High-Sensitivity Cardiac Troponin T in Prediction and Diagnosis of Early Postoperative Hypoxemia after Off-Pump Coronary Artery Bypass Grafting
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Population
2.2. Clinical Data Collection
2.3. Anesthesia and Cardiac Surgery Procedure
2.4. Routine Blood Testing and Serum High-Sensitivity Cardiac Troponin Measurement
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Correlation between Lowest PaO2/FiO2 and Baseline Characteristics
3.3. Cardiac Injury Measurements
3.4. Postoperative Events of Patients following OPCAB
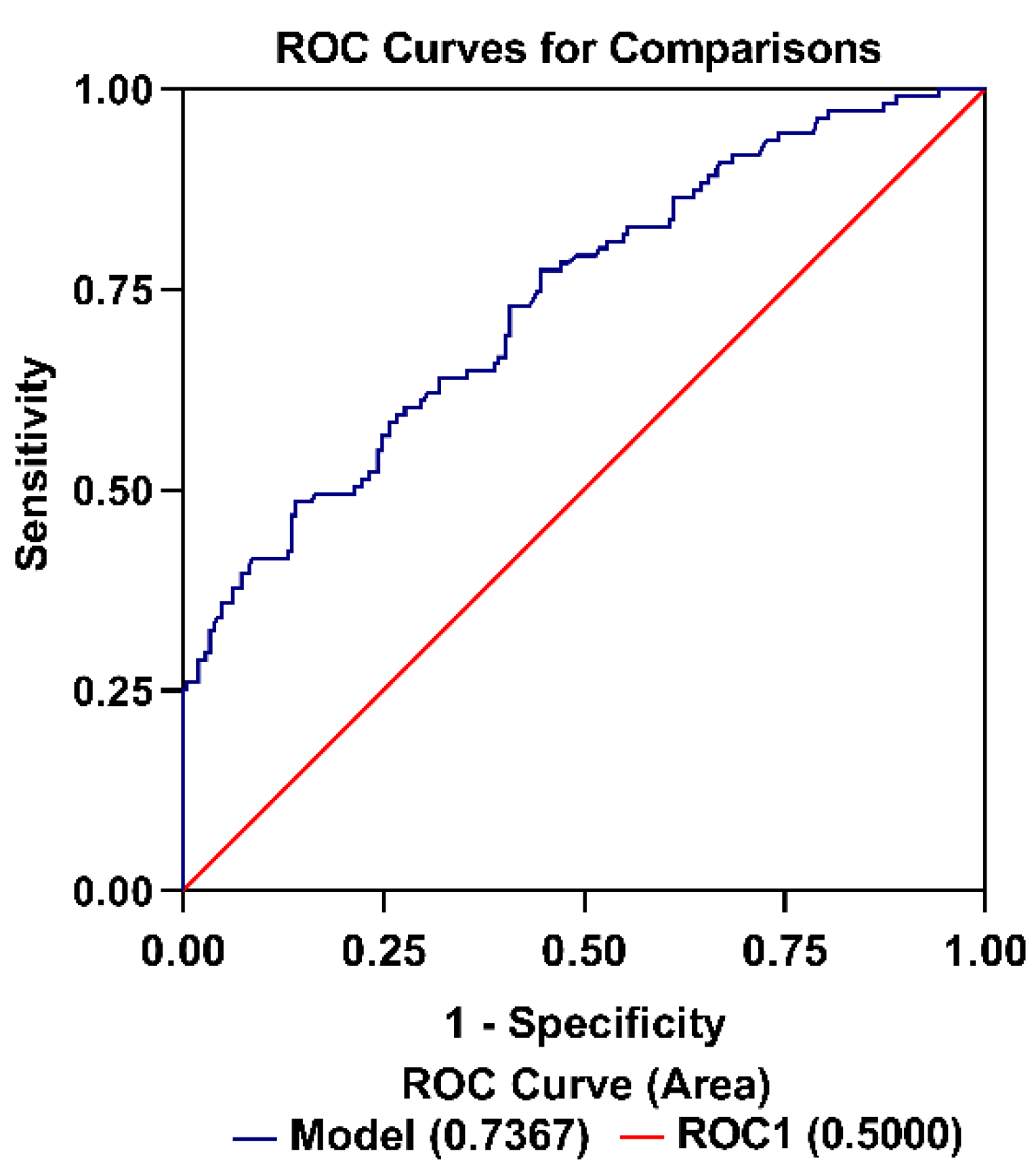
3.5. Association of Preoperative hs-cTnT with EPH
3.6. Factors Associated with EPH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| ACS | acute coronary syndrome |
| ALI | acute lung injury |
| ARDS | acute respiratory distress syndrome |
| BMI | body mass index |
| CABG | coronary artery bypass grafting |
| CAD | coronary artery disease |
| CPB | cardiopulmonary bypass |
| COPD | chronic obstructive pulmonary disease |
| EPH | early postoperative hypoxemia |
| Hs-cTnT | high-sensitivity cardiac troponin T |
| ICU | intensive care unit |
| I/R | ischemia and reperfusion |
| LVEDD | left ventricular end-diastolic diameter |
| LVEF | Left ventricular ejection fraction |
| LIMA | left internal mammary artery |
| MI NT-proBNP | myocardial infarction N-terminal pro-B-type natriuretic peptide |
| OPCAB | off-pump coronary artery bypass grafting |
| TTE | transthoracic echocardiography |
References
- Ng, C.S.; Wan, S.; Yim, A.P.; Arifi, A.A. Pulmonary dysfunction after cardiac surgery. Chest 2002, 121, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.S.; Shah, A.S.; Whitman, G.J. Lung injury and acute respiratory distress syndrome after cardiac surgery. Ann. Thorac. Surg. 2013, 95, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Palumbo, G.J.; Bignami, E.; Pavesi, M.; Ranucci, M.; Scolletta, S.; Pelosi, P.; Astuto, M. Acute Respiratory Distress Syndrome in the Perioperative Period of Cardiac Surgery: Predictors, Diagnosis, Prognosis, Management Options, and Future Directions. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Al Jaaly, E.; Zakkar, M.; Fiorentino, F.; Angelini, G.D. Pulmonary Protection Strategies in Cardiac Surgery: Are We Making Any Progress? Oxid. Med. Cell Longev. 2015, 2015, 416235. [Google Scholar] [CrossRef] [PubMed]
- Bartz, R.R.; Ferreira, R.G.; Schroder, J.N.; Davies, J.; Liu, W.W.; Camara, A.; Welsby, I.J. Prolonged pulmonary support after cardiac surgery: Incidence, risk factors and outcomes: A retrospective cohort study. J. Crit. Care 2015, 30, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Li, L.; Figueroa, P.; Mihaljevic, T.; Svensson, L.; Blackstone, E.H. Transfusion and pulmonary morbidity after cardiac surgery. Ann. Thorac. Surg. 2009, 88, 1410–1418. [Google Scholar] [CrossRef]
- Rocha, R.V.; Yanagawa, B.; Hussain, M.A.; Tu, J.V.; Fang, J.; Ouzounian, M.; Cusimano, R.J. Off-pump versus on-pump coronary artery bypass grafting in moderate renal failure. J. Thorac. Cardiovasc. Surg. 2020, 159, 1297–1304. [Google Scholar] [CrossRef]
- Montes, F.R.; Maldonado, J.D.; Paez, S.; Ariza, F. Off-pump versus on-pump coronary artery bypass surgery and postoperative pulmonary dysfunction. J. Cardiothorac. Vasc. Anesth. 2004, 18, 698–703. [Google Scholar] [CrossRef]
- Chiarenza, F.; Tsoutsouras, T.; Cassisi, C.; Santonocito, C.; Gerry, S.; Astuto, M.; George, S.; Sanfilippo, F. The Effects of On-Pump and Off-Pump Coronary Artery Bypass Surgery on Respiratory Function in the Early Postoperative Period. J. Intensive Care Med. 2019, 34, 126–132. [Google Scholar] [CrossRef]
- Lazo, M.; Rubin, J.; Clark, J.M.; Coresh, J.; Schneider, A.L.; Ndumele, C.; Hoogeveen, R.C.; Ballantyne, C.M.; Selvin, E. The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease. J. Hepatol. 2015, 62, 841–847. [Google Scholar] [CrossRef]
- Hoiseth, A.D.; Neukamm, A.; Karlsson, B.D.; Omland, T.; Brekke, P.H.; Soyseth, V. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax 2011, 66, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Murakami, M.M.; Pearce, L.A.; Herzog, C.A. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002, 106, 2941–2945. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Romero, D.; Vilchez, J.A.; Lahoz, A.; Romero-Aniorte, A.I.; Orenes-Pinero, E.; Caballero, L.; Jara-Rubio, R.; Arribas, J.M.; Garcia-Alberola, A.; Valdes, M.; et al. High-sensitivity troponin T as a biomarker for the development of atrial fibrillation after cardiac surgery. Eur. J. Cardiothorac. Surg. 2014, 45, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gao, Y.; Wang, L.; Liu, J.; Yuan, Z.; Yu, M. Elevated free fatty acid level is a risk factor for early postoperative hypoxemia after on-pump coronary artery bypass grafting: Association with endothelial activation. J. Cardiothorac. Surg. 2015, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ding, X.; Su, Y.; Yang, P.; Du, X.; Sun, M.; Huang, X.; Yue, Z.; Sun, F.; Xie, F.; et al. Incidence, Risk Factors, and Outcomes of Severe Hypoxemia After Cardiac Surgery. Front. Cardiovasc. Med. 2022, 9, 934533. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Yang, Y.L.; Chen, T.T.; Lee, C.I.; Tam, K.W. Recruitment maneuvers to reduce pulmonary atelectasis after cardiac surgery: A meta-analysis of randomized trials. J. Thorac. Cardiovasc. Surg. 2022, 164, 171–181.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.F.; Wang, D.J.; Liu, S.; Jia, M.; Jia, S.J. Efficacy and safety of noninvasive positive pressure ventilation in the treatment of acute respiratory failure after cardiac surgery. Chin. Med. J. 2013, 126, 4463–4469. [Google Scholar]
- Besch, G.; Perrotti, A.; Salomon du Mont, L.; Puyraveau, M.; Ben-Said, X.; Baltres, M.; Barrucand, B.; Flicoteaux, G.; Vettoretti, L.; Samain, E.; et al. Impact of intravenous exenatide infusion for perioperative blood glucose control on myocardial ischemia-reperfusion injuries after coronary artery bypass graft surgery: Sub study of the phase II/III ExSTRESS randomized trial. Cardiovasc. Diabetol. 2018, 17, 140. [Google Scholar] [CrossRef]
- Lai, C.C.; Huang, P.H.; Yang, A.H.; Chiang, S.C.; Tang, C.Y.; Tseng, K.W.; Huang, C.H. Baicalein Attenuates Lung Injury Induced by Myocardial Ischemia and Reperfusion. Am. J. Chin. Med. 2017, 45, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Magruder, J.T.; Grimm, J.C.; Crawford, T.C.; Johnston, L.; Santhanam, L.; Stephens, R.S.; Berkowitz, D.E.; Shah, A.S.; Bush, E.L.; Damarla, M.; et al. Imatinib Is Protective Against Ischemia-Reperfusion Injury in an Ex Vivo Rabbit Model of Lung Injury. Ann. Thorac. Surg. 2018, 105, 950–956. [Google Scholar] [CrossRef]
- Mehdiani, A.; Akhyari, P.; Kamiya, H.; Ahlers, J.; Godehardt, E.; Albert, A.; Boeken, U.; Lichtenberg, A. Prognostic value of the new high sensitive cardiac troponin T assay (hs-cTnT) after coronary artery bypass grafting. Acta Cardiol. 2017, 72, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.; Breitling, L.P.; Hahmann, H.; Wusten, B.; Brenner, H.; Rothenbacher, D. Cardiac troponin T measured by a high-sensitivity assay predicts recurrent cardiovascular events in stable coronary heart disease patients with 8-year follow-up. Clin. Chem. 2012, 58, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Angelini, G.D.; Antoniades, C.; Bakaeen, F.; Benedetto, U.; Calafiore, A.M.; di Franco, A.; di Mauro, M.; Fremes, S.E.; Girardi, L.N.; et al. Off-Pump Coronary Artery Bypass Grafting: 30 Years of Debate. J. Am. Heart Assoc. 2018, 7, e009934. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.T.; Bradley, L.A.; Dempsey, T.M.; Puro, A.C.; Adams, J.Y. Management of Mechanical Ventilation in Decompensated Heart Failure. J. Cardiovasc. Dev. Dis. 2016, 3, 33. [Google Scholar] [CrossRef]
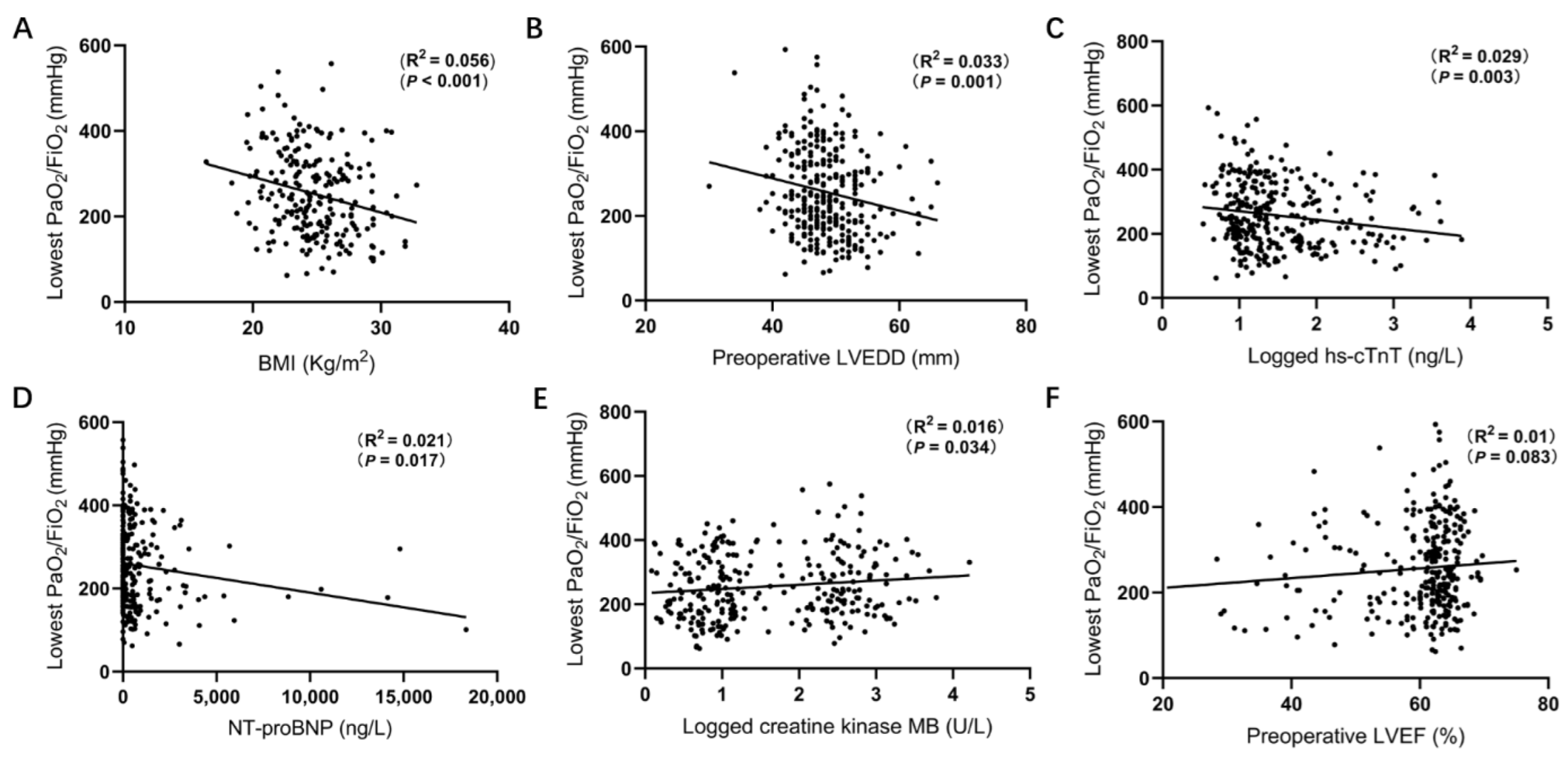
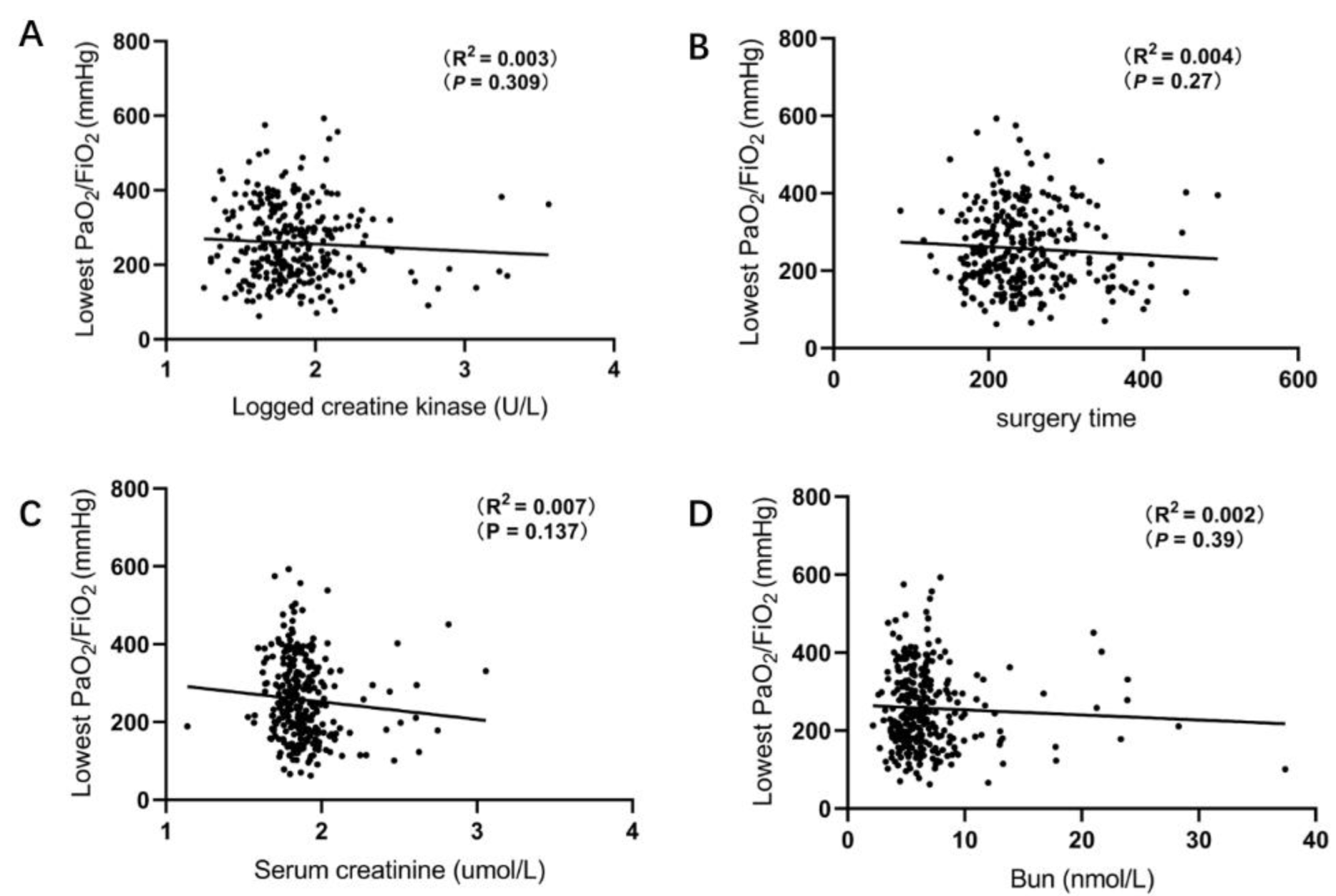

| Characteristics | ALL (n = 318) | Hypoxemia (n = 107) | Non-Hypoxemia (n = 211) | p Value |
|---|---|---|---|---|
| Age (y) | 65.5 ± 8.7 | 65.3 ± 8.8 | 65.7 ± 8.5 | 0.68 |
| Gender | ||||
| Female | 82 (25.8) | 23 (21.5) | 59 (28.0) | 0.21 |
| Male | 236 (74.2) | 84 (78.5) | 152 (72.0) | |
| BMI (kg/m2) | 24.7 ± 2.9 | 25.4 ± 2.6 | 24.4 ± 3.0 | 0.013 |
| Smoking status | ||||
| Never smoked | 168 (52.8) | 48 (44.9) | 120 (56.9) | 0.079 |
| Past smoker | 86 (27.0) | 31 (30.0) | 55 (26.1) | |
| Current smoker | 64 (20.1) | 28 (26.2) | 36 (17.1) | |
| Diabetes mellitus | 107 (33.6) | 38 (35.5) | 69 (32.7) | 0.616 |
| Hypertension | 217 (68.2) | 78 (72.9) | 139 (65.9) | 0.25 |
| Hyperlipidemia | 39 (12.3) | 12 (11.2) | 27 (12.8) | 0.685 |
| Preoperative LVEF (%) | 60.1 ± 7.7 | 59.3 ± 8.3 | 60.4 ± 7.3 | 0.21 |
| Preoperative LVEDD, mm | 48.3 ± 4.8 | 49.1 ± 4.0 | 48.0 ± 5.1 | 0.082 |
| EuroSCOREII | 2.2 ± 0.5 | 2.3 ± 0.4 | 2.2 ± 0.7 | 0.692 |
| Prior myocardial infarction | 96 (30.2) | 31 (29.0) | 65 (30.8) | 0.736 |
| Peripheral vascular disease | 33 (10.4) | 12 (11.2) | 21 (10.0) | 0.727 |
| Previous treatments | ||||
| Aspirin | 298 (93.7) | 97 (90.7) | 201 (95.3) | 0.109 |
| Beta blockers | 238 (74.8) | 82 (76.6) | 156 (73.9) | 0.6 |
| Statins | 216 (67.9) | 72 (67.3) | 144 (68.2) | 0.863 |
| ACE inhibitors | 221 (69.5) | 75 (70.1) | 146 (69.2) | 0.87 |
| CCS | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.79 |
| NYHA | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.578 |
| Left main coronary disease | 65 (20.4) | 30 (28.0) | 35 (16.6) | 0.017 |
| Preoperative biological data | ||||
| Serum creatinine, umol/L | 71.2 (61.0–86.0) | 76.0 (64.6–94.1) | 69.1 (59.6–83.2) | 0.002 |
| Bun, mmol/L | 6.6 ± 3.6 | 6.8 ± 4.1 | 6.6 ± 3.4 | 0.52 |
| Haemoglobin, g/L | 129.3 ± 19.3 | 130.3 ± 18.1 | 128.9 ± 19.7 | 0.24 |
| AST, U/L | 35.7 ± 34.6 | 32.48 ± 24.4 | 37.3 ± 38.6 | 0.41 |
| ALT, U/L | 40.2 ± 38.4 | 37.7 ± 27.7 | 41.5 ± 42.9 | 0.93 |
| LDH, U/L | 199 (175–229.2) | 201 (175–265) | 197 (174.5–222) | 0.11 |
| Creatine kinase, U/L | 65 (46.5–96.5) | 68 (47.2–103.5) | 63 (46–93) | 0.014 |
| Creatine kinase MB, U/L | 14.4 (5.6–316.92) | 11.7 (4.6–228.3) | 23.2 (6.8–366.6) | 0.033 |
| NT-proBNP, ng/L | 200.9 (1.88–652.5) | 393.9 (2.53–904.5) | 115 (1.79–549) | 0.007 |
| Hs-cTnT, ng/L | 19.77 (10.42–59.53) | 39.62 (18.1–287) | 16.16 (9.09–30.33) | <0.001 |
| Extent of CAD | ||||
| 1 VD | 26 (8.2) | 8 (7.5) | 18 (8.5) | 0.46 |
| 2 VD | 51 (20.1) | 21 (19.6) | 30 (14.2) | |
| 3 VD | 241 (75.8) | 78 (72.9) | 163 (77.3) |
| Variables | ALL (n = 318) | Hs-cTnT ≥ 14 (n = 198) | Hs-cTnT < 14 (n = 120) | p Value |
|---|---|---|---|---|
| PaO2 (mm Hg) | 126.1 ± 50.74 | 120.6 ± 42.1 | 135.6 ± 61.94 | * 0.012 |
| PaCO2 (mm Hg) | 38.05 ± 4.98 | 37.91 ± 5.23 | 38.28 ± 4.53 | 0.53 |
| PaO2/FiO2 Ratio (mm Hg) | 257.0 ± 101.6 | 247.1 ± 95.64 | 283.3 ± 124.6 | * 0.005 |
| SaO2 (%) | 97.33 ± 6.87 | 97.45 ± 4.2 | 97.12 ± 9.92 | 0.684 |
| Characteristics | ALL (n = 318) | Hypoxemia (n = 107) | Non-Hypoxemia (n = 211) | p Value |
|---|---|---|---|---|
| In-hospital stay after surgery (days) | 8.5 ± 2.1 | 8.7 ± 1.7 | 8.1 ± 2.6 | 0.009 |
| ICU stay (days) | 2.7 ± 1.5 | 2.8 ± 1.4 | 2.4 ± 1.6 | 0.008 |
| Extubated time (days) | 1.5 ± 1.0 | 1.6 ± 1.1 | 1.3 ± 0.6 | 0.021 |
| 30-day mortality | 5 (1.6) | 2 (1.9) | 3 (1.4) | 0.762 |
| Postoperative stroke | 7 (2.2) | 2 (1.9) | 5 (2.4) | 0.774 |
| Postoperative AF | 42 (13.21) | 14 (13.08) | 28 (13.27) | 0.963 |
| Deep sternal wound infection | 15 (4.7) | 6 (5.6) | 9 (4.3) | 0.594 |
| Hs-cTnT, ng/L | 132 (85–244) | 152 (91–278.5) | 124 (83.5–203.2) | 0.028 |
| NT-proBNP, ng/L | 920 (420–1863) | 997.5 (427.7–1943.2) | 877 (420–1807) | 0.62 |
| Creatine kinase, U/L | 325 (211–529) | 355 (214–573.5) | 313 (205–502) | 0.25 |
| Creatine kinase MB, U/L | 6 (4–11) | 6 (3–10) | 6 (4–11) | 0.45 |
| AST, U/L | 39.74 ± 29.17 | 35.57 ± 19.1 | 41.8 ± 32.88 | 0.068 |
| ALT, U/L | 32.77 ± 29.64 | 27.34 ± 19.66 | 35.34 ± 33.21 | 0.019 |
| LDH, U/L | 309 ± 91.24 | 317.9 ± 90.43 | 304.2 ± 91.56 | 0.227 |
| Serum creatinine, umol/L | 77.2 (63.08–99.53) | 85.4 (69.2–110.6) | 75.1 (62.1–91.5) | <0.001 |
| Bun, mmol/L | 6.7 ± 3.59 | 7.39 ± 4.4 | 6.37 ± 3.1 | 0.015 |
| Haemoglobin, g/L | 109.3 ± 16.79 | 110.2 ± 17.63 | 108.9 ± 16.38 | 0.52 |
| Variable | Patients with Event (n = 318) | Crude Odds Ratio hs-cTnT (Per ng/L) | 95% Confidence Interval | p Value |
|---|---|---|---|---|
| EPH | 107 (33.6) | 1.86 | (1.30–2.68) | <0.001 |
| 30-day mortality | 5 (1.6) | 0.73 | (0.10–2.69) | 0.683 |
| Postoperative AF | 42 (13.2) | 1.17 | (0.70–1.86) | 0.541 |
| Postoperative stroke | 7 (2.2) | 0.90 | (0.21–2.60) | 0.866 |
| Deep sternal wound infection | 15 (4.7) | 0.72 | (0.25–1.64) | 0.465 |
| Length of In-hospital stay after surgery > 9 d | 94 (29.6) | 1.42 | (0.98–2.04) | 0.065 |
| ICU stay > 3 d | 97 (30.5) | 1.58 | (1.08–2.32) | 0.019 |
| Extubated time > 1 d | 90 (28.3) | 1.63 | (1.15–2.34) | 0.007 |
| Effect | Ajusted Odd Ratio | 95% Wald Confidence Limits | p Value |
|---|---|---|---|
| Preoperative hs-cTnT | |||
| <14 ng/L | Reference | ||
| ≥14 ng/L | 0.45 | 0.23–0.86 | 0.016 |
| Smoking status | |||
| Never smoked | Reference | ||
| Past smoker | 0.84 | 0.41–1.72 | 0.64 |
| Current smoker | 1.24 | 0.55–2.77 | 0.59 |
| BMI | |||
| <24 kg/m2 | Reference | ||
| ≥24 kg/m2 | 2.66 | 1.35–5.41 | 0.006 |
| Hypertension | 0.96 | 0.49–1.85 | 0.90 |
| NT-proBNP | |||
| ≤100 ng/L | Reference | ||
| >100 ng/L | 1.06 | 0.54–2.10 | 0.87 |
| Left main coronary disease | 0.66 | 0.33–1.36 | 0.257 |
| Extent of CAD | |||
| 1 VD | Reference | ||
| 2 VD | 2.16 | 0.61–8.29 | 0.244 |
| 3 VD | 1.11 | 0.37–3.60 | 0.86 |
| Preoperative creatinine | |||
| ≤133 umol/L | Reference | ||
| >133 umol/L | 0.8 | 0.19–2.92 | 0.745 |
| Preoperative LVEF (per %) | 0.96 | 0.91–1.0 | 0.098 |
| Preoperative LVEDD (per mm) | 1.00 | 0.92–1.08 | 0.938 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Lu, X.; Li, B.; Wang, C.; Wang, X.; Ji, Y.; Liu, Z.; Li, X.; Yi, C.; Song, M.; et al. High-Sensitivity Cardiac Troponin T in Prediction and Diagnosis of Early Postoperative Hypoxemia after Off-Pump Coronary Artery Bypass Grafting. J. Cardiovasc. Dev. Dis. 2022, 9, 416. https://doi.org/10.3390/jcdd9120416
Lu P, Lu X, Li B, Wang C, Wang X, Ji Y, Liu Z, Li X, Yi C, Song M, et al. High-Sensitivity Cardiac Troponin T in Prediction and Diagnosis of Early Postoperative Hypoxemia after Off-Pump Coronary Artery Bypass Grafting. Journal of Cardiovascular Development and Disease. 2022; 9(12):416. https://doi.org/10.3390/jcdd9120416
Chicago/Turabian StyleLu, Peng, Xiaohu Lu, Ben Li, Chufan Wang, Xufeng Wang, Yumeng Ji, Zhaoyang Liu, Xiangyu Li, Chenlong Yi, Meijuan Song, and et al. 2022. "High-Sensitivity Cardiac Troponin T in Prediction and Diagnosis of Early Postoperative Hypoxemia after Off-Pump Coronary Artery Bypass Grafting" Journal of Cardiovascular Development and Disease 9, no. 12: 416. https://doi.org/10.3390/jcdd9120416
APA StyleLu, P., Lu, X., Li, B., Wang, C., Wang, X., Ji, Y., Liu, Z., Li, X., Yi, C., Song, M., & Wang, X. (2022). High-Sensitivity Cardiac Troponin T in Prediction and Diagnosis of Early Postoperative Hypoxemia after Off-Pump Coronary Artery Bypass Grafting. Journal of Cardiovascular Development and Disease, 9(12), 416. https://doi.org/10.3390/jcdd9120416







