. Selection of Ablation Targets
2.3.3. Domain
2.3.4. Boundary Conditions
- Electrical Boundary Conditions
- In the computation of the temperature distribution, the amplitude of the is 1000 V, and the total PFA duration is 1 s, containing five pulse trains and five pulse train intervals; each pulse train contains eight pulses and eight pulse intervals, each pulse width and pulse interval are both 100 μs, and the pulse train interval is 198.4 ms, to consist with the parameters of PFA generators used in the animal experiment [28]. The waveform of in the computation of the temperature distribution is shown in Figure 4.
- Thermal Boundary Conditions
- Initial temperature boundary condition: At t = 0, the initial temperature of the myocardium T0 and the blood Tb was set to 37 °C.
- The second type of thermal boundary condition (constant heat flux boundary condition): The boundary of the plastic catheter and the epicardium represented the zero heat flux conditions and is expressed as:
- CFD Boundary Conditions
2.3.5. Material Properties
2.4. Statistical Analysis
3. Results
3.1. Estimated Fiber Orientation
3.2. Surface Ablation Area
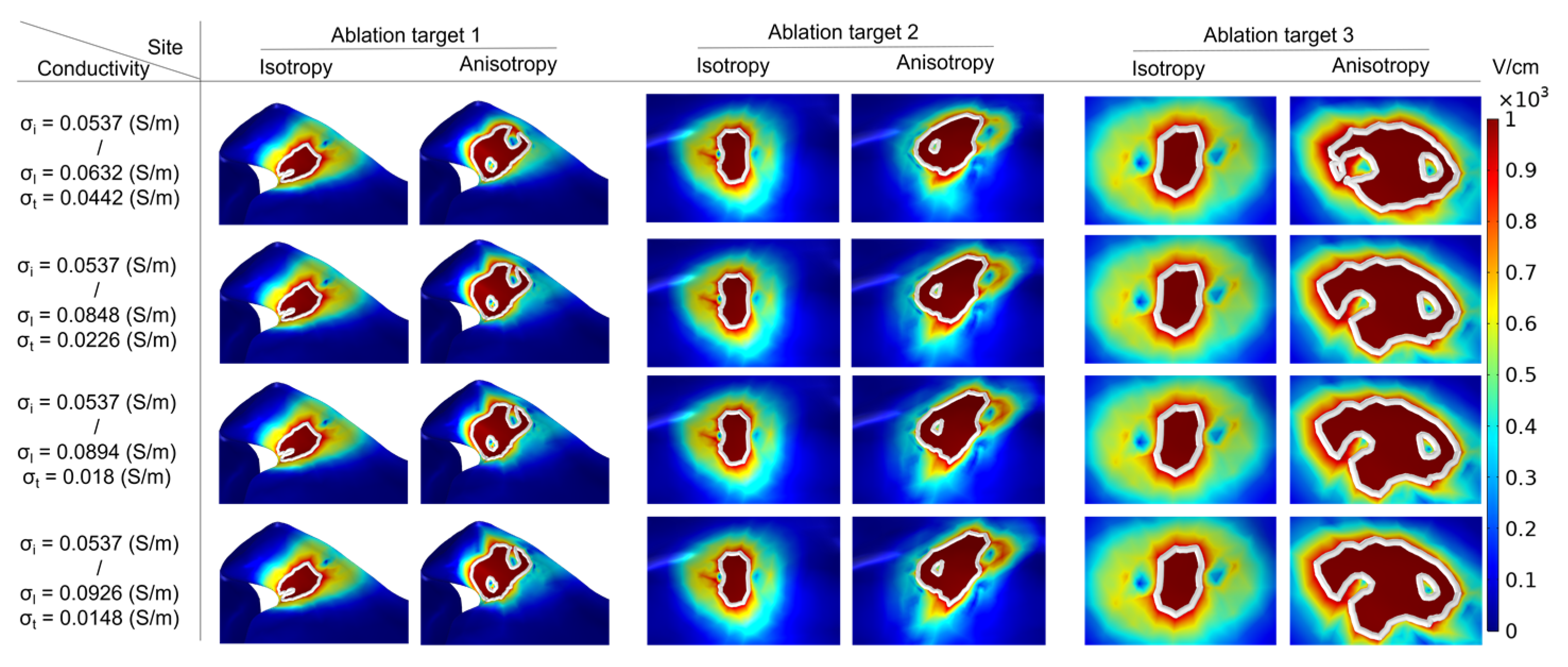
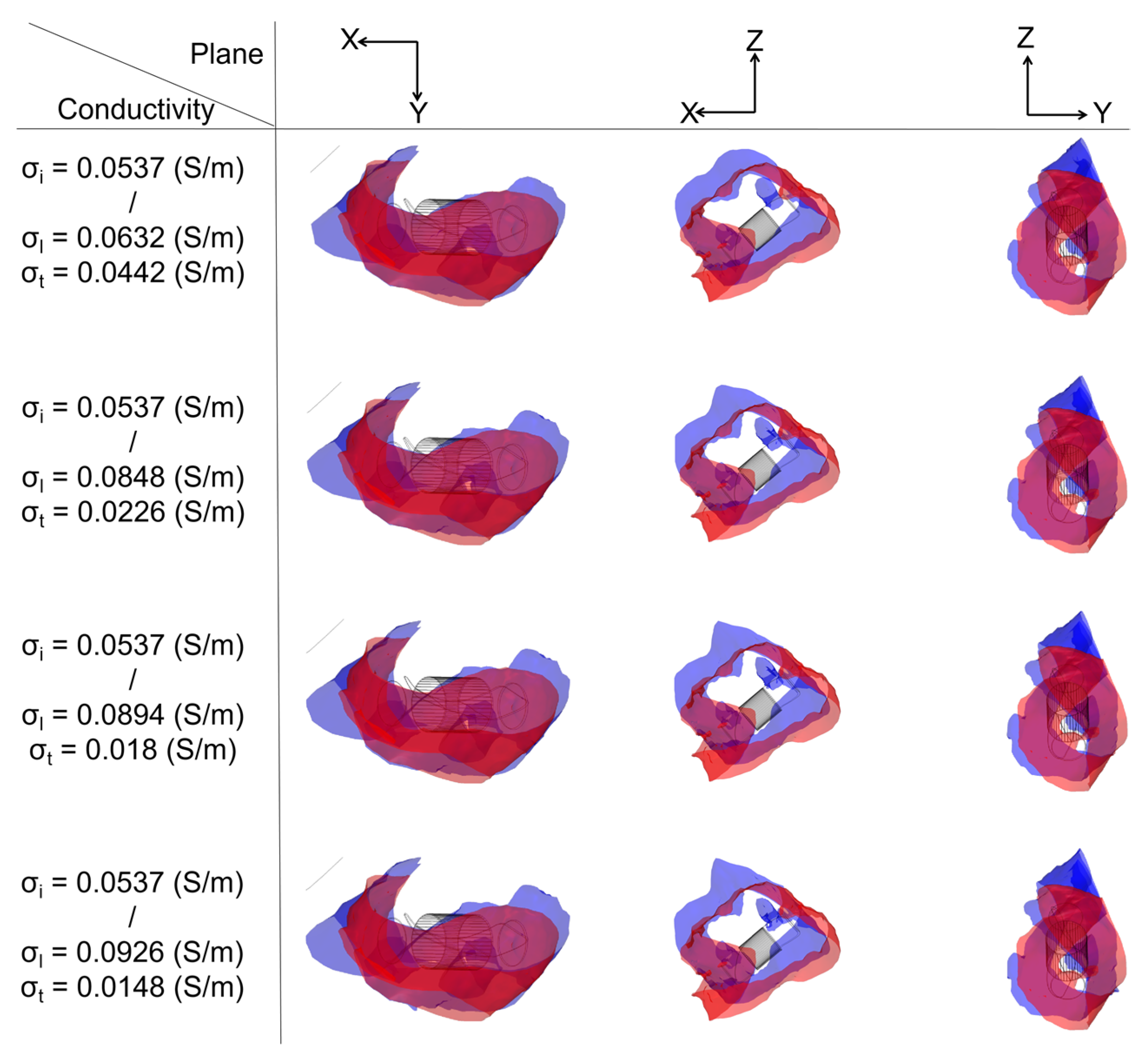
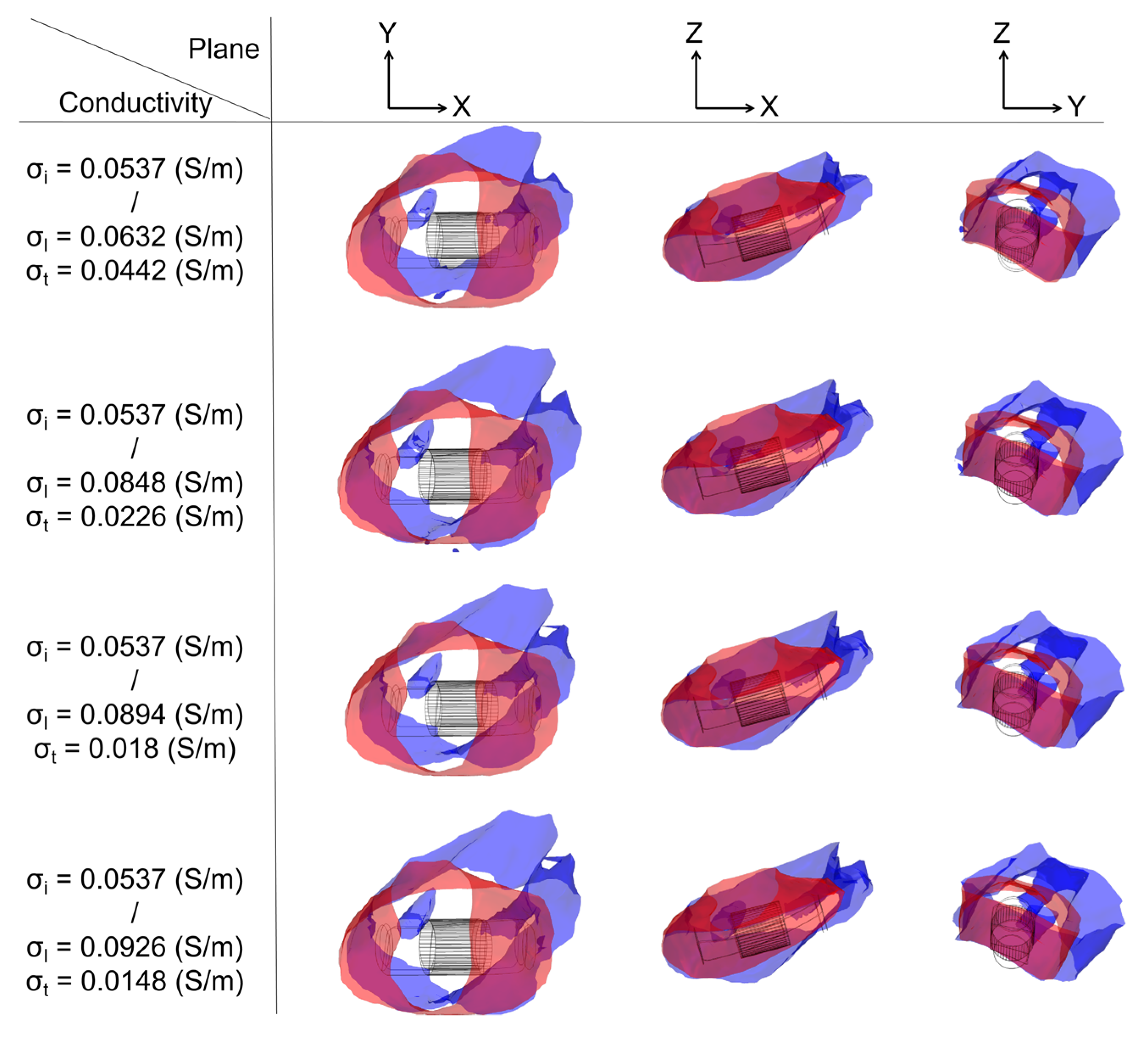
3.3. Ablation Isosurface and Ablation Volume
3.3.1. Ablation Isosurface
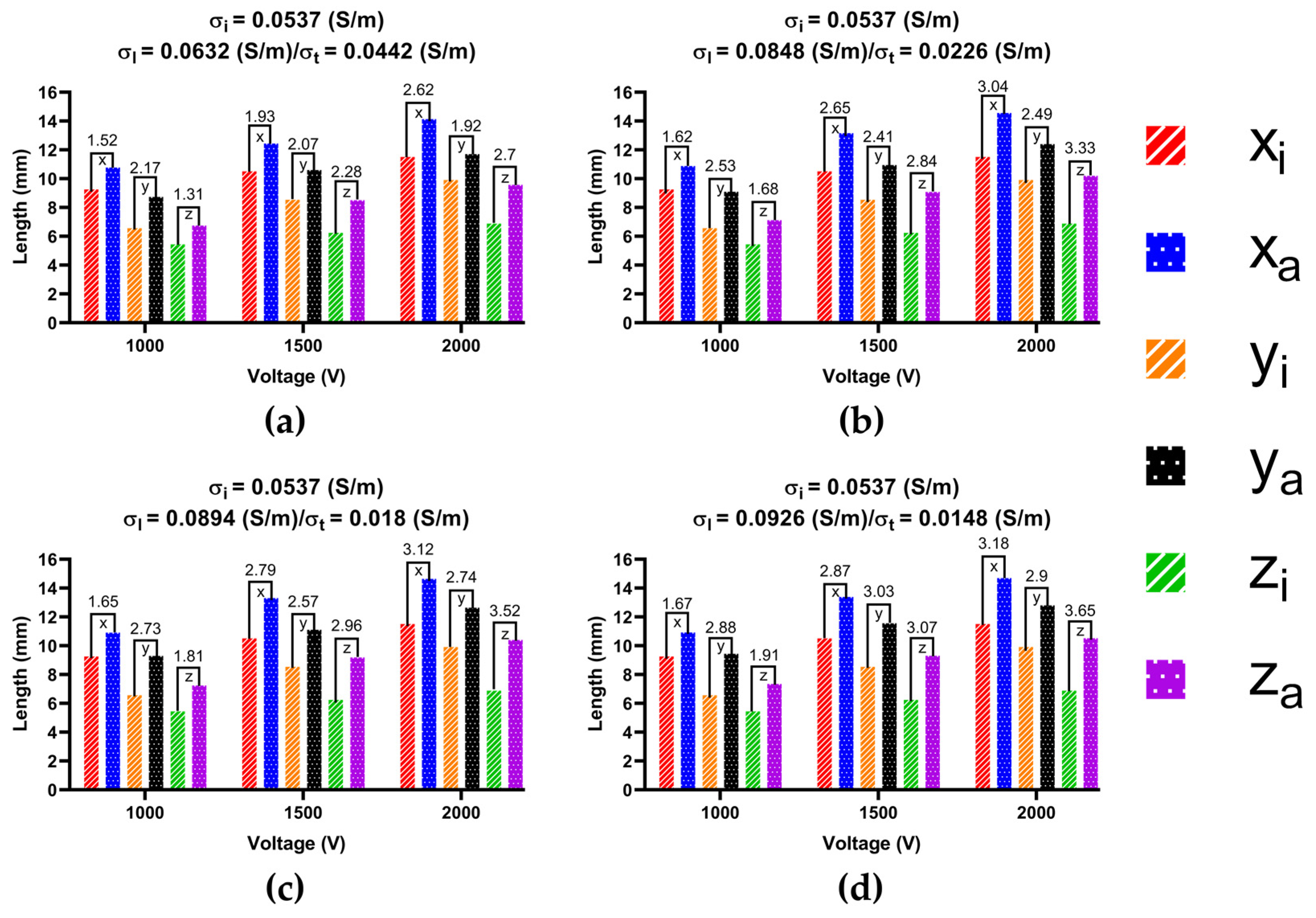
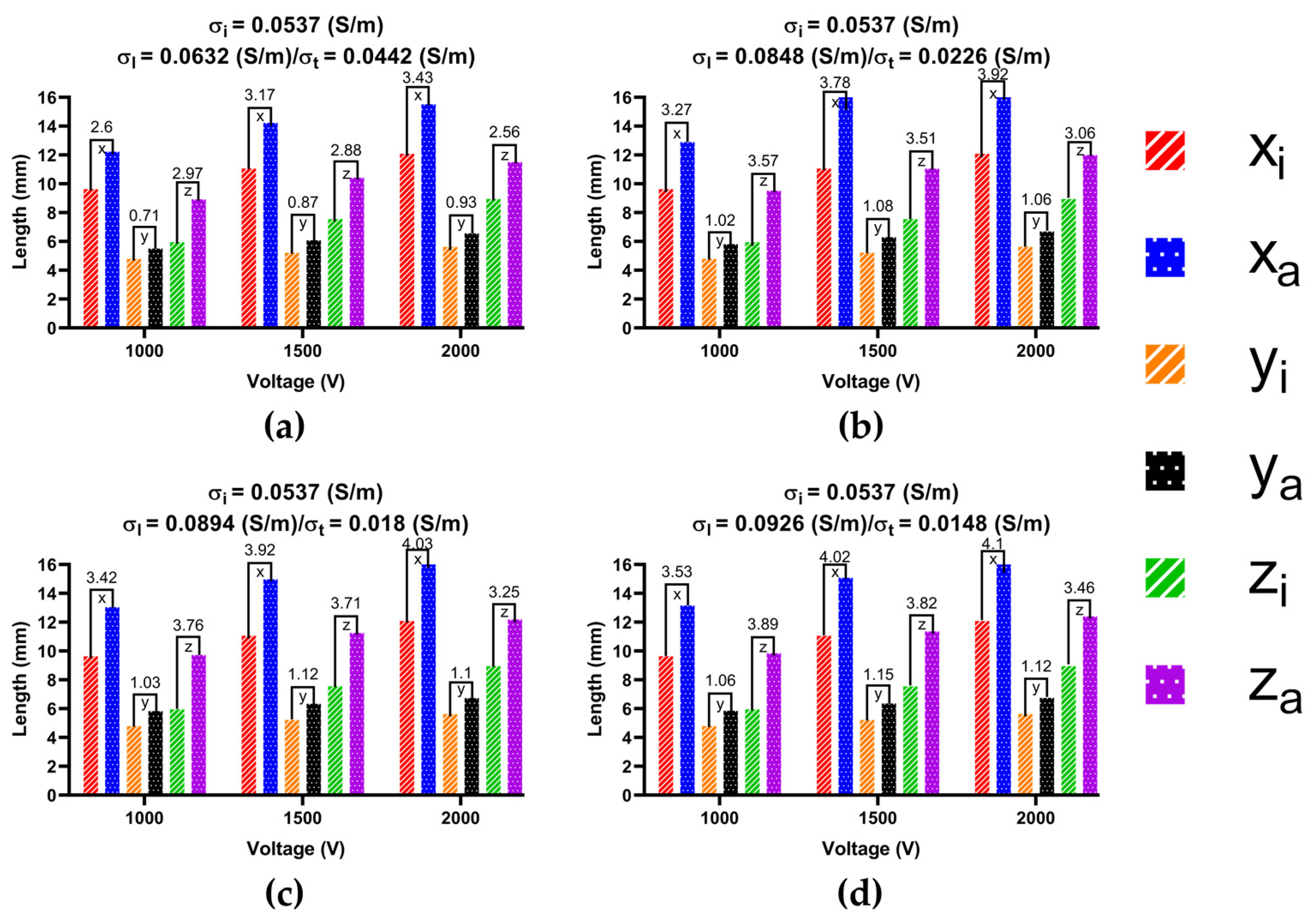
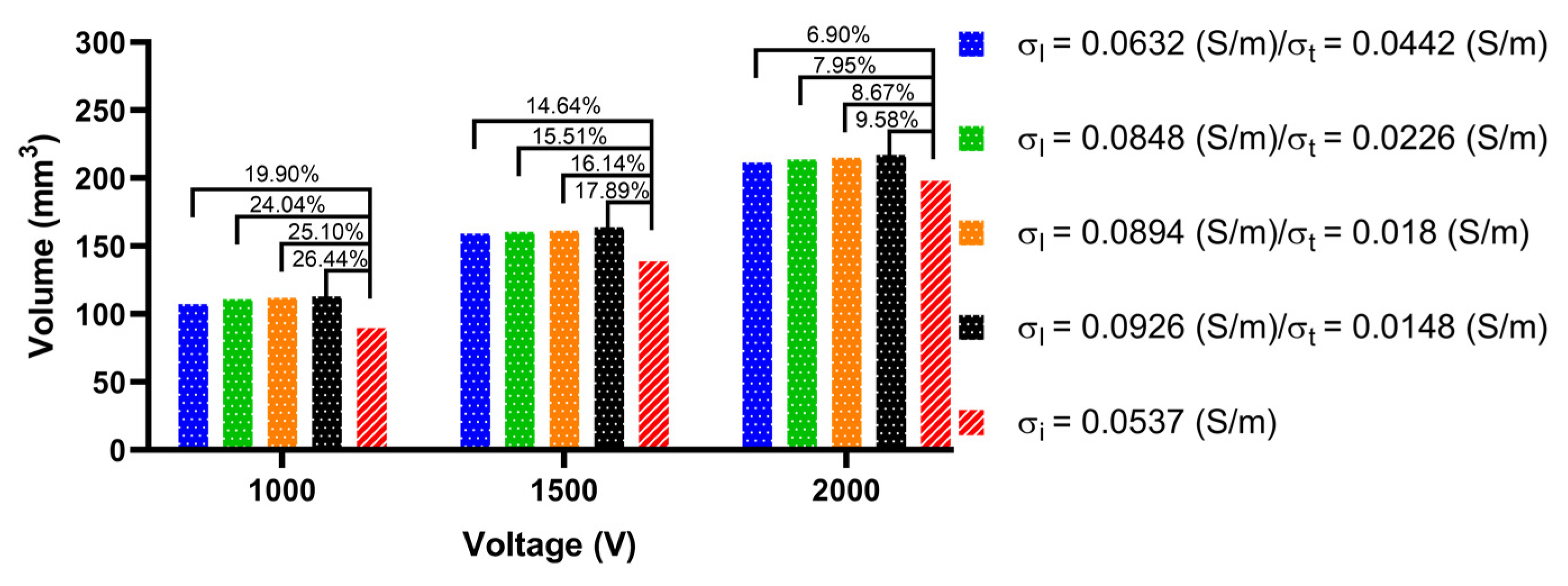
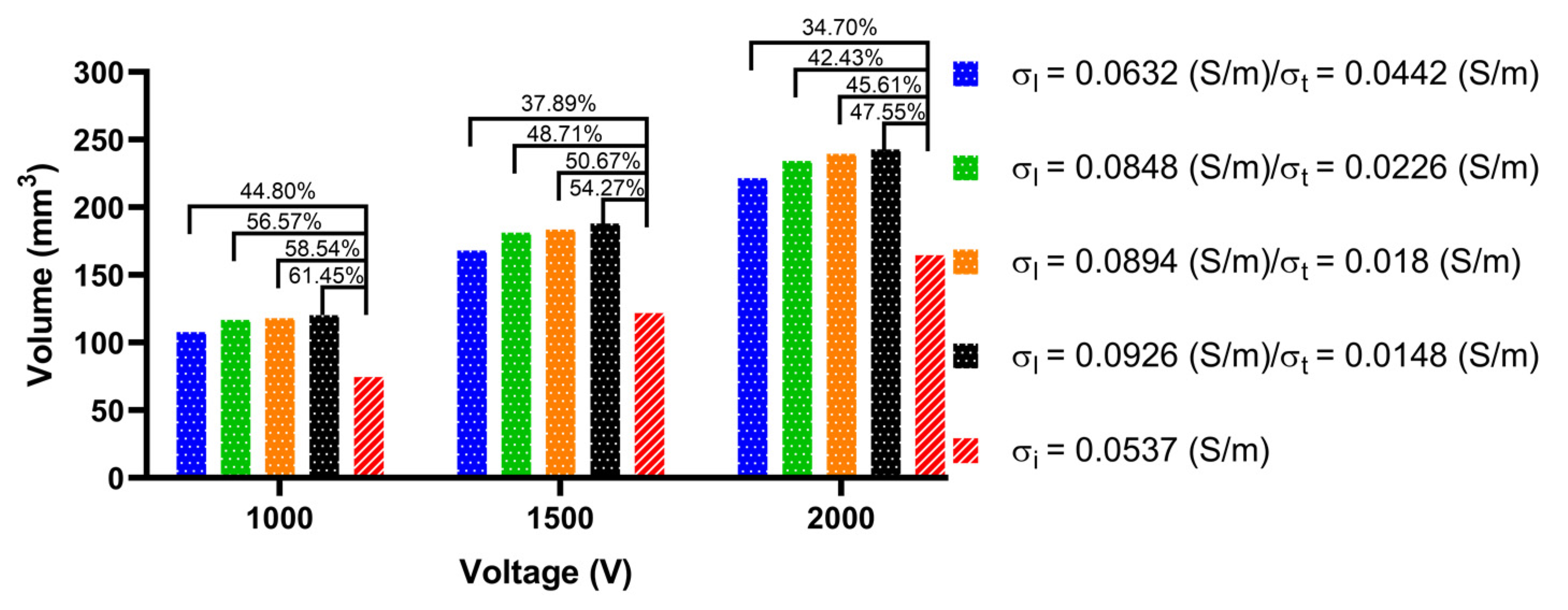
3.3.2. Ablation Volume
3.4. Temperature Increase
4. Discussion
4.1. Importance of Fiber Orientation
4.2. Effect of Different Ablation Targets
4.3. Effect of Different Electrical Conductivity
4.4. The Differences of Male/Female in Model Construction
4.5. Compared with the Experimental Results
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, F.; Zemlin, C.W. Effect of Twisted Fiber Anisotropy in Cardiac Tissue on Ablation with Pulsed Electric Fields. PLoS ONE 2016, 11, e0152262. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Asivatham, S.J.; Deneke, T.; Castellvi, Q.; Neal, R.E., 2nd. Primer on Pulsed Electrical Field Ablation: Understanding the Benefits and Limitations. Circ. Arrhythmia Electrophysiol. 2021, 14, e010086. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.T.; Haines, D.E.; Miklavcic, D.; Kos, B.; Kirchhof, N.; Barka, N.; Mattison, L.; Martien, M.; Onal, B.; Howard, B.; et al. Safety and chronic lesion characterization of pulsed field ablation in a Porcine model. J. Cardiovasc. Electrophysiol. 2021, 32, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Belalcazar, A. Safety and efficacy aspects of pulsed field ablation catheters as a function of electrode proximity to blood and energy delivery method. Heart rhythm O2 2021, 2, 560–569. [Google Scholar] [CrossRef]
- Tsai, J.Z.; Will, J.A.; Vorperian, V.R.; Hubbard-van Stelle, S.; Cao, H.; Tungjitkusolmun, S.; Choy, Y.B.; Webster, J.G. In vitro measurement of myocardial impedivity anisotropy with a miniature rectangular tube. IEEE Trans. Biomed. Eng. 2003, 50, 528–532. [Google Scholar] [CrossRef]
- Loewe, A.; Krueger, M.W.; Platonov, P.G.; Holmqvist, F.; Dössel, O.; Seemann, G. Left and Right Atrial Contribution to the P-wave in Realistic Computational Models. In Proceedings of the Functional Imaging and Modeling of the Heart, Maastricht, The Netherlands, 25–27 June 2015; pp. 439–447. [Google Scholar]
- Boyle, P.M.; Zghaib, T.; Zahid, S.; Ali, R.L.; Deng, D.; Franceschi, W.H.; Hakim, J.B.; Murphy, M.J.; Prakosa, A.; Zimmerman, S.L.; et al. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat. Biomed. Eng. 2019, 3, 870–879. [Google Scholar] [CrossRef]
- Zhao, J.; Butters, T.D.; Zhang, H.; Pullan, A.J.; LeGrice, I.J.; Sands, G.B.; Smaill, B.H. An image-based model of atrial muscular architecture: Effects of structural anisotropy on electrical activation. Circ. Arrhythmia Electrophysiol. 2012, 5, 361–370. [Google Scholar] [CrossRef]
- Kaminska, I.; Kotulska, M.; Stecka, A.; Saczko, J.; Drag-Zalesinska, M.; Wysocka, T.; Choromanska, A.; Skolucka, N.; Nowicki, R.; Marczak, J.; et al. Electroporation-induced changes in normal immature rat myoblasts (H9C2). Gen. Physiol. Biophys. 2012, 31, 19–25. [Google Scholar] [CrossRef]
- Razeghi, O.; Solis-Lemus, J.A.; Lee, A.W.C.; Karim, R.; Corrado, C.; Roney, C.H.; de Vecchi, A.; Niederer, S.A. CemrgApp: An interactive medical imaging application with image processing, computer vision, and machine learning toolkits for cardiovascular research. SoftwareX 2020, 12, 100570. [Google Scholar] [CrossRef]
- Xiong, Z.; Xia, Q.; Hu, Z.; Huang, N.; Bian, C.; Zheng, Y.; Vesal, S.; Ravikumar, N.; Maier, A.; Yang, X.; et al. A global benchmark of algorithms for segmenting the left atrium from late gadolinium-enhanced cardiac magnetic resonance imaging. Med. Image Anal. 2021, 67, 101832. [Google Scholar] [CrossRef]
- Varela, M.; Morgan, R.; Theron, A.; Dillon-Murphy, D.; Chubb, H.; Whitaker, J.; Henningsson, M.; Aljabar, P.; Schaeffter, T.; Kolbitsch, C.; et al. Novel MRI Technique Enables Non-Invasive Measurement of Atrial Wall Thickness. IEEE Trans. Med. Imaging 2017, 36, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, R.; Funamoto, K.; Hayase, T.; Kanke, Y.; Shibata, M.; Shiraishi, Y.; Yambe, T. Numerical analysis of hemodynamic changes in the left atrium due to atrial fibrillation. J. Biomech. 2015, 48, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Geuzaine, C.; Remacle, J.-F. Gmsh: A 3-D finite element mesh generator with built-in pre- and post-processing facilities. Int. J. Numer. Methods Eng. 2009, 79, 1309–1331. [Google Scholar] [CrossRef]
- McDowell, K.S.; Vadakkumpadan, F.; Blake, R.; Blauer, J.; Plank, G.; Macleod, R.S.; Trayanova, N.A. Mechanistic inquiry into the role of tissue remodeling in fibrotic lesions in human atrial fibrillation. Biophys. J. 2013, 104, 2764–2773. [Google Scholar] [CrossRef]
- Wachter, A.; Loewe, A.; Krueger, M.W.; Dössel, O.; Seemann, G. Mesh structure-independent modeling of patient-specific atrial fiber orientation. Curr. Dir. Biomed. Eng. 2015, 1, 409–412. [Google Scholar] [CrossRef]
- Fyrenius, A.; Wigström, L.; Ebbers, T.; Karlsson, M.; Engvall, J.; Bolger, A.F. Three dimensional flow in the human left atrium. Heart 2001, 86, 448–455. [Google Scholar] [CrossRef]
- De Marchi, S.F.; Bodenmüller, M.; Lai, D.L.; Seiler, C. Pulmonary venous flow velocity patterns in 404 individuals without cardiovascular disease. Heart 2001, 85, 23–29. [Google Scholar] [CrossRef][Green Version]
- Fluckiger, J.U.; Goldberger, J.J.; Lee, D.C.; Ng, J.; Lee, R.; Olsen, A.B.; Carr, J.; Markl, M. Quantification of left atrial flow velocity distribution in atrial fibrillation using 4D flow MRI. J. Cardiovasc. Magn. Reson. 2013, 15, 261. [Google Scholar] [CrossRef]
- MIT. Steady Ohmic Conduction. Available online: http://web.mit.edu/6.013_book/www/chapter7/7.2.html (accessed on 25 May 2022).
- Gu, K.; Yan, S.; Wu, X. Effect of anisotropy in myocardial electrical conductivity on lesion characteristics during radiofrequency cardiac ablation: A numerical study. Int. J. Hyperth. 2022, 39, 120–133. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1998, 85, 5–34. [Google Scholar] [CrossRef]
- Parés, C.; Berjano, E.; González-Suárez, A. Effect of intracardiac blood flow pulsatility during radiofrequency cardiac ablation: Computer modeling study. Int. J. Hyperth. 2021, 38, 316–325. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; Chen, S.A.; Crijns, H.J.; Damiano, R.J., Jr.; Davies, D.W.; DiMarco, J.; et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012, 9, 632–696.e621. [Google Scholar] [CrossRef] [PubMed]
- Proietti, R.; Santangeli, P.; Di Biase, L.; Joza, J.; Bernier, M.L.; Wang, Y.; Sagone, A.; Viecca, M.; Essebag, V.; Natale, A. Comparative effectiveness of wide antral versus ostial pulmonary vein isolation: A systematic review and meta-analysis. Circ. Arrhythmia Electrophysiol. 2014, 7, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.R.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef]
- Lavee, J.; Onik, G.; Mikus, P.; Rubinsky, B. A novel nonthermal energy source for surgical epicardial atrial ablation: Irreversible electroporation. Heart Surg. Forum 2007, 10, E162–E167. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, Y.H.; Mao, Y.K.; Jiang, C.Y.; Fan, J.R.; Luo, K. Numerical prediction of thrombosis risk in left atrium under atrial fibrillation. Math. Biosci. Eng. 2020, 17, 2348–2360. [Google Scholar] [CrossRef]
- González-Suárez, A.; Irastorza, R.M.; Deane, S.; O’Brien, B.; O’Halloran, M.; Elahi, A. Full torso and limited-domain computer models for epicardial pulsed electric field ablation. Comput. Methods Programs Biomed. 2022, 221, 106886. [Google Scholar] [CrossRef]
- Hasgall, P.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. Available online: www.itis.ethz.ch/database (accessed on 3 August 2022).
- Krueger, M.W.; Rhode, K.S.; O’Neill, M.D.; Rinaldi, C.A.; Gill, J.; Razavi, R.; Seemann, G.; Doessel, O. Patient-specific modeling of atrial fibrosis increases the accuracy of sinus rhythm simulations and may explain maintenance of atrial fibrillation. J. Electrocardiol. 2014, 47, 324–328. [Google Scholar] [CrossRef]
- Yan, S.; Wu, X.; Wang, W. A simulation study to compare the phase-shift angle radiofrequency ablation mode with bipolar and unipolar modes in creating linear lesions for atrial fibrillation ablation. Int. J. Hyperthermia 2016, 32, 231–238. [Google Scholar] [CrossRef][Green Version]
- Labarthe, S.; Coudiere, Y.; Henry, J.; Cochet, H. A semi-automatic method to construct atrial fibre structures: A tool for atrial simulations. In Proceedings of the Computers in Cardiology (CinC), Krakow, Poland, 9–12 September 2012; pp. 881–884. [Google Scholar]
- González-Suárez, A.; Pérez, J.J.; Berjano, E. Should fluid dynamics be included in computer models of RF cardiac ablation by irrigated-tip electrodes? Biomed. Eng. Online 2018, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.; Eliakim, M. The Junction Between the Left Atrium and the Pulmonary Veins: An Anatomic Study of Human Hearts. Circulation 1966, 34, 412. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.S.; Anderson, R.H.; Damián, S.Q. Atrial structure and fibres: Morphologic bases of atrial conduction. Cardiovasc. Res. 2002, 54, 325–336. [Google Scholar] [CrossRef]
- Ho, S.Y.; Sanchez-Quintana, D. The importance of atrial structure and fibers. Clin. Anat. 2009, 22, 52–63. [Google Scholar] [CrossRef]
- Zhao, L.; Rasko, A.; Drescher, C.; Maleki, S.; Cejnar, M.; McEwan, A. Preliminary Validation of Electroporation-Electrolysis (E2) for Cardiac Ablation Using a Parameterisable In-Vivo Model. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 289–293. [Google Scholar] [CrossRef]
- Avazzadeh, S.; O’Brien, B.; Coffey, K.; O’Halloran, M.; Keane, D.; Quinlan, L.R. Establishing Irreversible Electroporation Electric Field Potential Threshold in A Suspension In Vitro Model for Cardiac and Neuronal Cells. J. Clin. Med. 2021, 10, 5443. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, H.; Zang, L.; Yan, S.; Wu, X. The Effect of Discharge Mode on the Distribution of Myocardial Pulsed Electric Field-A Simulation Study for Pulsed Field Ablation of Atrial Fibrillation. J. Cardiovasc. Dev. Dis. 2022, 9, 95. [Google Scholar] [CrossRef]
- Pashakhanloo, F.; Herzka, D.A.; Ashikaga, H.; Mori, S.; Gai, N.; Bluemke, D.A.; Trayanova, N.A.; McVeigh, E.R. Myofiber Architecture of the Human Atria as Revealed by Submillimeter Diffusion Tensor Imaging. Circ. Arrhythmia Electrophysiol. 2016, 9, e004133. [Google Scholar] [CrossRef]
- Varela, M.; Zhao, J.; Aslanidi, O.V. Determination of Atrial Myofibre Orientation Using Structure Tensor Analysis for Biophysical Modelling. In Proceedings of the Functional Imaging and Modeling of the Heart, London, UK, 20–22 June 2013; pp. 425–432. [Google Scholar]
- Wei, W.; Ge, J.B.; Zou, Y.; Lin, L.; Cai, Y.; Liu, X.B.; Zhu, W.Q. Anatomical characteristics of pulmonary veins for the prediction of postoperative recurrence after radiofrequency catheter ablation of atrial fibrillation. PLoS ONE 2014, 9, e93817. [Google Scholar] [CrossRef]
- Yang, X.; Yang, G.; Yao, X.; Han, B. Measurment of the normal left atrial volume by SCT. Chin Clin. Med. Imaging 2010, 21, 353–354. [Google Scholar]
- Ben-David, E.; Ahmed, M.; Faroja, M.; Moussa, M.; Wandel, A.; Sosna, J.; Appelbaum, L.; Nissenbaum, I.; Goldberg, S.N. Irreversible electroporation: Treatment effect is susceptible to local environment and tissue properties. Radiology 2013, 269, 738–747. [Google Scholar] [CrossRef]
- Sugrue, A.; Maor, E.; Del-Carpio Munoz, F.; Killu, A.M.; Asirvatham, S.J. Cardiac ablation with pulsed electric fields: Principles and biophysics. Europace 2022, 24, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Peyman, A.; Grant, E.H. Electrical conductivity of tissue at frequencies below 1 MHz. Phys. Med. Biol. 2009, 54, 4863–4878. [Google Scholar] [CrossRef] [PubMed]
- Baena-Montes, J.M.; O’Halloran, T.; Clarke, C.; Donaghey, K.; Dunne, E.; O’Halloran, M.; Quinlan, L.R. Electroporation Parameters for Human Cardiomyocyte Ablation In Vitro. J. Cardiovasc. Dev. Dis. 2022, 9, 240. [Google Scholar] [CrossRef] [PubMed]









| Element/Material | ||||
|---|---|---|---|---|
| Electrode | 21,500 | 132 | 71 | 4.6 × 106 |
| Plastic Catheter | 70 | 1045 | 0.026 | 1 × 10−5 |
| Blood | 1000 | 4180 | 0.54 | 0.99 |
| Myocardium | 1200 | 3200 | 0.53 | Table 2 |
| Group/Electrical Conductivity | AC | IC | Reference | ||
|---|---|---|---|---|---|
| * | ** | *** | **** | ||
| 1 | 0.0632 | 0.0442 | 1.429 | 0.0537 | [21] |
| 2 | 0.0848 | 0.0226 | 3.75 | 0.0537 | [6] |
| 3 | 0.0894 | 0.018 | 4.98 | 0.0537 | [7] |
| 4 | 0.0926 | 0.0148 | 6.25 | 0.0537 | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, L.; Gu, K.; Ji, X.; Zhang, H.; Yan, S.; Wu, X. Effect of Anisotropic Electrical Conductivity Induced by Fiber Orientation on Ablation Characteristics of Pulsed Field Ablation in Atrial Fibrillation Treatment: A Computational Study. J. Cardiovasc. Dev. Dis. 2022, 9, 319. https://doi.org/10.3390/jcdd9100319
Zang L, Gu K, Ji X, Zhang H, Yan S, Wu X. Effect of Anisotropic Electrical Conductivity Induced by Fiber Orientation on Ablation Characteristics of Pulsed Field Ablation in Atrial Fibrillation Treatment: A Computational Study. Journal of Cardiovascular Development and Disease. 2022; 9(10):319. https://doi.org/10.3390/jcdd9100319
Chicago/Turabian StyleZang, Lianru, Kaihao Gu, Xingkai Ji, Hao Zhang, Shengjie Yan, and Xiaomei Wu. 2022. "Effect of Anisotropic Electrical Conductivity Induced by Fiber Orientation on Ablation Characteristics of Pulsed Field Ablation in Atrial Fibrillation Treatment: A Computational Study" Journal of Cardiovascular Development and Disease 9, no. 10: 319. https://doi.org/10.3390/jcdd9100319
APA StyleZang, L., Gu, K., Ji, X., Zhang, H., Yan, S., & Wu, X. (2022). Effect of Anisotropic Electrical Conductivity Induced by Fiber Orientation on Ablation Characteristics of Pulsed Field Ablation in Atrial Fibrillation Treatment: A Computational Study. Journal of Cardiovascular Development and Disease, 9(10), 319. https://doi.org/10.3390/jcdd9100319






