A Putative NADPH Oxidase Gene in Unicellular Pathogenic Candida glabrata Is Required for Fungal ROS Production and Oxidative Stress Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Cultural Conditions
2.2. Plasmid Construction and Transformation of C. glabrata
2.3. Measurement of Fungal Intercellular and Extracellular ROS Production
2.4. Spot Assay to Test Sensitivity to Oxidizing Agents
2.5. 2,3,5-Triphenyltetrazolium Chloride (TTC) Reduction Overlay Assay
2.6. Extracellular Ferric Reductase Assay
2.7. XTT Assay and SEM Observation of Biofilms
2.8. Measurement of Hepatocyte (HC) Cellular Transglutaminase (TG) Activity and ROS Yield during Fungal Co-Incubation
2.9. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.10. Statistical Analysis
3. Results
3.1. Identification of the Putative C. glabrata NADPH Oxidase Family Gene
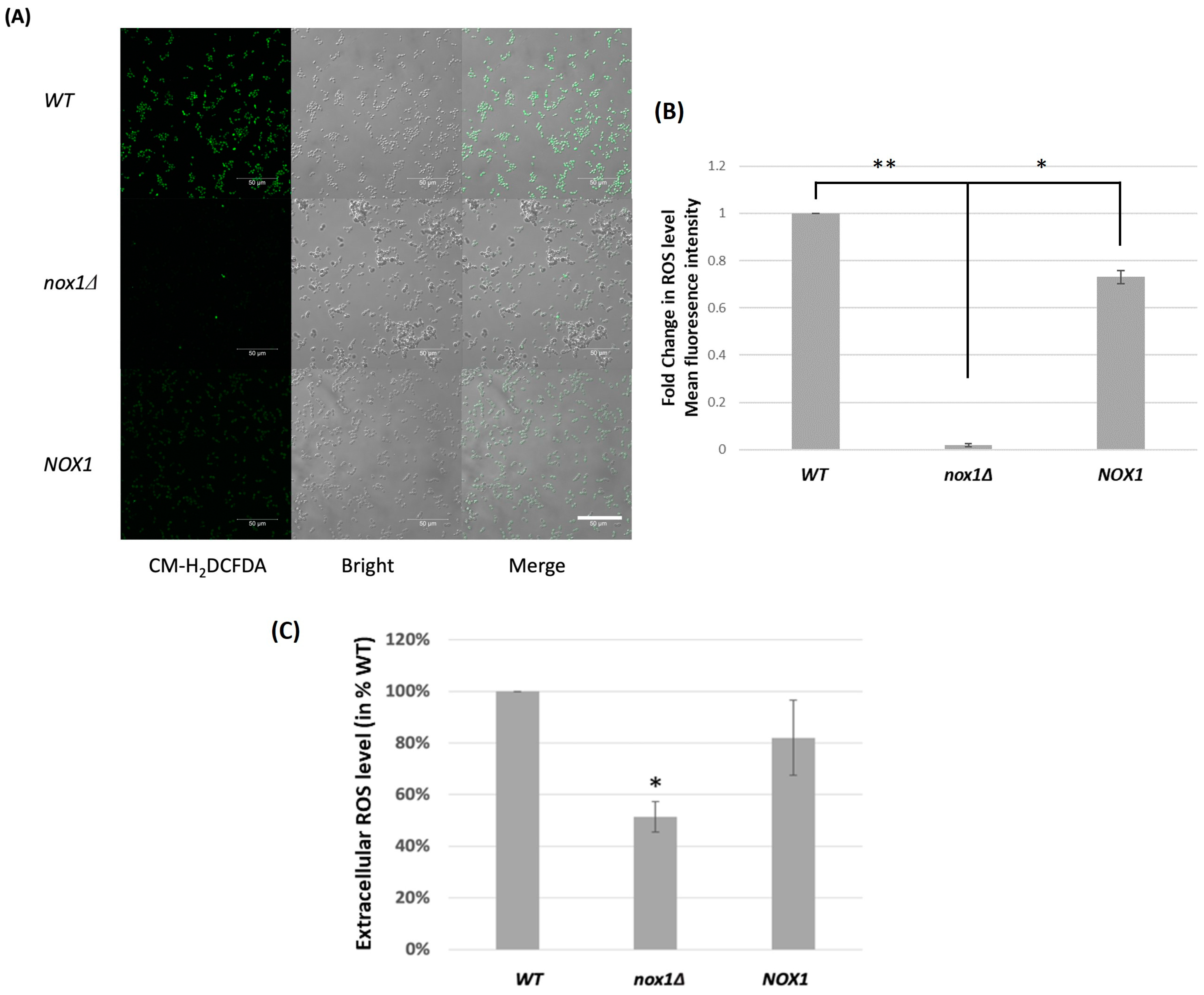
3.2. Role of CgNOX1 in C. glabrata ROS Production
3.3. Role of CgNOX1 in the Oxidative Stress Response of C. glabrata
3.4. Role of CgNOX1 in C. glabrata Ferric Reductase Activity
3.5. Role of CgNOX1 in C. glabrata Biofilm Formation
3.6. Role of CgNOX1 in Co-Incubation with Human Hepatocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Roudbary, M.; Brás, S.; Tafaj, S.; Rodrigues, C.F. Candida auris: A Quick Review on Identification, Current Treatments, and Challenges. Int. J. Mol. Sci. 2021, 22, 4470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, A.; Roudbary, M.; Mohammadi, R.; Cernakova, L.; Rodrigues, C.F. Overview on the Infections Related to Rare Candida Species. Pathogens 2022, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Mechanism of Candida Pathogenesis: Revisiting the Vital Drivers. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1797–1819. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Herrera, E.; Frias-De-Leon, M.G.; Hernandez-Castro, R.; Garcia-Salazar, E.; Arenas, R.; Ocharan-Hernandez, E.; Rodriguez-Cerdeira, C. Antifungal Resistance in Clinical Isolates of Candida glabrata in Ibero-America. J. Fungi 2022, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH Oxidases: An Overview from Structure to Innate Immunity-Associated Pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Takemoto, D.; Tanaka, A.; Scott, B. NADPH Oxidases in Fungi: Diverse Roles of Reactive Oxygen Species in Fungal Cellular Differentiation. Fungal Genet. Biol. 2007, 44, 1065–1076. [Google Scholar] [CrossRef]
- Malagnac, F.; Lalucque, H.; Lepere, G.; Silar, P. Two NADPH Oxidase Isoforms Are Required for Sexual Reproduction and Ascospore Germination in the Filamentous Fungus Podospora Anserina. Fungal Genet. Biol. 2004, 41, 982–997. [Google Scholar] [CrossRef]
- Lalucque, H.; Silar, P. NADPH Oxidase: An Enzyme for Multicellularity? Trends Microbiol. 2003, 11, 9–12. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Buettner, S.; Laun, P.; Heeren, G.; Felder, T.K.; Klinger, H.; Weinberger, M.; Stolze, K.; Grousl, T.; Hasek, J.; et al. Yno1p/Aim14p, a NADPH-Oxidase Ortholog, Controls Extramitochondrial Reactive Oxygen Species Generation, Apoptosis, and Actin Cable Formation in Yeast. Proc. Natl. Acad. Sci. USA 2012, 109, 8658–8663. [Google Scholar] [CrossRef]
- Rossi, D.C.P.; Gleason, J.E.; Sanchez, H.; Schatzman, S.S.; Culbertson, E.M.; Johnson, C.J.; McNees, C.A.; Coelho, C.; Nett, J.E.; Andes, D.R.; et al. Candida albicans FRE8 Encodes a Member of the NADPH Oxidase Family That Produces a Burst of ROS during Fungal Morphogenesis. PLoS Pathog. 2017, 13, e1006763. [Google Scholar] [CrossRef]
- Huang, Y.; Fujii, K.; Chen, X.; Iwatani, S.; Chibana, H.; Kojima, S.; Kajiwara, S. Fungal NOX Is an Essential Factor for Induction of TG2 in Human Hepatocytes. Med. Mycol. 2020, 58, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Iwatani, S.; Kitamoto, T.; Chibana, H.; Kajiwara, S. The Lack of SNARE Protein Homolog Syn8 Influences Biofilm Formation of Candida glabrata. Front. Cell Dev. Biol. 2021, 9, 607188. [Google Scholar] [CrossRef]
- Shrestha, R.; Shrestha, R.; Qin, X.-Y.; Kuo, T.-F.; Oshima, Y.; Iwatani, S.; Teraoka, R.; Fujii, K.; Hara, M.; Li, M.; et al. Fungus-Derived Hydroxyl Radicals Kill Hepatic Cells by Enhancing Nuclear Transglutaminase. Sci. Rep. 2017, 7, 4746. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Wang, Y.; Jin, L.; Abiko, Y.; Samaranayake, L.P. Candida albicans Biofilm Formation Is Associated with Increased Anti-Oxidative Capacities. Proteomics 2008, 8, 2936–2947. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-C.; Yang, C.-Y.; Lan, C.-Y. Candida albicans Hap43 Is a Repressor Induced under Low-Iron Conditions and Is Essential for Iron-Responsive Transcriptional Regulation and Virulence. Eukaryot. Cell 2011, 10, 207–225. [Google Scholar] [CrossRef]
- Jeeves, R.E.; Mason, R.P.; Woodacre, A.; Cashmore, A.M. Ferric Reductase Genes Involved in High-Affinity Iron Uptake Are Differentially Regulated in Yeast and Hyphae of Candida albicans. Yeast 2011, 28, 629–644. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Takada, A.; Iwatani, S.; Oka, C.; Kitamoto, T.; Kajiwara, S. The Role of Bgl2p in the Transition to Filamentous Cells during Biofilm Formation by Candida albicans. Mycoses 2017, 60, 96–103. [Google Scholar] [CrossRef]
- Collart, M.A.; Oliviero, S. Preparation of Yeast RNA. Curr. Protoc. Mol. Biol. 1993, 23, 13.12.1–13.12.5. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gerwien, F.; Safyan, A.; Wisgott, S.; Brunke, S.; Kasper, L.; Hube, B. The Fungal Pathogen Candida glabrata Does Not Depend on Surface Ferric Reductases for Iron Acquisition. Front. Microbiol. 2017, 8, 1055. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Ríos-Momberg, M.; Hewitt, D.; Hansberg, W. Reactive Oxygen Species and Development in Microbial Eukaryotes. Trends Microbiol. 2005, 13, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, W.; Bartosz, G. 2,7-Dichlorofluorescin Oxidation and Reactive Oxygen Species: What Does It Measure? Cell Biol. Int. 2000, 24, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Maghzal, G.J.; Krause, K.-H.; Stocker, R.; Jaquet, V. Detection of Reactive Oxygen Species Derived from the Family of NOX NADPH Oxidases. Free Radic. Biol. Med. 2012, 53, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Schroter, C.; Hipler, U.C.; Wilmer, A.; Kunkel, W.; Wollina, U. Generation of Reactive Oxygen Species by Candida albicans in Relation to Morphogenesis. Arch. Dermatol. Res. 2000, 292, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.G.; Tan, S.-X.; Dawes, I.W. Reactive Oxygen Species and Yeast Apoptosis. Biochim. Biophys. Acta-Mol. Cell Res. 2008, 1783, 1354–1368. [Google Scholar] [CrossRef]
- Vangalis, V.; Papaioannou, I.A.; Markakis, E.A.; Knop, M.; Typas, M.A. The NADPH Oxidase A of Verticillium Dahliae Is Essential for Pathogenicity, Normal Development, and Stress Tolerance, and It Interacts with Yap1 to Regulate Redox Homeostasis. J. Fungi 2021, 7, 740. [Google Scholar] [CrossRef]
- Shakoury-Elizeh, M.; Tiedeman, J.; Rashford, J.; Ferea, T.; Demeter, J.; Garcia, E.; Rolfes, R.; Brown, P.O.; Botstein, D.; Philpott, C.C. Transcriptional Remodeling in Response to Iron Deprivation in Saccharomyces Cerevisiae. Mol. Biol. Cell 2004, 15, 1233–1243. [Google Scholar] [CrossRef]
- Meneghini, R. Iron Homeostasis, Oxidative Stress, and DNA Damage. Free Radic. Biol. Med. 1997, 23, 783–792. [Google Scholar] [CrossRef]
- Xu, N.; Cheng, X.; Yu, Q.; Zhang, B.; Ding, X.; Xing, L.; Li, M. Identification and Functional Characterization of Mitochondrial Carrier Mrs4 in Candida albicans. FEMS Yeast Res. 2012, 12, 844–858. [Google Scholar] [CrossRef]
- De Freitas, J.M.; Kim, J.H.; Poynton, H.; Su, T.; Wintz, H.; Fox, T.; Holman, P.; Loguinov, A.; Keles, S.; van der Laan, M.; et al. Exploratory and Confirmatory Gene Expression Profiling of Mac1 Delta. J. Biol. Chem. 2004, 279, 4450–4458. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Casadio, R.; Bergamini, C.M. Transglutaminases: Nature’s Biological Glues. Biochem. J. 2002, 368, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, H.; Hitomi, K. Role of Transglutaminase 2 in Cell Death, Survival, and Fibrosis. Cells 2021, 10, 1842. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, H.; Fukaya, Y.; Frampton, G.; Martinez-Fuentes, A.; Suzuki, K.; Kuo, T.-F.; Nagatsuma, K.; Shimokado, K.; Okuno, M.; Wu, J.; et al. Role of Transglutaminase 2 in Liver Injury via Cross-Linking and Silencing of Transcription Factor Sp1. Gastroenterology 2009, 136, 1783–1795. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.P.; Lim, Y.-C.; Hwang, J.; Na, S.; Kim, Y.-M.; Ha, K.-S. C-Peptide Prevents Hyperglycemia-Induced Endothelial Apoptosis through Inhibition of Reactive Oxygen Species-Mediated Transglutaminase 2 Activation. Diabetes 2013, 62, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.W.; Kwon, S.M.; Kim, S.W.; Yi, S.J.; Kim, Y.M.; Ha, K.S. Activation of in Situ Tissue Transglutaminase by Intracellular Reactive Oxygen Species. Biochem. Biophys. Res. Commun. 2003, 305, 633–640. [Google Scholar] [CrossRef]
- Ren, T.; Zhu, H.; Tian, L.; Yu, Q.; Li, M. Candida albicans Infection Disturbs the Redox Homeostasis System and Induces Reactive Oxygen Species Accumulation for Epithelial Cell Death. FEMS Yeast Res. 2020, 20, foz081. [Google Scholar] [CrossRef]
- Amorim-Vaz, S.; Tran, V.D.T.; Pradervand, S.; Pagni, M.; Coste, A.T.; Sanglard, D. RNA Enrichment Method for Quantitative Transcriptional Analysis of Pathogens In Vivo Applied to the Fungus Candida Albicans. mBio 2015, 6, e00942-15. [Google Scholar] [CrossRef]
- Schrevens, S.; Durandau, E.; Tran, V.D.T.; Sanglard, D. Using In Vivo Transcriptomics and RNA Enrichment to Identify Genes Involved in Virulence of Candida glabrata. Virulence 2022, 13, 1285–1303. [Google Scholar] [CrossRef]
- Eix, E.F.; Nett, J.E. How Biofilm Growth Affects Candida-Host Interactions. Front. Microbiol. 2020, 11, 1437. [Google Scholar] [CrossRef]




| Name | Parent | Genotype | Source or Reference |
|---|---|---|---|
| CBS138 | Wild type strain | [13] | |
| 2000H | 2001U | Δhis3::ScURA3Δura3 | [13] |
| KUE100 | 2000H | his3 yku80::SAT1 flipper | [13] |
| nox1Δ | KUE100 | his3 yku80::FRT nox1::CgHIS3 | [14] |
| NOX1 | nox1Δ | His3 yku80::FRT NOX1::CgHIS3 | This study |
| Primer Name | Sequence 5′-3′ |
|---|---|
| pHIScheckF | AGAAAACCAGCCTCACGATG |
| pHIScheckR | GTTCTTCTAGGGGAGCTAGTAGGGG |
| pNox1compF | GCTCTAGATAGGACTAGATGTAATTGAGCC |
| pNox1compR | GCTCTAGAGTTGAAGCATTCGGTATTAAC |
| pChr606 F1 | AAGAATGCCAACCAAGGATTCACAATAATCCGAAGC |
| pChr606 R1 | TTAGGCAAAGCATTTGTAAACCATTACAAGCACTC |
| pZeoORFcheckF1 | AAGTTGACCAGTGCCGTTCCGGTG |
| pchrF606kcheck | CTAATGGGGATATAGAAAGATAGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Huang, Y.; Orihara, K.; Chibana, H.; Kajiwara, S.; Chen, X. A Putative NADPH Oxidase Gene in Unicellular Pathogenic Candida glabrata Is Required for Fungal ROS Production and Oxidative Stress Response. J. Fungi 2024, 10, 16. https://doi.org/10.3390/jof10010016
Lin M, Huang Y, Orihara K, Chibana H, Kajiwara S, Chen X. A Putative NADPH Oxidase Gene in Unicellular Pathogenic Candida glabrata Is Required for Fungal ROS Production and Oxidative Stress Response. Journal of Fungi. 2024; 10(1):16. https://doi.org/10.3390/jof10010016
Chicago/Turabian StyleLin, Maoyi, Yao Huang, Kanami Orihara, Hiroji Chibana, Susumu Kajiwara, and Xinyue Chen. 2024. "A Putative NADPH Oxidase Gene in Unicellular Pathogenic Candida glabrata Is Required for Fungal ROS Production and Oxidative Stress Response" Journal of Fungi 10, no. 1: 16. https://doi.org/10.3390/jof10010016
APA StyleLin, M., Huang, Y., Orihara, K., Chibana, H., Kajiwara, S., & Chen, X. (2024). A Putative NADPH Oxidase Gene in Unicellular Pathogenic Candida glabrata Is Required for Fungal ROS Production and Oxidative Stress Response. Journal of Fungi, 10(1), 16. https://doi.org/10.3390/jof10010016







