The Changes, Aggregation Processes, and Driving Factors for Soil Fungal Communities during Tropical Forest Restoration
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Designs
2.2. Soil Physicochemical Analysis
2.3. DNA Extraction, Amplification, Sequencing Data Processing
2.4. Data Analysis
3. Results
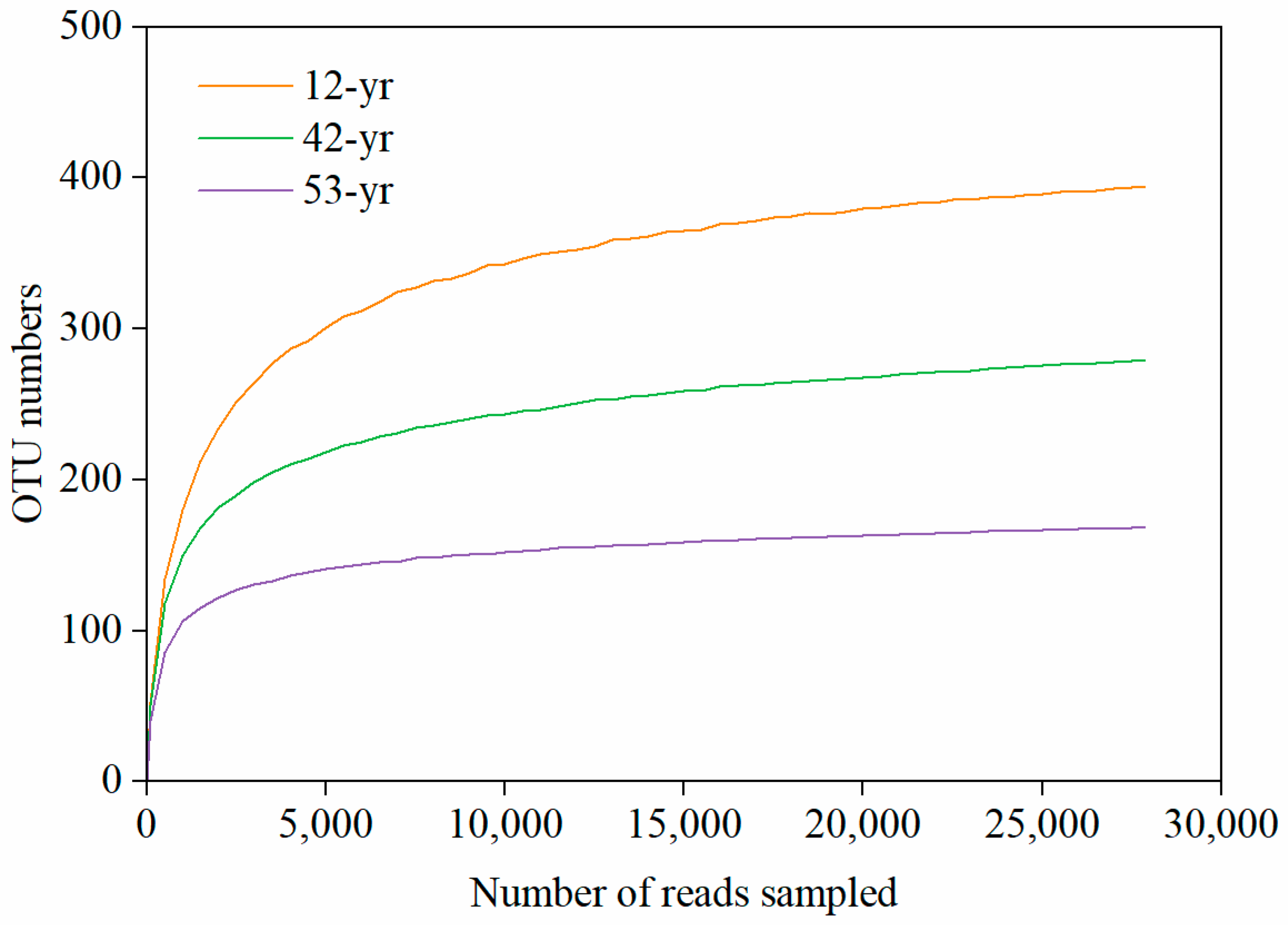
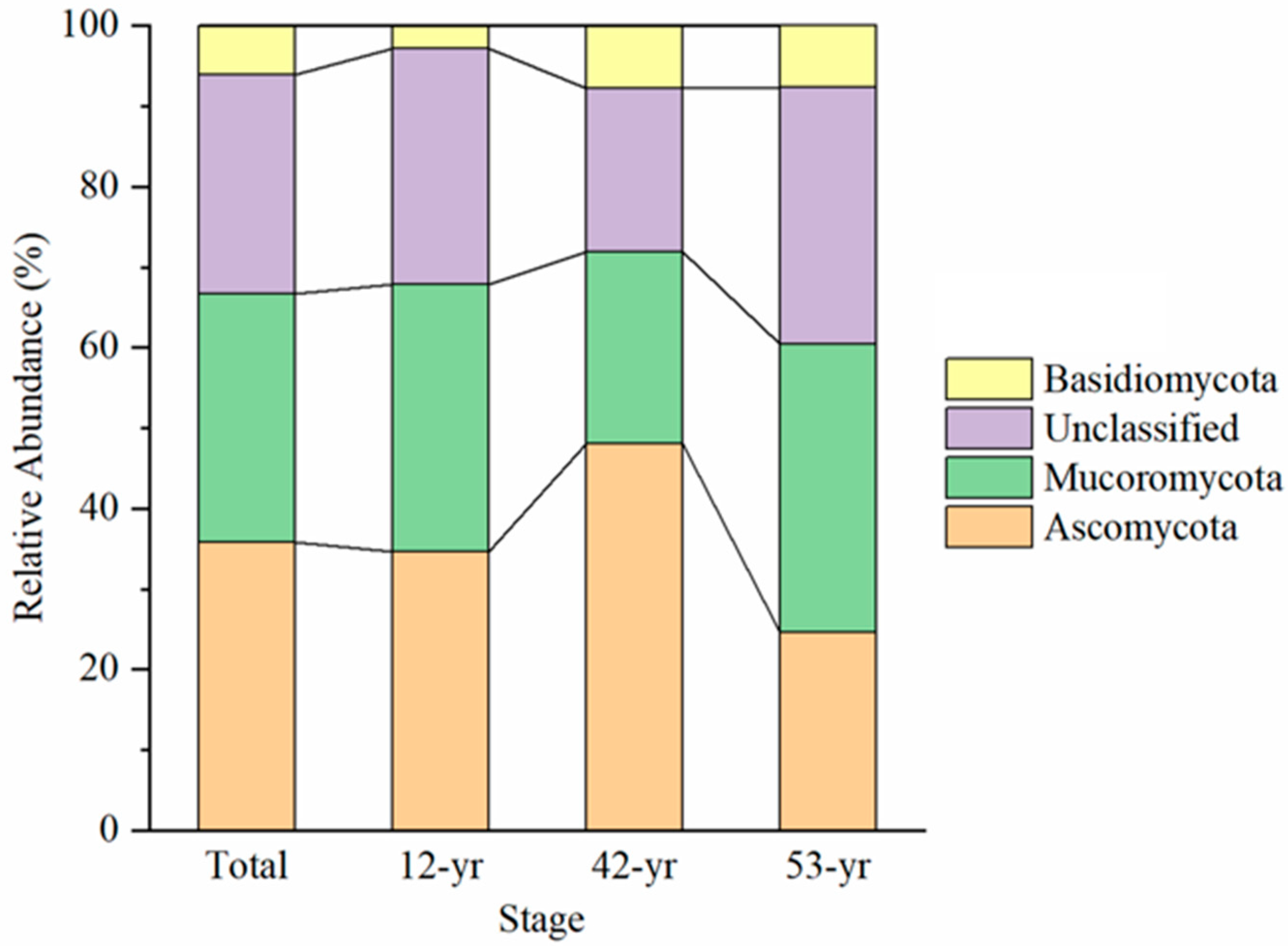
3.1. Changes in Fungal Diversity and Structure during Tropical Forest Restoration
3.2. Deterministic and Stochastic Processes of Soil Fungal Community Assembly
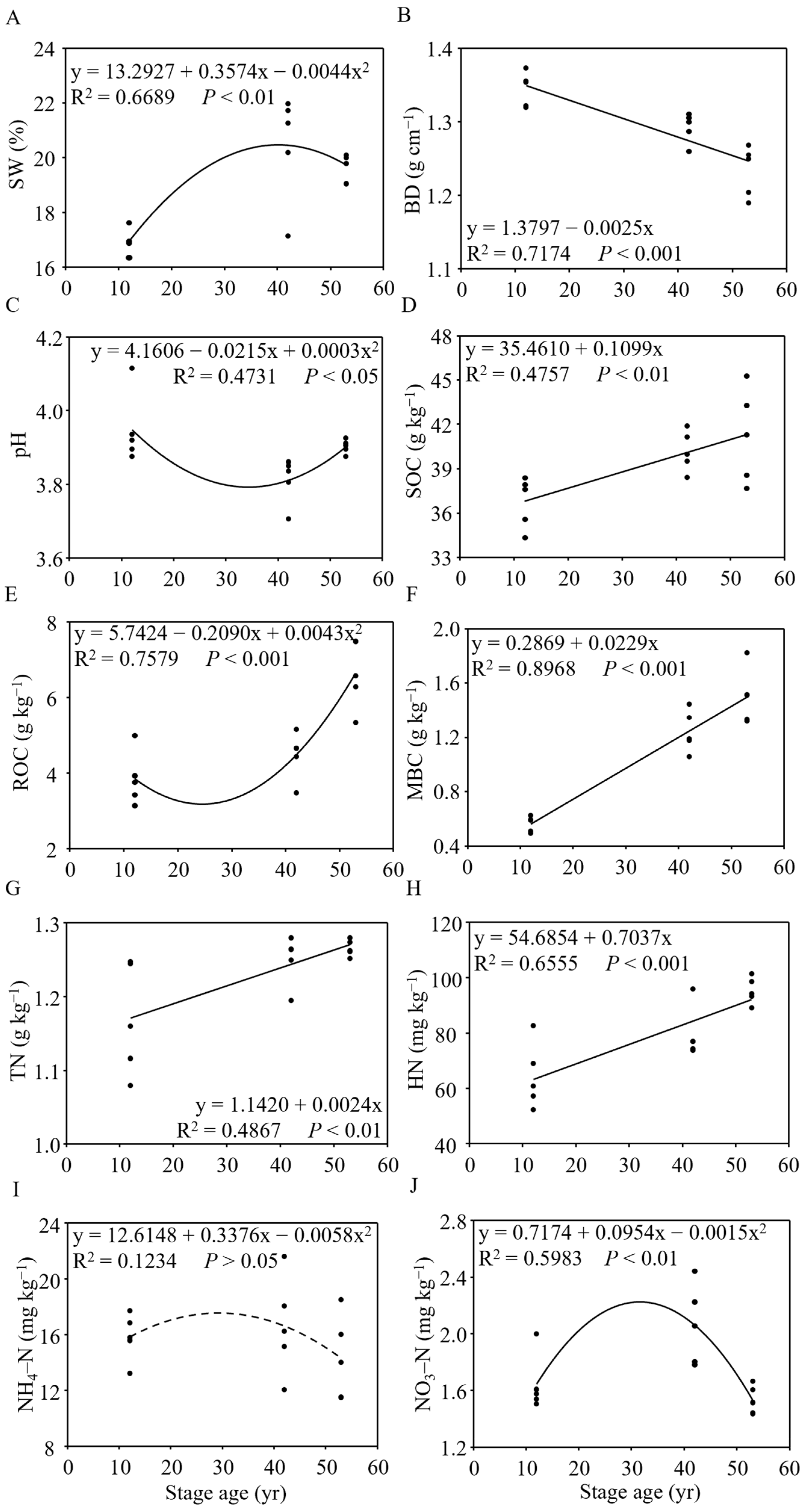
3.3. Shifts in Soil Properties during Tropical Forest Restoration
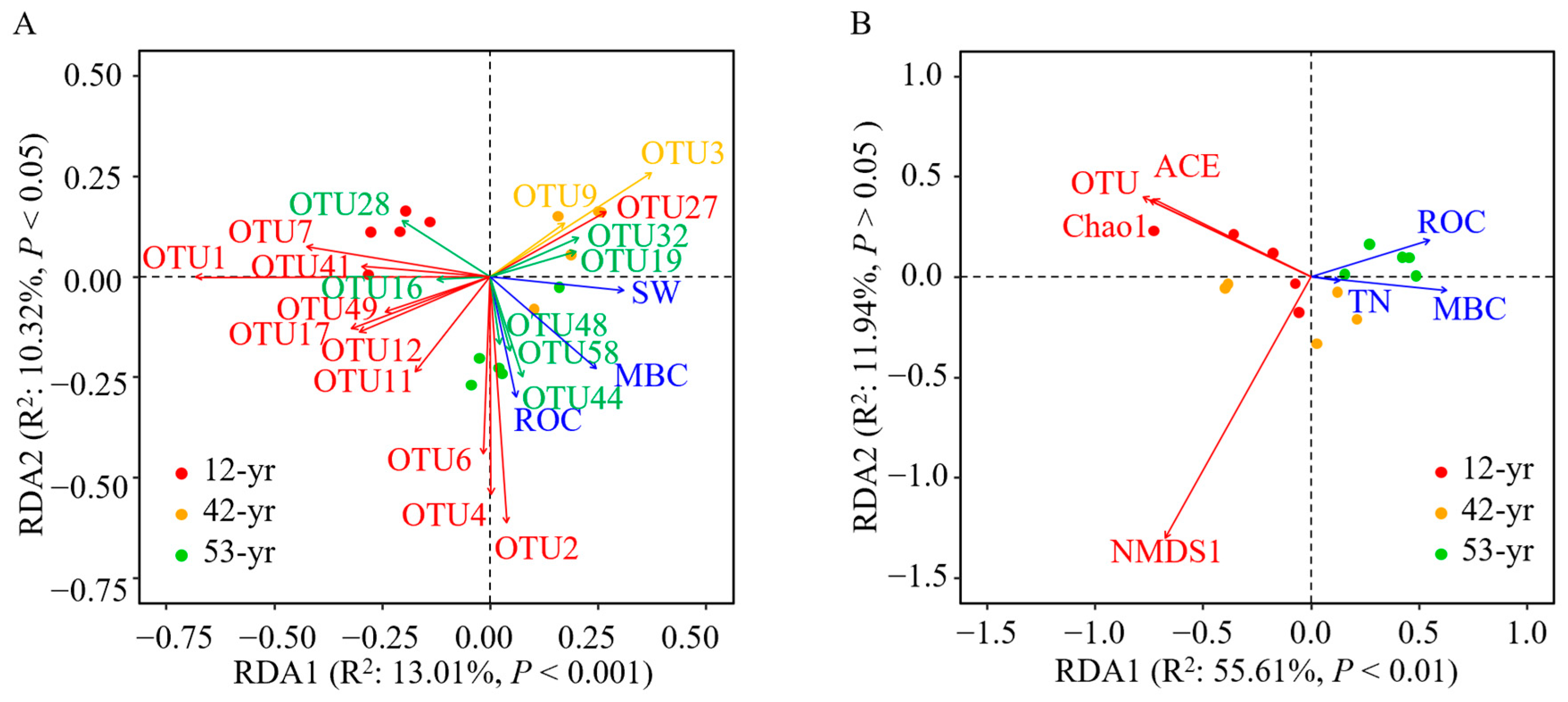
3.4. Linking Fungal Communities to Soil Properties
4. Discussion
4.1. Effect of Tropical Forest Restoration on Fungi Community Structure
4.2. Effect of Tropical Forest Restoration on Fungi Community Diversity
4.3. Effect of Stochastic and Deterministic Processes on Fungi Assemblages
4.4. Effect of Restoration-Driven Alterations of Soil Variables on Fungi Assemblages
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.D.; Yu, M.K.; Cheng, X.R. Abundant fungal and rare bacterial taxa jointly reveal soil nutrient cycling and multifunctionality in uneven-aged mixed plantations. Ecol. Indic. 2021, 129, 107932. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wang, J.Y.; Meng, Z.X.; Xu, R.; Chen, J.; Zhang, Y.J.; Hu, T.M. Fertility-related interplay between fungal guilds underlies plant richness-productivity relationships in natural grasslands. New Phytol. 2020, 226, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Huang, X.B.; Shen, J.Y.; Xu, F.D.; Su, J.R. Effects of plant diversity and soil properties on soil fungal community structure with secondary succession in the Pinus yunnanensis forest. Geoderma 2020, 379, 114646. [Google Scholar] [CrossRef]
- Wang, K.; Bi, Y.L.; Cao, Y.; Peng, S.P.; Christie, P.; Ma, S.P.; Zhang, J.Y.; Xie, L.L. Shifts in composition and function of soil fungal communities and edaphic properties during the reclamation chronosequence of an open-cast coal mining dump. Sci. Total. Environ. 2021, 767, 144465. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.M.; Cardoso, I.M.; Bianchi, F.; Silva, A.D.; Jamme, D.; Pena-Claros, M. Linking vegetation and soil functions during secondary forest succession in the Atlantic forest. For. Ecol. Manag. 2020, 457, 117696. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, M.K.; Cao, R.; Cao, Q.B.; Zuo, Q.Q.; Wang, P.; Yang, B.; Zhao, S. Contribution of plant litter and soil variables to organic carbon pools following tropical forest development after slash-and-burn agriculture. Land Degrad. Dev. 2020, 31, 1071–1077. [Google Scholar] [CrossRef]
- Kara, Ö.; Bolat, İ.; Çakıroğlu, K.; Öztürk, M. Plant canopy effects on litter accumulation and soil microbial biomass in two temperate forests. Biol. Fert. Soils 2008, 45, 193–198. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.J.; Liu, G.C.; Hu, C.Y.; Wang, X.C.; Yan, G.Y.; Wang, Q.G. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Zhao, W.; Yin, Y.L.; Li, S.X.; Dong, Y.L.; Su, S.F. Changes in soil fungal community composition and functional groups during the succession of Alpine grassland. Plant Soil 2023, 484, 201–216. [Google Scholar] [CrossRef]
- Visser, S. Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytol. 1995, 129, 389–401. [Google Scholar] [CrossRef]
- Artz, R.R.E.; Anderson, I.C.; Chapman, S.J.; Hagn, A.; Schloter, M.; Potts, J.M.; Campbell, C.D. Changes in fungal community composition in response to vegetational succession during the natural regeneration of cutover peatlands. Microb. Ecol. 2007, 54, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.X.; Dong, S.K.; Xu, Y.D.; Li, Y.; Li, S.; Wu, S.J.; Shen, H.; Liu, S.L.; Fry, E.L. Revegetation significantly increased the bacterial-fungal interactions in different successional stages of alpine grasslands on the Qinghai-Tibetan Plateau. Catena 2021, 205, 105385. [Google Scholar] [CrossRef]
- Hu, Y.G.; Zhang, Z.S.; Huang, L.; Qi, Q.; Liu, L.C.; Zhao, Y.; Wang, Z.R.; Zhou, H.K.; Lv, X.Y.; Mao, Z.C.; et al. Shifts in soil microbial community functional gene structure across a 61-year desert revegetation chronosequence. Geoderma 2019, 347, 126–134. [Google Scholar] [CrossRef]
- Chen, L.F.; He, Z.B.; Zhao, W.Z.; Liu, J.L.; Zhou, H.; Li, J.; Meng, Y.Y.; Wang, L.S. Soil structure and nutrient supply drive changes in soil microbial communities during conversion of virgin desert soil to irrigated cropland. Eur. J. Soil Sci. 2020, 71, 768–781. [Google Scholar] [CrossRef]
- Twieg, B.D.; Durall, D.M.; Simard, S.W. Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol. 2007, 176, 437–447. [Google Scholar] [CrossRef]
- Navarrete, A.A.; Aburto, F.; Gonzalez-Rocha, G.; Guzman, C.M.; Schmidt, R.; Scow, K. Anthropogenic degradation alter surface soil biogeochemical pools and microbial communities in an Andean temperate forest. Sci. Total Environ. 2023, 854, 158508. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.; Shi, N.N.; Zheng, Y.; Chen, L.; Wubet, T.; Bruelheide, H.; Both, S.; Buscot, F.; Ding, Q.; et al. Community assembly of ectomycorrhizal fungi along a subtropical secondary forest succession. New Phytol. 2015, 205, 771–785. [Google Scholar] [CrossRef]
- Garcia De Leon, D.; Neuenkamp, L.; Moora, M.; Öpik, M.; Davison, J.; Peña-Venegas, C.P.; Vasar, M.; Jairus, T.; Zobel, M. Arbuscular mycorrhizal fungal communities in tropical rain forest are resilient to slash-and-burn agriculture. J. Trop. Ecol. 2018, 34, 186–199. [Google Scholar] [CrossRef]
- Adamo, I.; Ortiz-Malavasi, E.; Chazdon, R.; Chaverri, P.; Ter Steege, H.; Geml, J. Soil Fungal Community Composition Correlates with Site-Specific Abiotic Factors, Tree Community Structure, and Forest Age in Regenerating Tropical Rainforests. Biology 2021, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.L.; Zhang, W.; Kuzyakov, Y.; Xiao, L.M.; Xiao, D.; Xu, L.; Chen, H.S.; Zhao, J.; Wang, K.L. Linking bacterial life strategies with soil organic matter accrual by karst vegetation restoration. Soil Biol. Biochem. 2023, 177, 108925. [Google Scholar] [CrossRef]
- Bastian, F.; Bouziri, L.; Nicolardot, B.; Ranjard, L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Jiao, S.; Chu, H.Y.; Zhang, B.G.; Wei, X.R.; Chen, W.M.; Wei, G.H. Linking soil fungi to bacterial community assembly in arid ecosystems. iMeta 2022, 1, e2. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.X.; Wang, B.R.; Xue, Z.J.; Wang, Y.Q.; An, S.S.; Chang, S.X. Deciphering factors driving soil microbial life-history strategies in restored grasslands. iMeta 2023, 2, e66. [Google Scholar] [CrossRef]
- Wang, S.J.; Zhao, S.; Yang, B.; Zhang, K.F.; Fan, Y.X.; Zhang, L.L.; Yang, X.D. The carbon and nitrogen stoichiometry in litter-soil-microbe continuum rather than plant diversity primarily shapes the changes in bacterial communities along a tropical forest restoration chronosequence. Catena 2022, 213, 106202. [Google Scholar] [CrossRef]
- Yang, T.; Adams, J.M.; Shi, Y.; He, J.S.; Jing, X.; Chen, L.T.; Tedersoo, L.; Chu, H.Y. Soil fungal diversity in natural grasslands of the Tibetan Plateau: Associations with plant diversity and productivity. New Phytol. 2017, 215, 756–765. [Google Scholar] [CrossRef]
- Waring, B.G.; Alvarez-Cansino, L.; Barry, K.E.; Becklund, K.K.; Dale, S.; Gei, M.G.; Keller, A.B.; Lopez, O.R.; Markesteijn, L.; Mangan, S.; et al. Pervasive and strong effects of plants on soil chemistry: A meta-analysis of individual plant ‘Zinke’ effects. Proc. R. Soc. B-Biol. Sci. 2015, 282, 91–98. [Google Scholar] [CrossRef]
- Ren, C.J.; Chen, J.; Deng, J.; Zhao, F.Z.; Han, X.H.; Yang, G.H.; Tong, X.G.; Feng, Y.Z.; Shelton, S.; Ren, G.X. Response of microbial diversity to C:N:P stoichiometry in fine root and microbial biomass following afforestation. Biol. Fertil. Soils 2017, 53, 457–468. [Google Scholar] [CrossRef]
- Lu, M.; Ren, Y.L.; Wang, S.J.; Tian, K.; Sun, X.Y.; Peng, S.X. Contribution of soil variables to bacterial community composition following land use change in Napahai plateau wetlands. J. Environ. Manag. 2019, 246, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, W.J.; Zhong, Z.K.; Zhang, Q.Y.; Wang, X.; Han, X.H.; Ren, C.J.; Yang, G.H. Response of soil microbial community to C:N:P stoichiometry along a Caragana korshinskii restoration gradient on the Loess Plateau, China. Forests 2020, 11, 823. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.Y.; Yan, W.M.; Zhong, Y.Q.W.; Shangguan, Z.P. Changes in soil microbial community structure during long-term secondary succession. Land Degrad. Dev. 2020, 31, 1151–1166. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Krause, S.M.B.; Li, S.P.; Wang, X.; Zang, Z.C.; Shen, M.W.; Yang, Q.S.; Lian, J.Y.; Wang, X.H.; et al. Changes in assembly processes of soil microbial communities during secondary succession in two subtropical forests. Soil Biol. Biochem. 2021, 154, 108144. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B 2011, 366, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Yan, G.Y.; Luo, X.; Huang, B.B.; Wang, H.L.; Sun, X.Y.; Gao, H.L.; Zhou, M.X.; Xing, Y.J.; Wang, Q.G. Assembly processes, driving factors, and shifts in soil microbial communities across secondary forest succession. Land Degrad. Dev. 2023, 34, 3130–3143. [Google Scholar] [CrossRef]
- Mahnert, A.; Moissl-Eichinger, C.; Berg, G. Microbiome interplay: Plants alter microbial abundance and diversity within the built environment. Front. Microbiol. 2015, 6, 887. [Google Scholar] [CrossRef]
- Shao, P.S.; Liang, C.; Rubert-Nason, K.; Li, X.Z.; Xie, H.T.; Bao, X.L. Secondary successional forests undergo tightly-coupled changes in soil microbial community structure and soil organic matter. Soil Biol. Biochem. 2019, 128, 56–65. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Deng, Y.; Zhang, P.; Xue, K.; Liang, Y.T.; Van Nostrand, J.D.; Yang, Y.F.; He, Z.L.; Wu, L.Y.; Stahl, D.A.; et al. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc. Natl. Acad. Sci. USA 2014, 111, E836–E845. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y.H. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob. Chang. Biol. 2020, 26, 4506–4520. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; Van Der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Kardol, P.; Bezemer, T.M.; Van Der Putten, W.H. Temporal variation in plant-soil feedback controls succession. Ecol. Lett. 2006, 9, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Powell, J.R.; Hamonts, K.; Reith, F.; Mele, P.; Brown, M.V.; Dennis, P.G.; Ferrari, B.C.; Fitzgerald, A.; Young, A.; et al. Circular linkages between soil biodiversity, fertility and plant productivity are limited to topsoil at the continental scale. New Phytol. 2017, 215, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Bannert, A.; Kleineidam, K.; Wissing, L.; Mueller-Niggemann, C.; Vogelsang, V.; Welzl, G.; Cao, Z.H.; Schloter, M. Changes in Diversity and Functional Gene Abundances of Microbial Communities Involved in Nitrogen Fixation, Nitrification, and Denitrification in a Tidal Wetland versus Paddy Soils Cultivated for Different Time Periods. Appl. Environ. Microbiol. 2011, 77, 6109–6116. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.Q.; Eichenberg, D.; Bruelheide, H.; Krober, W.; Kuhn, P.; Li, Y.; Von Oheimb, G.; Purschke, O.; Scholten, T.; Buscot, F.; et al. Soil and tree species traits both shape soil microbial communities during early growth of Chinese subtropical forests. Soil Biol. Biochem. 2016, 96, 180–190. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Jia, X.; Han, L.; Liu, Z.; Kang, S.Z.; Zhao, Y.H. Fungal community diversity in soils along an elevation gradient in a Quercus aliena var. acuteserrata forest in Qinling Mountains, China. Appl. Soil Ecol. 2021, 167, 104014. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, S.Y.; Huang, X.Q.; Zhao, Y.; Zhao, J.; Cheng, Y.; Cai, Z.C.; Zhang, J.B. pH-induced changes in fungal abundance and composition affects soil heterotrophic nitrification after 30 days of artificial pH manipulation. Geoderma 2020, 366, 114255. [Google Scholar] [CrossRef]
- Shang, R.G.; Li, S.F.; Huang, X.B.; Liu, W.D.; Lang, X.D.; Su, J.R. Effects of soil properties and plant diversity on soil microbial community composition and diversity during secondary succession. Forests 2021, 12, 805. [Google Scholar] [CrossRef]
- Wang, J.T.; Zheng, Y.M.; Hu, H.W.; Zhang, L.M.; Li, J.; He, J.Z. Soil pH determines the alpha diversity but not beta diversity of soil fungal community along altitude in a typical Tibetan forest ecosystem. J. Soils Sediments 2015, 15, 1224–1232. [Google Scholar] [CrossRef]
- Li, J.R.; Chen, L.; Wang, H.; Ouyang, S.; Liu, X.H.; Lu, J. Pattern and drivers of soil fungal community along elevation gradient in the Abies georgei forests of Segila mountains, Southeast Tibet. Glob. Ecol. Conserv. 2022, 39, e02291. [Google Scholar] [CrossRef]
- Wang, X.D.; Feng, J.G.; Ao, G.K.L.; Qin, W.K.; Han, M.G.; Shen, Y.W.; Liu, M.L.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Beck, T.; Joergensen, R.G.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.R.; Scheu, S. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible organic carbon fractions and soil quality changes in an Oxic Paleustalf under different pasture leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar]
- Johnson, J.; Evans, C.; Brown, N.; Skeates, S.; Watkinson, S.; Bass, D. Molecular analysis shows that soil fungi from ancient semi-natural woodland exist in sites converted to non-native conifer plantations. Forestry 2014, 87, 705–717. [Google Scholar] [CrossRef][Green Version]
- Zhou, L.; Liu, L.; Chen, W.Y.; Sun, J.J.; Hou, S.W.; Kuang, T.X.; Wang, W.X.; Huang, X.D. Stochastic determination of the spatial variation of potentially pathogenic bacteria communities in a large subtropical river. Environ. Pollut. 2020, 264, 114683. [Google Scholar] [CrossRef]
- Ning, D.L.; Deng, Y.; Tiedje, J.M.; Zhou, J.Z. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef]
- Jumpponen, A. Soil fungal community assembly in a primary successional glacier forefront ecosystem as inferred from rDNA sequence analyses. New Phytol. 2003, 158, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, D.B.; Clipson, N.; Melville, K.; Gadd, G.M.; McDermott, F.P. Characterization of fungal community structure on a weathered pegmatitic granite. Microb. Ecol. 2005, 50, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Huang, S.K.; Abdel-Wahab, M.A.; Daranagama, D.A.; Dayarathne, M.; D’Souza, M.J.; Goonasekara, I.D.; et al. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 2015, 72, 199–301. [Google Scholar] [CrossRef]
- Wang, H.Y.; Guo, S.Y.; Huang, M.R.; Thorsten, H.L.; Wei, J.C. Ascomycota has faster evolutionary rate and higher species diversity than Basidiomycota. Sci. China Life Sci. 2010, 53, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, A.; Luster, J.; Goransson, H.; Smittenberg, R.H.; Brunner, I.; Bernasconi, S.M.; Zeyer, J.; Frey, B. Bacterial, Archaeal and Fungal Succession in the Forefield of a Receding Glacier. Microb. Ecol. 2012, 63, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Li, S.Y.; Bol, R.; Zhu, P.; Peng, C.; An, T.T.; Cheng, N.; Liu, X.; Li, T.Y.; Xu, Z.Q.; et al. Differential long-term fertilization alters residue-derived labile organic carbon fractions and microbial community during straw residue decomposition. Soil Till. Res. 2021, 213, 105120. [Google Scholar] [CrossRef]
- Yelle, D.J.; Ralph, J.; Lu, F.C.; Hammel, K.E. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ. Microbiol. 2008, 10, 1844–1849. [Google Scholar] [CrossRef]
- Yan, B.S.; Sun, L.P.; Li, J.J.; Liang, C.Q.; Wei, F.R.; Xue, S.; Wang, G.L. Change in composition and potential functional genes of soil bacterial and fungal communities with secondary succession in Quercus liaotungensis forests of the Loess Plateau, western China. Geoderma 2020, 364, 114199. [Google Scholar] [CrossRef]
- Brinkmann, N.; Schneider, D.; Sahner, J.; Ballauff, J.; Edy, N.; Barus, H.; Irawan, B.; Budi, S.W.; Qaim, M.; Daniel, R.; et al. Intensive tropical land use massively shifts soil fungal communities. Sci. Rep. 2019, 9, 3403. [Google Scholar] [CrossRef]
- Köninger, J.; Ballabio, C.; Panagos, P.; Jones, A.; Schmid, M.W.; Orgiazzi, A.; Briones, M.J. Ecosystem type drives soil eukaryotic diversity and composition in Europe. Glob. Chang. Biol. 2023, 29, 5706–5719. [Google Scholar] [CrossRef]
- Hui, N.; Liu, X.X.; Jumpponen, A.; Setala, H.; Kotze, D.J.; Biktasheva, L.; Romantschuk, M. Over twenty years farmland reforestation decreases fungal diversity of soils, but stimulates the return of ectomycorrhizal fungal communities. Plant Soil 2018, 427, 231–244. [Google Scholar] [CrossRef]
- Labouyrie, M.; Ballabio, C.; Romero, F.; Panagos, P.; Jones, A.; Schmid, M.W.; Mikryukov, V.; Dulya, O.; Tedersoo, L.; Bahram, M. Patterns in soil microbial diversity across Europe. Nat. Commun. 2023, 14, 3311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, G.B.; Xue, S.; Wang, G.L. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Chase, J.M. Stochastic Community Assembly Causes Higher Biodiversity in More Productive Environments. Science 2010, 328, 1388–1391. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, K.E.; Finlay, R.D.; Dahlberg, A.; Stenlid, J.; Wardle, D.A.; Lindahl, B.D. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, J.; Hou, W.P.; Stubbendieck, R.M.; Xiong, H.; Jin, J.; Gong, J.Y.; Cheng, C.; Tang, X.X.; Liu, Y.L.; et al. Structural variability in the bulk soil, rhizosphere, and root endophyte fungal communities of Themeda japonica plants under different grades of karst rocky desertification. Plant Soil 2022, 475, 105–122. [Google Scholar] [CrossRef]
- Wagner, L.; Stielow, B.; Hoffmann, K.; Petkovits, T.; Papp, T.; Vagvolgyi, C.; De Hoog, G.S.; Verkley, G.; Voigt, K. A comprehensive molecular phylogeny of the Mortierellales (Mortierellomycotina) based on nuclear ribosomal DNA. Persoonia 2013, 30, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Redmile-Gordon, M.; Zhang, J.B.; Zhang, C.Z.; Ning, Q.; Li, W. Mortierella elongata’s roles in organic agriculture and crop growth promotion in a mineral soil. Land Degrad. Dev. 2018, 29, 1642–1651. [Google Scholar] [CrossRef]
- Koechli, C.; Campbell, A.N.; Pepe-Ranney, C.; Buckley, D.H. Assessing fungal contributions to cellulose degradation in soil by using high-throughput stable isotope probing. Soil Biol. Biochem. 2019, 130, 150–158. [Google Scholar] [CrossRef]
- Kaiser, M.; Kleber, M.; Berhe, A.A. How air-drying and rewetting modify soil organic matter characteristics: An assessment to improve data interpretation and inference. Soil Biol. Biochem. 2015, 80, 324–340. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Gao, C.; Zheng, Y.; He, X.H.; Yang, W.; Chen, L.; Wan, S.Q.; Guo, L.D. Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a semiarid steppe ecosystem. Mycorrhiza 2015, 25, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Eisenhauer, N.; Scheu, S.; Jousset, A. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol. Lett. 2012, 15, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Garbeva, P.; Liu, X.J.; Gunnewiek, P.; Clocchiatti, A.; Hundscheid, M.P.J.; Wang, X.X.; De Boer, W. Volatile-mediated antagonism of soil bacterial communities against fungi. Environ. Microbiol. 2020, 22, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Toyota, K.; Kimura, M. Colonization of chlamydospores of Fusarium oxysporum f. sp. raphani by soil bacteria and their effects on germination. Soil Biol. Biochem. 1993, 25, 193–197. [Google Scholar] [CrossRef]
- Munimbazi, C.; Bullerman, L.B. Isolation and partial characterization of antifungal metabolites of Bacillus pumilus. J. Appl. Microbiol. 1998, 84, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Xavier, L.J.C.; Germida, J.J. Bacteria associated with Glomus clarum spores influence mycorrhizal activity. Soil Biol. Biochem. 2003, 35, 471–478. [Google Scholar] [CrossRef]

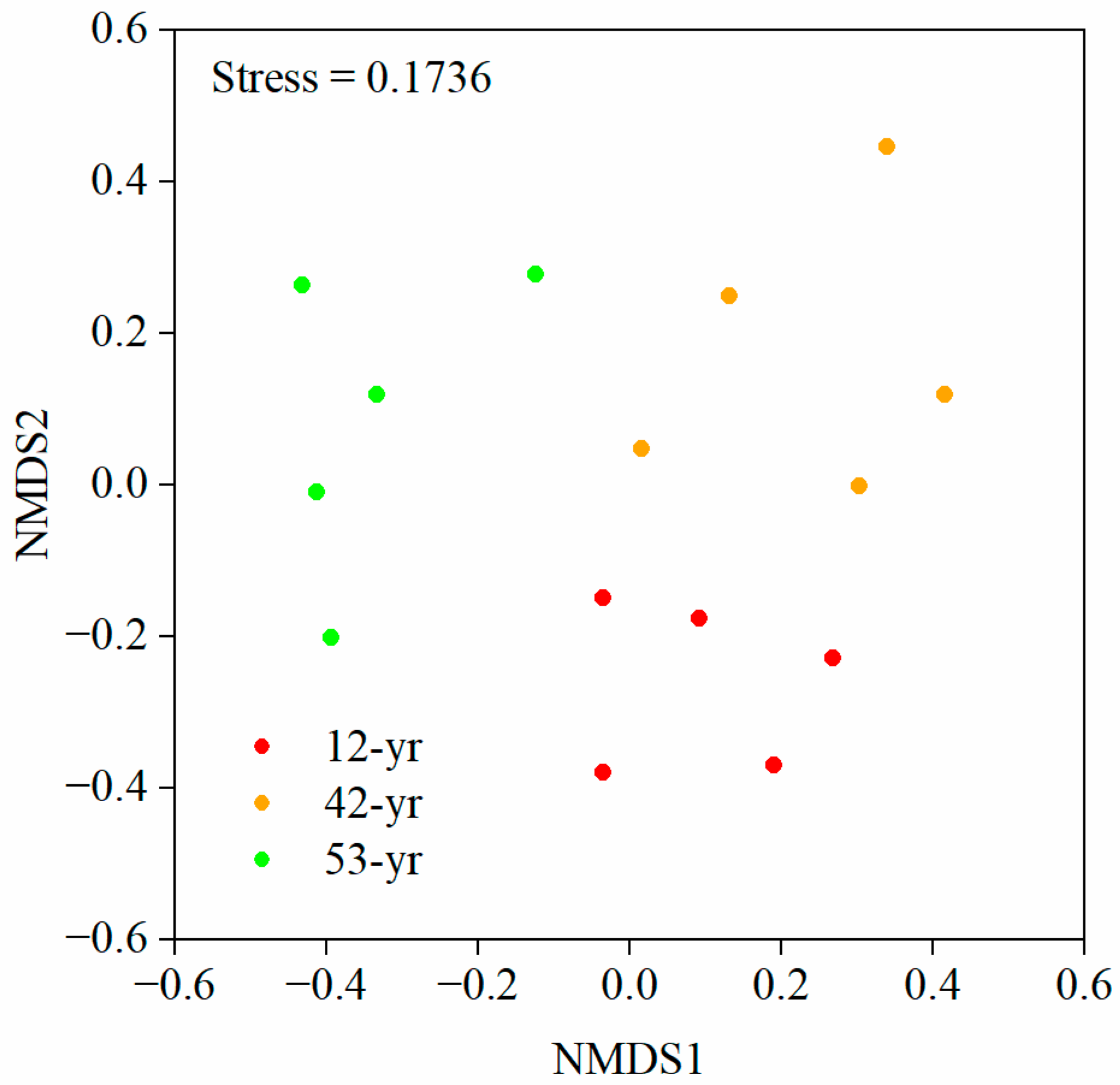
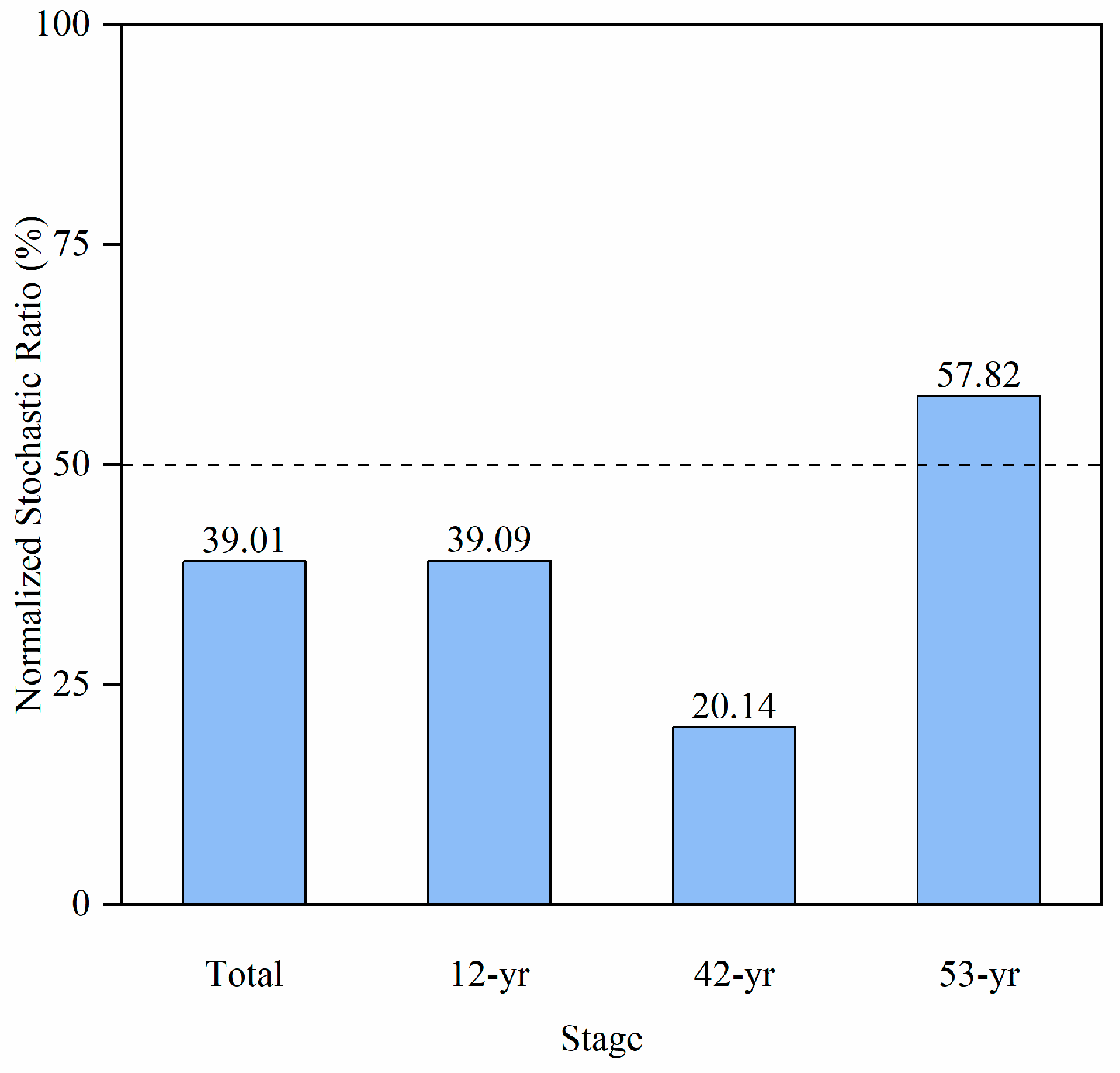
| Stage | R | p | Significance | Description |
|---|---|---|---|---|
| 12-yr vs. 42-yr | 0.61 | 0.008 | ** | significantly separated but overlapped |
| 12-yr vs. 53-yr | 0.52 | 0.008 | ** | significantly separated but overlapped |
| 42-yr vs. 53-yr | 0.47 | 0.020 | * | separated but strongly overlapped |
| overall | 0.50 | 0.001 | *** | significantly separated but overlapped |
| Variations | Variables | MBC | ROC | TN | SW | Residuals | Total |
|---|---|---|---|---|---|---|---|
| Fungal composition | R2 | 0.115 | 0.096 | - | 0.091 | 0.698 | 1.000 |
| R2 (Cumulative) | 0.115 | 0.211 | - | 0.302 | 1.000 | 1.000 | |
| p | 0.003 | 0.010 | - | 0.027 | - | - | |
| Fungal diversity | R2 | 0.400 | 0.140 | 0.136 | - | 0.324 | 1.000 |
| R2 (Cumulative) | 0.400 | 0.540 | 0.676 | - | 1.000 | 1.000 | |
| p | 0.003 | 0.043 | 0.022 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wang, S.; Wang, C.; Lan, M.; Yang, S.; Luo, S.; Li, R.; Xia, J.; Xiao, B.; Xie, L.; et al. The Changes, Aggregation Processes, and Driving Factors for Soil Fungal Communities during Tropical Forest Restoration. J. Fungi 2024, 10, 27. https://doi.org/10.3390/jof10010027
Guo X, Wang S, Wang C, Lan M, Yang S, Luo S, Li R, Xia J, Xiao B, Xie L, et al. The Changes, Aggregation Processes, and Driving Factors for Soil Fungal Communities during Tropical Forest Restoration. Journal of Fungi. 2024; 10(1):27. https://doi.org/10.3390/jof10010027
Chicago/Turabian StyleGuo, Xiaofei, Shaojun Wang, Chen Wang, Mengjie Lan, Shengqiu Yang, Shuang Luo, Rui Li, Jiahui Xia, Bo Xiao, Lingling Xie, and et al. 2024. "The Changes, Aggregation Processes, and Driving Factors for Soil Fungal Communities during Tropical Forest Restoration" Journal of Fungi 10, no. 1: 27. https://doi.org/10.3390/jof10010027
APA StyleGuo, X., Wang, S., Wang, C., Lan, M., Yang, S., Luo, S., Li, R., Xia, J., Xiao, B., Xie, L., Wang, Z., & Guo, Z. (2024). The Changes, Aggregation Processes, and Driving Factors for Soil Fungal Communities during Tropical Forest Restoration. Journal of Fungi, 10(1), 27. https://doi.org/10.3390/jof10010027






