A Study of Soil-Borne Fusarium Wilt in Continuous Cropping Chrysanthemum Cultivar ‘Guangyu’ in Henan, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Investigation and Soil Sample Collection
2.2. Isolation and Identification of Pathogen
2.3. Pathogenicity Test
2.4. Observation of Colonization Process and Disease Assessment
2.5. Plant-Cell-Wall-Degrading Enzyme (CWDE) Activity of Pathogen
2.6. Physiological Response of Plants after Infection by Pathogen
2.7. Soil DNA Extraction, PCR Amplification, and Sequencing
2.8. Microbiome Profiling
2.9. Statistical Analysis
3. Results
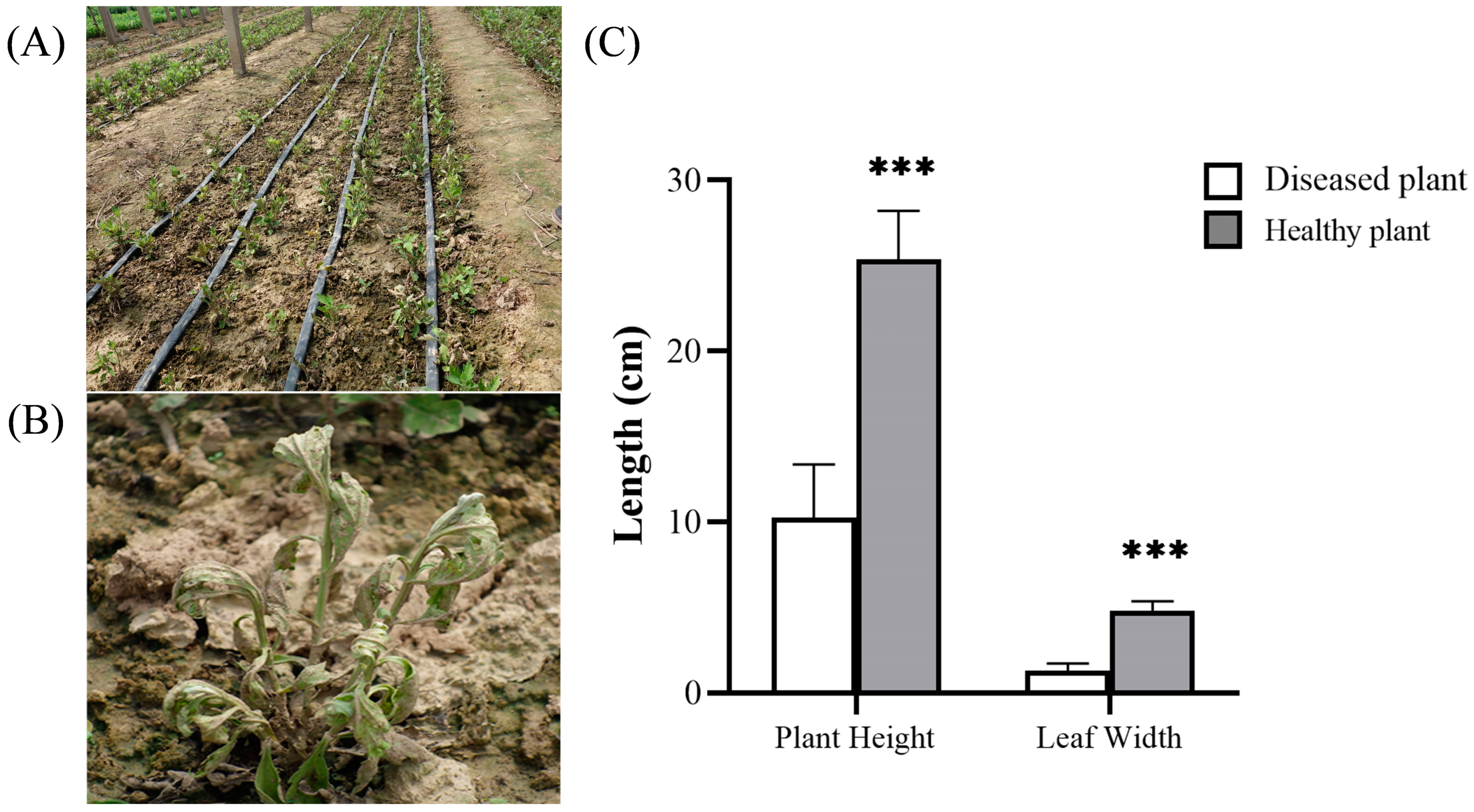
3.1. Chrysanthemum Disease Symptoms in the Field
3.2. Isolation and Identification of Fusarium Pathogens
3.3. Pathogenicity Determination of Pathogen
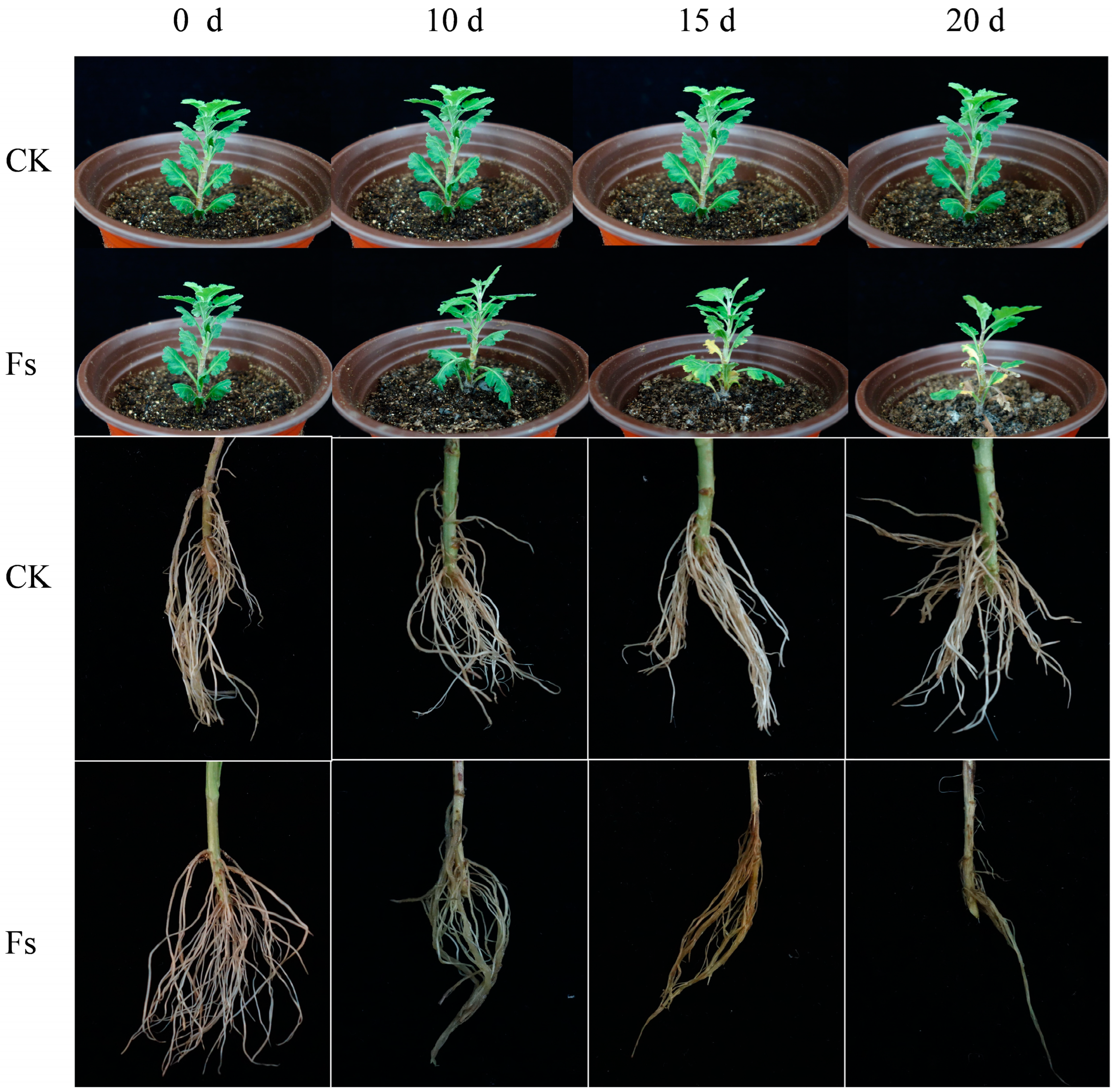
3.4. Invasion and Colonization of F. solani in Chrysanthemum Root
3.5. Chrysanthemum’s Physiological Response to F. solani Inoculation
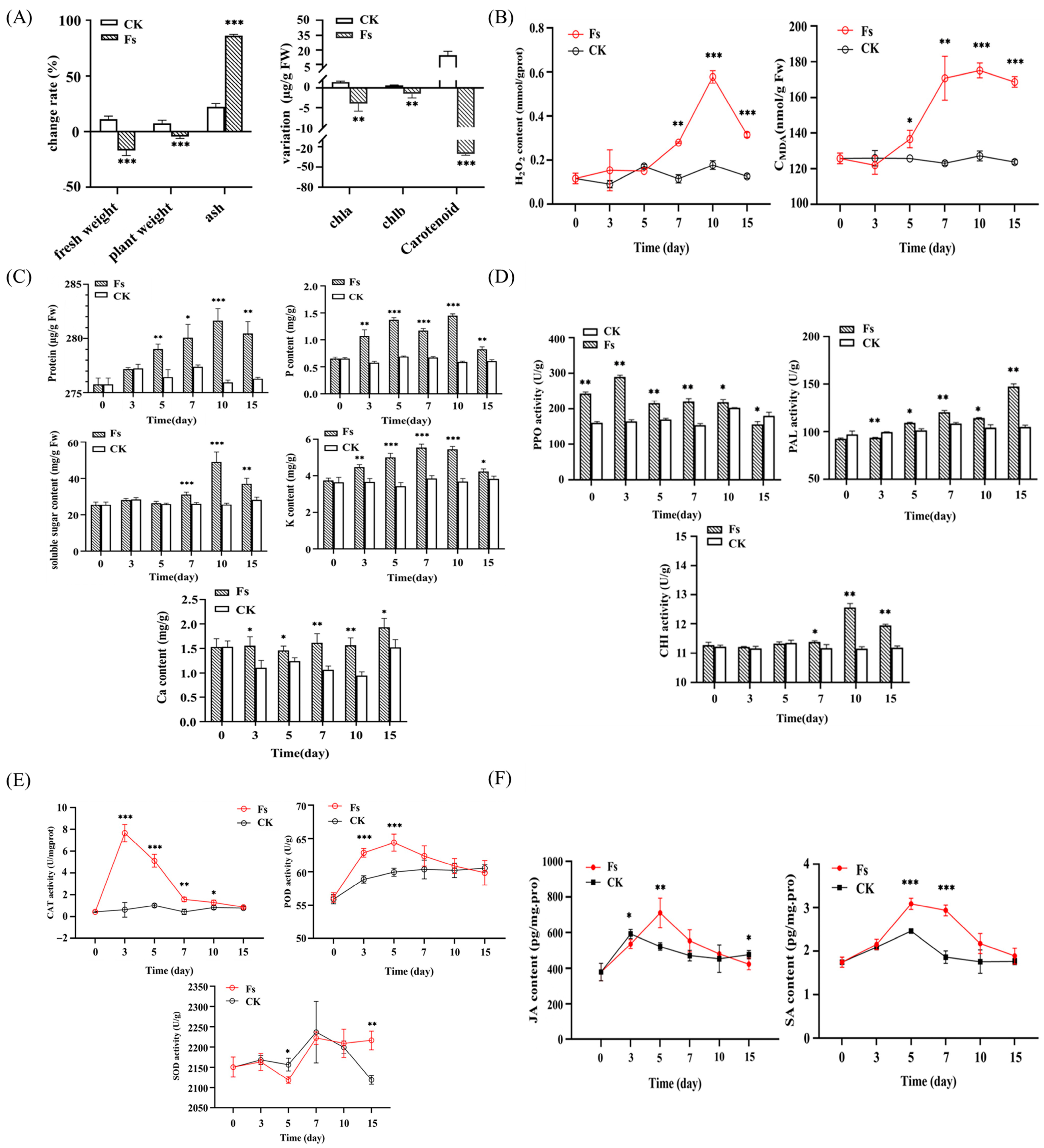
3.5.1. Effects on Plant Nutrition and Growth
3.5.2. Oxidative Stress on Plants
3.5.3. Changes in Defense-Related Enzyme Activity
3.5.4. Plant Hormone Level
3.6. Rhizosphere Microbial Community Diversity Changes during Continuous Monocropping
3.6.1. Changes in Bacterial Community Composition
3.6.2. Changes in the Fungal Community Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shibata, M. Importance of genetic transformation in ornamental plant breeding. Plant Biotechnol. 2008, 25, 3–8. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.C.; Li, Y.; Zhang, K.; Wang, H.L. Possible mechanisms of control of Fusarium wilt of cut chrysanthemum by Phanerochaete chrysosporium in continuous cropping fields: A case study. Sci. Rep. 2017, 7, 15994. [Google Scholar] [CrossRef]
- Song, A.P.; Zhao, S.; Chen, S.S.; Jiang, J.F.; Chen, S.M.; Li, H.Y.; Chen, Y.; Chen, X.; Fang, W.M.; Chen, F.D. The abundance and diversity of soil fungi in continuously monocropped chrysanthemum. Sci. World J. 2013, 2013, 632920. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Kerry, B.R. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-pathogenic fungi. Ann. Rev. Phytopahtol. 2000, 38, 423–441. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Li, J.; Meng, F.; Jiang, M.; Zhang, H.; Chu, G.; Tao, R. Assembly and co-occurrence patterns of rhizosphere bacterial communities are closely linked to soil fertility during continuous cropping of cut chrysanthemum (Chrysanthemum morifolium Ramat). J. Appl. Microbiol. 2023, 134, lxad175. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Carrión, V.J.; Revillini, D.; Yin, S.; Dong, Y.; Zhang, T.; Wang, X.; Delgado-Baquerizo, M. Acidification suppresses the natural capacity of soil microbiome to fight pathogenic Fusarium infections. Nat. Commun. 2023, 14, 5090. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current Status of Fusarium oxysporum Formae Speciales and Races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, Systematic Mycology & Microbiology Laboratory, ARS, USDA, USA. Available online: http://nt.ars-grin.gov/fungaldatabases/ (accessed on 17 March 2023).
- Mun, H.Y.; Jeong, J.Y.; Kim, C.J.; Lee, H.B. First report of chrysanthemum (Chrysanthemum morifolium) crown rot caused by Fusarium solani in Korea. Plant Pathol. J. 2012, 28, 49–54. [Google Scholar] [CrossRef]
- Mosher, J.J.; Bowman, B.; Bernberg, E.L.; Shevchenko, O.; Kan, J.; Korlach, J.; Kaplan, L.A. Improved performance of the PacBio SMRT technology for 16S rDNA sequencing. J. Microbiol. Meth. 2014, 104, 59–60. [Google Scholar] [CrossRef]
- Pootakham, W.; Mhuantong, W.; Yoocha, T.; Sangsrakru, D.; Kongkachana, W.; Sonthirod, C.; Naktang, C.; Jomchai, N.; U-Thoomporn, S.; Yeemin, T.; et al. Taxonomic profiling of Symbiodiniaceae and bacterial communities associated with Indo-Pacific corals in the gulf of Thailand using PacBio sequencing of full-length ITS and 16S rRNA genes. Genomics 2021, 113, 2717–2729. [Google Scholar] [CrossRef]
- Lei, H.; Liu, A.; Hou, Q.; Zhao, Q.; Guo, J.; Wang, Z. Diversity patterns of soil microbial communities in the Sophora flavescens rhizosphere in response to continuous monocropping. BMC Microbiol. 2020, 20, 272. [Google Scholar] [CrossRef]
- Romero, A.; Carrion, G.; Rico-Gray, V. Fungal latent pathogens and endophytes from leaves of Parthenium hysterophorus (Asteraceae). Fungal Divers. 2001, 7, 81–87. [Google Scholar]
- Al-Sadi, A.M.; Al-Masoodi, R.S.; Al-Ismaili, M.; Al-Mahmooli, I.H. Population Structure and Development of Resistance to Hymexazol Among Fusarium solani Populations from Date Palm, Citrus and Cucumber. J. Phytopathol. 2015, 163, 947–955. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Thompson, J.D.; Gigson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal X windows interface: Flexible strategies for multiple sequence alignment aided by qualityanalysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Wang, S.; Zhang, L.; Shen, G.; Teng, H.; Yang, C.; Song, C.; Xiang, W.; Wang, X.; et al. Identification, Pathogenicity, and Genetic Diversity of Fusarium spp. Associated with Maize Sheath Rot in Heilongjiang Province, China. Int. J. Mol. Sci. 2022, 23, 10821. [Google Scholar] [CrossRef] [PubMed]
- Getha, K.; Vikineswary, S.; Wong, W.H.; Seki, T.; Ward, A.; Goodfellow, M. Evaluation of Streptomyces sp. strain g10 for suppression of Fusarium wilt and rhizosphere colonization in pot-grown banana plantlets. J. Ind. Microbiol. Biotechnol. 2005, 32, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Qi, A.; Liu, Y.; Mendoza, L.E.D.R.; Liu, Z.; Lin, Z.; Khan, M.F.R. Evaluating Inoculation Methods to Infect Sugar Beet with Fusarium oxysporum f. betae and F. secorum. Plant Dis. 2020, 104, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Alkher, H.; El Hadrami, A.; Rashid, K.Y.; Adam, L.R.; Daayf, F. Cross-pathogenicity of Verticillium dahliae between potato and sunflower. Eur. J. Plant Pathlo. 2009, 3, 505–519. [Google Scholar] [CrossRef]
- Boamah, S.; Zhang, S.W.; Xu, B.L.; Li, T.; Calderón-Urrea, A. Trichoderma longibrachiatum (TG1) Enhances Wheat Seedlings Tolerance to Salt Stress and Resistance to Fusarium pseudograminearum. Front. Plant Sci. 2021, 12, 741231. [Google Scholar] [CrossRef] [PubMed]
- Mitic, M.; Berry, D.; Brasell, E.; Green, K.; Young, C.A.; Saikia, S.; Rakonjac, J.; Scott, B. Disruption of calcineurin catalytic subunit (cnaA) in Epichloë festucae induces symbiotic defects and intrahyphal hyphae formation. Mol. Plant. Pathol. 2018, 19, 1414–1426. [Google Scholar] [CrossRef]
- Helal, G.A.; Khalil, R.R.; Galal, Y.G.; Soliman, S.M.; Abd Elkader, R.S. Studies on cellulases of some cellulose-degrading soil fungi. Arch. Microbiol. 2021, 204, 65. [Google Scholar] [CrossRef] [PubMed]
- Pocock, T.; Król, M.; Huner, N.P. The determination and quantification of photosynthetic pigments by reverse phase high-performance liquid chromatography, thin-layer chromatography, and spectrophotometry. Methods Mol. Biol. 2004, 274, 137–148. [Google Scholar] [PubMed]
- Chang, D.; Liu, H.; An, M.; Hong, D.; Fan, H.; Wang, K.; Li, Z. Integrated Transcriptomic and Metabolomic Analysis of the Mechanism of Foliar Application of Hormone-Type Growth Regulator in the Improvement of Grape (Vitis vinifera L.) Coloration in Saline-Alkaline Soil. Plants 2022, 11, 2115. [Google Scholar] [CrossRef]
- Vieira, E.G.; Henriques, S.B. Comparison of the Lowry and Coomassie Blue methods for the determination of protein concentration. Braz. J. Med. Biol. Res. 1992, 25, 583–591. [Google Scholar]
- Schwerbel, K.; Tüngerthal, M.; Nagl, B.; Niemann, B.; Drößer, C.; Bergelt, S.; Uhlig, K.; Höpfner, T.; Greiner, M.; Lindtner, O.; et al. Results of the BfR MEAL Study: The food type has a stronger impact on calcium, potassium and phosphorus levels than factors such as seasonality, regionality and type of production. Food Chem. X 2022, 13, 100221. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhu, Y.L.; Ji, P.Y.; Li, A.Q.; Qiu, Z.Y.; Cheng, Y.Y.; Wang, R.; Ma, C.H.; Song, J.K.; Cui, Z.H.; et al. Mineral and Metabolome Analyses Provide Insights into the Cork Spot Disorder on ‘Akizuki’ Pear Fruit. Horticulturae 2023, 9, 818. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove Essential Oil as an Alternative Approach to Control Postharvest Blue Mold Caused by Penicillium italicum in Citrus Fruit. Biomolecules. 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.F.; Wang, J.Y.; Gong, J.X.; Zhang, Z.L.; Wang, S.; Sun, J.; Li, Q.Q.; Gu, X.; Jiang, J.H.; Qi, S.L. The Arabidopsis thaliana trehalose-6-phosphate phosphatase gene AtTPPI improve chilling tolerance through accumulating soluble sugar and JA. Environ. Exp. Bot. 2023, 205, 105117. [Google Scholar] [CrossRef]
- She, X.M.; He, Z.F.; Tang, Y.F.; Du, Z.G.; Lan, G.B. First Report of Potato Blackleg Disease Caused by Pectobacterium atrosepticum in Guangdong China. Plant Dis. 2013, 97, 1652. [Google Scholar] [CrossRef]
- Kikot, G.E.; Hours, R.A.; Alconada, T.M. Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: A review. J. Basic Microbiol. 2009, 49, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.H.; Yang, Y.R.; Wu, M.T.; Huang, G.; Ge, L.J.; Liu, Y.; Guan, Z.Y.; Chen, S.M.; Fang, W.M.; Chen, F.D.; et al. Potential pathways and genes expressed in Chrysanthemum in response to early fusarium oxysporum infection. BMC Plant Biol. 2023, 23, 312. [Google Scholar] [CrossRef] [PubMed]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 23, 187. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox. Sign. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Fareed, G.; Atiq, M.; Abbas, M.; Usman, M.; Qamar, S.H. Varietal reaction of Cucumber (Cucumis sativus L.) germplasm for management of Fusarium wilt of cucumber (FWC). Adv. Zool. Bot. 2017, 5, 1–3. [Google Scholar] [CrossRef]
- Małolepsza, U.; Nawrocka, J.; Szczech, M. Trichoderma virens 106 inoculation stimulates defence enzyme activities and enhances phenolic levels in tomato plants leading to lowered Rhizoctonia solani infection. Biocontrol Sci. Techn. 2017, 27, 180–199. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Zheng, Y.; Luo, J.M.; Yang, X.R.; Wang, X.L. Quantitative changes of plant defense enzymes and phytohormone in biocontrol of cucumber Fusarium wilt by Bacillus subtilis B579. World J. Microb. Biot. 2010, 26, 675–684. [Google Scholar] [CrossRef]
- Anothai, J.; Chairin, T. Soil physicochemical properties closely associated with fungal enzymes and plant defense enzymes in ganoderma-infected oil palm orchards. Plant Soil. 2020, 456, 99–112. [Google Scholar] [CrossRef]
- Sasaki, C.; Yokoyama, A.; Itoh, Y.; Hashimoto, M.; Watanabe, T.; Fukamizo, T. Comparative study of the reaction mechanism of eamily 18 chitinases from plants and microbes. J. Biochem. 2002, 131, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Chandrasekaran, A.; Kuttalam, S.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Association of some plant defense enzyme activities with systemic resistance to early leaf blight and leaf spot induced in tomato plants by azoxystrobin and Pseudomonas fluorescens. J. Plant Interact. 2007, 2, 233–244. [Google Scholar] [CrossRef]
- Chen, W.; He, J.; Wang, B.; Wang, X.J.; Luo, T.Z.; Liu, J.J. Effect of potato glycoalkaloids on induced resistance of Fusarium fruit rot on Lycium barbarum and activities of related defense enzymes. J. Plant Prot. 2018, 45, 1129–1136. [Google Scholar]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [PubMed]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, S.; Zhang, K.; Zhao, J.; Jiang, J.; Chen, F.; Fang, W. Evaluation of Soil-Applied Chemical Fungicide and Biofungicide for Control of the Fusarium Wilt of Chrysanthemum and Their Effects on Rhizosphere Soil Microbiota. Agriculture 2018, 8, 184. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, X.; Deng, S.; Dong, X.; Song, A.; Yao, J.; Fang, W.; Chen, F. The Effects of Fungicide, Soil Fumigant, Bio-Organic Fertilizer and Their Combined Application on Chrysanthemum Fusarium Wilt Controlling, Soil Enzyme Activities and Microbial Properties. Molecules 2016, 21, 526. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Jin, Y.; Chen, M.; Lian, H.; Liu, Y.; Yin, Q.; Wang, H. A Study of Soil-Borne Fusarium Wilt in Continuous Cropping Chrysanthemum Cultivar ‘Guangyu’ in Henan, China. J. Fungi 2024, 10, 14. https://doi.org/10.3390/jof10010014
Liu L, Jin Y, Chen M, Lian H, Liu Y, Yin Q, Wang H. A Study of Soil-Borne Fusarium Wilt in Continuous Cropping Chrysanthemum Cultivar ‘Guangyu’ in Henan, China. Journal of Fungi. 2024; 10(1):14. https://doi.org/10.3390/jof10010014
Chicago/Turabian StyleLiu, Lei, Yaqiong Jin, Miaomiao Chen, Huijuan Lian, Yanyan Liu, Qianxi Yin, and Hailei Wang. 2024. "A Study of Soil-Borne Fusarium Wilt in Continuous Cropping Chrysanthemum Cultivar ‘Guangyu’ in Henan, China" Journal of Fungi 10, no. 1: 14. https://doi.org/10.3390/jof10010014
APA StyleLiu, L., Jin, Y., Chen, M., Lian, H., Liu, Y., Yin, Q., & Wang, H. (2024). A Study of Soil-Borne Fusarium Wilt in Continuous Cropping Chrysanthemum Cultivar ‘Guangyu’ in Henan, China. Journal of Fungi, 10(1), 14. https://doi.org/10.3390/jof10010014




