First Record of Flavocillium subprimulinum (Cordycipitaceae, Hypocreales) in Mexico: Morphological and Molecular Characterisation, Nematocidal Activity of Its Liquid Culture Filtrates against Haemonchus contortus and Protease Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location
2.2. Biological Material
2.3. Fungal Traditional Taxonomy (Morphometrics)
2.4. Fungal Taxonomy by Molecular Techniques
2.5. Phylogenetic Tree
2.6. Nematodes
2.6.1. Panagrellus Redivivus
2.6.2. Haemonchus contortus Infective Larvae
2.7. Flavocillium subprimulinum Culture Filtrates
2.8. Assessment of the Lethal Activity of Fungal Liquid Culture Filtrates against Haemonchus contortus Infective Larvae
2.9. Protein Profile and Protease Activity Analysis Using Liquid Culture Filtrates
2.10. Microscopic Analysis of Haemonchus contortus Infective Larvae Exposed to Flavocillium subprimulinum Liquid Culture Filtrates
2.11. Statistical Analysis
3. Results
3.1. Fungal Traditional Taxonomy (Morphometrics)
3.2. Fungal Molecular Taxonomic Identification
3.3. Assessment of the Lethal Activity of Fungal Liquid Culture Filtrates against Haemonchus contortus Infective Larvae
3.4. Microscopic Analysis of Haemonchus contortus Infective Larvae Exposed to Flavocillium subprimulinum Liquid Culture Filtrates
3.5. Protein Profile and Protease Activity Analysis from Liquid Culture Filtrates
4. Discussion
4.1. Fungal Traditional and Molecular Taxonomy
4.2. Assessment of the Lethal Activity of Fungal Liquid Culture Filtrates against Haemonchus contortus Infective Larvae
4.3. Microscopic Analysis of Haemonchus contortus Infective Larvae Exposed to Flavocillium subprimulinum Liquid Culture Filtrates
4.4. Protein Profile and Protease Activity Analysis of Fungal Liquid Culture Filtrates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setälä, H.; McLean, M.A. Decomposition rate of organic substrates in relation to the species diversity of soil saprophytic fungi. Oecologia 2004, 139, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, T.S.A.; Gouveia, A.S.; Balbino, H.M.; Morgan, T.; de Freitas, L.G. Duddingtonia. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil-Kumar, M., Annapurina, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 683–694. [Google Scholar] [CrossRef]
- Akram, S.; Ahmed, A.; He, P.; He, P.; Liu, Y.; Wu, Y.; Munir, S.; He, Y. Uniting the role of endophytic fungi against plant pathogens and their interaction. J. Fungi 2023, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, M.R.; Zare, R. Fungi as biological control agents of plant-parasitic nematodes. In Plant Defense: Biological Control. Progress in Biological Control, 2nd ed.; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2020; Volume 22. [Google Scholar] [CrossRef]
- Vanitha, S.; Padma, A.S. Mycobiota-Role in soil health and as biocontrol agent. In Sustainable Utilization of Fungi in Agriculture and Industry; Ajmera, S., Bhima, B., Krishnappa, M., Merugu, R., Eds.; Bentham Science Publishers: Singapore, 2022; Volume 4, pp. 15–34. [Google Scholar]
- Ye, L.F.; Liu, H.Y.; Deng, H.D.; Zheng, Y.P.; Han, Y.W.; Gao, X.T.; Abbott, L.K.; Zhao, C.M.; Li, J.H. Effects of decadal nitrogen and phosphorus fertilization on microbial taxonomic and functional attributes associated with soil organic carbon decomposition and concentration in an alpine meadow. Ecol. Indic. 2023, 146, 109790. [Google Scholar] [CrossRef]
- Wei, D.P.; Gentekaki, E.; Wanasinghe, D.N.; Tang, S.M.; Hyde, K.D. Diversity, molecular dating and ancestral characters state reconstruction of entomopathogenic fungi in Hypocreales. Mycosphere 2022, 13, 281–351. [Google Scholar] [CrossRef]
- Nicoletti, R.; Becchimanzi, A. Endophytism of Lecanicillium and Akanthomyces. Agriculture 2020, 10, 205. [Google Scholar] [CrossRef]
- Reddy, S.G.E. Lecanicillium spp. for the management of aphids, whiteflies, thrips, scales and mealy bugs. In Arthropods-Are They Beneficial for Mankind? Ranz, R.E.R., Ed.; IntechOpen: London, UK, 2021; pp. 181–199. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Hlaoua, W.; Rhouma, A.; Al-Judaibi, A.A.; Arcos, S.C.; Robertson, L.; Ciordia, S.; Horrigue-Raouani, N.; Navas, A.; Abdel-Azeem, A.M. Biological and proteomic analysis of a new isolate of the nematophagous fungus Lecanicillium sp. BMC Microbiol. 2023, 23, 108. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I.; Kitsiou, F.; Eliopoulos, P.A. Entomopathogenic action of wild fungal strains against stored product beetle pests. Insects 2023, 14, 91. [Google Scholar] [CrossRef]
- Huang, S.; Maharachchikumbura, S.S.N.; Jeewon, R.; Jayarama, B.; Phookamsak, R.; Hyde, K.D.; Al-Sadi, A.M.; Kang, J. Lecanicillium subprimulinum (Cordycipitaceae, Hypocreales), a novel species from Baoshan, Yunnan. Phytotaxa 2018, 348, 99–108. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, Y.; Fan, Q.; Duan, D.E.; Zhang, G.D.; Dai, R.Q.; Yu, H. Multigene phylogeny of the family Cordycipitaceae (Hypocreales): New taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Divers 2020, 103, 1–46. [Google Scholar] [CrossRef]
- Barron, G.L. The Nematode-Destroying Fungi; Canadian Biological Publication: Guelph, ON, Canada, 1977. [Google Scholar]
- Chen, S.A.; Lin, H.C.; Hsueh, Y.P. The cAMP-PKA pathway regulates prey sensing and trap morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora. G3 2022, 12, jkac217. [Google Scholar] [CrossRef]
- Norbring-Hertz, B.; Jansson, H.B.; Tunlid, A. Nematophagous Fungi. In Encyclopedia of Life Sciences; John Willey & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Hastuti, L.D.S.; Berliani, K.; Mulya, M.B.; Hartanto, A.; Pahlevi, S. Mini review: Extracellular enzymes and proteins produced by nematophagous fungi. IOP Conf. Ser. Earth Environ. Sci. 2022, 1115, 012063. [Google Scholar] [CrossRef]
- Goh, J.; Oh, Y.; Park, Y.H.; Mun, H.Y.; Park, S.; Cheon, W. Isolation and Characterization of Previously Undescribed Seventeen Fungal Species Belonging to the order Hypocreales in Korea. Korean J. Mycol. 2022, 50, 1–29. [Google Scholar]
- Rouatbi, M.; Gharbi, M.; Rjeibi, M.R.; Salem, I.B.; Akkari, H.; Lassoued, N.; Rekik, M. Effect of the infection with the nematode Haemonchus contortus (Strongylida: Trichostrongylidae) on the haematological, biochemical, clinical and reproductive traits in rams. Onderstepoort J. Vet. Res. 2016, 83, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Flay, K.J.; Hill, F.I.; Muguiro, D.H. A Review: Haemonchus contortus infection in pasture-based sheep production systems, with a focus on the pathogenesis of anaemia and changes in haematological parameters. Animals 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- GPS Coordinates. Latitude and Longitude of Tetela del Volcán, Morelos, Mexico. 2023. Available online: https://latitude.to/map/mx/mexico/cities/tetela-del-volcan (accessed on 12 July 2023).
- Sánchez-Martínez, E.; Aguilar-Marcelino, L.; Hernandez-Romano, J.; Castaneda-Ramirez, G.S.; Mendoza-de-Gives, P. Taxonomic and biological characterization and predatory activity of four nematophagous fungi isolates of Arthrobotrys species from Tapachula, Chiapas, Mexico. Arch. Agron. Soil Sci. 2021, 69, 327–343. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Stielow, J.B.; Levesque, C.A.; Seifert, K.A.; Meyer, W.; Irinyi, L.; Smits, D.; Robert, V. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia-Mol. Phylogeny Evol. Fungi 2015, 35, 242–263. [Google Scholar] [CrossRef]
- de Lara, R.; Castro, T.; Castro, J.; Castro, G. Cultivo del nematodo Panagrellus redivivus (Goodey, 1945) en un medio de avena enriquecida con Spirulina sp. Rev. Biol. Mar. Oceanogr. 2007, 42, 29–36. [Google Scholar] [CrossRef]
- NOM-052-ZOO-1995. Norma Oficial Mexicana. Available online: http://www.senasica.gob.mx (accessed on 8 August 2023).
- Ley Federal de Sanidad Animal (Federal Law for Animal Health). DOF, Diario Oficial de la Federación, 07-06-2012. Available online: https://www.gob.mx/cms/uploads/attachment/file/118761/LFSA.pdf (accessed on 8 August 2023).
- Cedillo-Borda, M.; López-Arellano, M.E.; Reyes-Guerrero, D.E. In vitro assessment of ivermectin resistance and gene expression profiles of P-glycoprotein genes from Haemonchus contortus (L3). Bio-Protocol 2020, 101, e3851. [Google Scholar] [CrossRef]
- Olmedo-Juárez, A.; Rojo-Rubio, R.; Zamilpa, A.; Mendoza-de Gives, P.; Arece-García, J.; López-Arellano, M.E.; von Son-de Fernex, E. In vitro larvicidal effect of a hydroalcoholic extract from Acacia cochliacantha leaf against ruminant parasitic nematodes. Vet. Res. Commun. 2017, 41, 227–232. [Google Scholar] [CrossRef]
- Camas-Pereyra, R.; Bautista-García, G.A.; Avila, G.; Alcala-Canto, Y.; Maza-Lopez, J.; Reyes-Guerrero, D.E.; Higuera-Piedrahita, R.I.; López-Arellano, M.E. In silico analysis of two Haemonchus spp. serine protease peptides (S28) and their immunomodulatory activity in vitro. Mol. Biochem. Parasitol. 2023, 253, 111545. [Google Scholar] [CrossRef]
- Pérez-Anzúrez, G.; Olmedo-Juárez, A.; von Son-de Fernex, E.; Alonso-Díaz, M.Á.; Delgado-Núñez, E.J.; López-Arellano, M.E.; González-Cortázar, M.; Zamilpa, A.; Ocampo-Gutiérrez, A.Y.; Paz-Silva, A.; et al. Arthrobotrys musiformis (Orbiliales) kills Haemonchus contortus infective larvae (Trichostronylidae) through its predatory activity and its fungal culture filtrates. Pathogens 2022, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-de Gives, P.; Delgado-Núñez, E.J.; Salinas-Sánchez, D.O.; Olmedo-Juárez, A.; González-Cortázar, M. Prosopis Genus: Potential uses and applications with special reference to Public and Veterinary Medicine. In Prosopis: Properties, Uses and Diversity; Batista, R., Ed.; Nova Science Publisher Inc.: New York, NY, USA, 2021; Chapter 3; pp. 109–150. [Google Scholar]
- Delgado-Núñez, E.J.; Zamilpa, A.; González-Cortazar, M.; Olmedo-Juárez, A.; Cardoso-Taketa, A.; Sánchez-Mendoza, E.; Tapia-Maruri, D.; Salinas-Sánchez, D.O.; Mendoza-de Gives, P. Isorhamnetin: A nematocidal flavonoid from Prosopis laevigata leaves against Haemonchus contortus eggs and larvae. Biomolecules 2020, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Morales, J.A.; Zamilpa, A.; Salinas-Sánchez, D.O.; González-Cortazar, M.; Tapia-Maruri, D.; Mendoza-de Gives, P.; Rivas-González, J.M.; Olmedo-Juárez, A. In vitro ovicidal effect of p-coumaric acid from Acacia bilimekii aerial parts against Haemonchus contortus. Vet. Parasitol. 2023, 320, 109971. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, J.C.; Djian, C.; Pijarowski, L. Study of the nematicidal properties of the culture filtrate of the nematophagous fungus Paecilomyces lilacinus. Rev. Nematol. 1989, 12, 331–336. [Google Scholar]
- Djian, C.; Pijarowski, L.; Ponchet, M.; Arpin, N.; Favre-Bonvin, J. Acetic acid: A selective nematicidal metabolite from culture filtrates of Paecilomyces lilacinus (Thom) Samson and Trichoderma longibrachiatum Rifai. Nematologica 1991, 37, 101–112. [Google Scholar]
- Regaieg, H.; Ciancio, A.; Raouani, N.H.; Grasso, G.; Rosso, L. Effects of culture filtrates from the nematophagous fungus Verticillium leptobactrum on viability of the root-knot nematode Meloidogyne incognita. World J. Microbiol. Biotechnol. 2010, 26, 2285–2289. [Google Scholar] [CrossRef]
- Kasim, A.A. Influence of bioactive metabolites extracted from three species of nematode-trapping fungi on the growth of Escherichia coli and Staphylococcus aureus. World J. Biol. Sci. 2016, 4, 09–14. [Google Scholar]
- Lafta, A.A.; Kasim, A.A. Effect of nematode-trapping fungi, Trichoderma harzianum and pseudomonas fluorescens in controlling Meloidogyne spp. Plant Arch. 2019, 19, 1163–1168. [Google Scholar]
- Chen, Y.H.; Liu, X.; Dai, R.; Ou, X.; Xu, Z.F.; Zhang, K.Q.; Niu, X.M. Novel polyketide-terpenoid hybrid metabolites and increased fungal nematocidal ability by disruption of genes 277 and 279 in nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2020, 68, 7870–7879. [Google Scholar] [CrossRef]
- Ángeles-Hernández, S.; Torres-Hernández, G.; Alonso-Díaz, M.A.; von Son-de-Fernex, E.; Aguilar-Marcelino, L.; González-Garduño, R.; Becerril-Pérez, C.M.; Alcántara-Carbajal, J.L.; Vargas-López, S.; Olmedo-Juárez, A.; et al. Effect of an Arthrobotrys musiformis (Fungi: Orbiliales) culture filtrate on the population of gastrointestinal parasitic nematode eggs in faeces of grazing lambs. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100565. [Google Scholar] [CrossRef]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-de Gives, P.; Rodríguez-Labastida, M.; Olmedo-Juárez, A.; Gamboa-Angulo, M.M.; Reyes-Estebanez, M. A nematode crude extract acts as an elicitor of the nematocidal activity of nematophagous fungi liquid culture filtrates against Haemonchus contortus (Nematoda: Trichostrongylidae). Acta Parasitol. 2022, 67, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Saharan, R.; Patil, J.A.; Yadav, S.; Kumar, A.; Goyal, V. The nematicidal potential of novel fungus, Trichoderma asperellum FbMi6 against Meloidogyne incognita. Sci. Rep. 2023, 13, 6603. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.G.; Jarman, T.B.; Rickards, R.W. Structures and absolute configurations of antibiotics of the oligosporon group from the nematode-trapping fungus Arthrobotrys oligospora. J. Antibiot. 1995, 48, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.G.; Rickards, R.W.; Lacey, E. Structures of flagranones A, B and C, cyclohexenoxide antibiotics from the nematode-trapping fungus Duddingtonia flagrans. J. Antibiot. 1999, 52, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. Appl. Microbiol. Biotechnol. 2016, 100, 3799–3812. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.L.; Atkinson, H.J. Physiology of Nematodes, 2nd ed.; Columbia University Press: New York, NY, USA, 1977; p. 14. [Google Scholar]
- Chen, L.L.; Liu, L.J.; Shi, M.; Song, X.Y.; Zheng, C.Y.; Chen, X.L.; Zhang, Y.Z. Characterization and gene cloning of a novel serine protease with nematicidal activity from Trichoderma pseudokoningii SMF2. FEMS Microbiol. Lett. 2009, 299, 135–142. [Google Scholar] [CrossRef]
- Segers, R.; Butt, T.M.; Kerry, B.R.; Peberdy, J.F. The nematophagous fungus Verticillium chlamydosporium produces a chymoelastase-like protease which hydrolyses host nematode proteins in situ. Microbiology 1994, 140, 2715–2723. [Google Scholar] [CrossRef]
- Tikhonov, V.E.; Lopez-Llorca, L.V.; Salinas, J.; Jansson, H.B. Purification and characterization of chitinases from the nematophagous fungi Verticillium chlamydosporium and V. suchlasporium. Fungal Genet. Biol. 2002, 35, 67–78. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Hlaoua, W.; Al-Judaibi, A.A.; Rhouma, A.; Horrigue-Raouani, N.; Abdel-Azeem, A.M. Comparative effectiveness of filamentous fungi in biocontrol of Meloidogyne javanica and activated defense mechanisms on tomato. J. Fungi 2022, 9, 37. [Google Scholar] [CrossRef]
- Shetty, P. Glucoamylase from the predacious fungus Arthrobotrys conoides: A cationic enzyme with high debranching activity and raw starch digestibility. Appl. Biochem. Microbiol. 2016, 52, 176–182. [Google Scholar] [CrossRef]
- Lopez-Llorca, L.V.; Gómez-Vidal, S.; Monfort, E.; Larriba, E.; Casado-Vela, J.; Elortza, F.; Jansson, H.B.; Salinas, J.; Martín-Nieto, J. Expression of serine proteases in egg-parasitic nematophagous fungi during barley root colonization. Fungal Genet. Biol. 2010, 47, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Meng, Z.; Ye, F.; Yang, J.; Liu, S.; Sun, Y.; Guo, Y.; Mi, Q.; Huang, X.; Zou, C.; et al. The crystal structures of two cuticle–degrading proteases from nematophagous fungi and their contribution to infection against nematodes. FASEB J. 2010, 24, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.B.; Yang, J.K.; Lin, C.; Zhang, Y.; Zhang, K.Q. Purification and characterization of an extracellular serine protease from the nematode-trapping fungus Dactylella shizishanna. Lett. Appl. Microbiol. 2006, 42, 589–594. [Google Scholar] [CrossRef]
- Soares, F.E.; Braga, F.R.; Araújo, J.V.; Geniêr, H.L.; Gouveia, A.S.; Queiroz, J.H. Nematicidal activity of three novel extracellular proteases of the nematophagous fungus Monacrosporium sinense. Parasitol. Res. 2013, 112, 1557–1565. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Wu, W.; Liu, X.; Li, S. Purification, characterization, and gene cloning of an alkaline serine protease from a highly virulent strain of the nematode-endoparasitic fungus Hirsutella rhossiliensis. Microbiol. Res. 2009, 164, 665–673. [Google Scholar] [CrossRef]


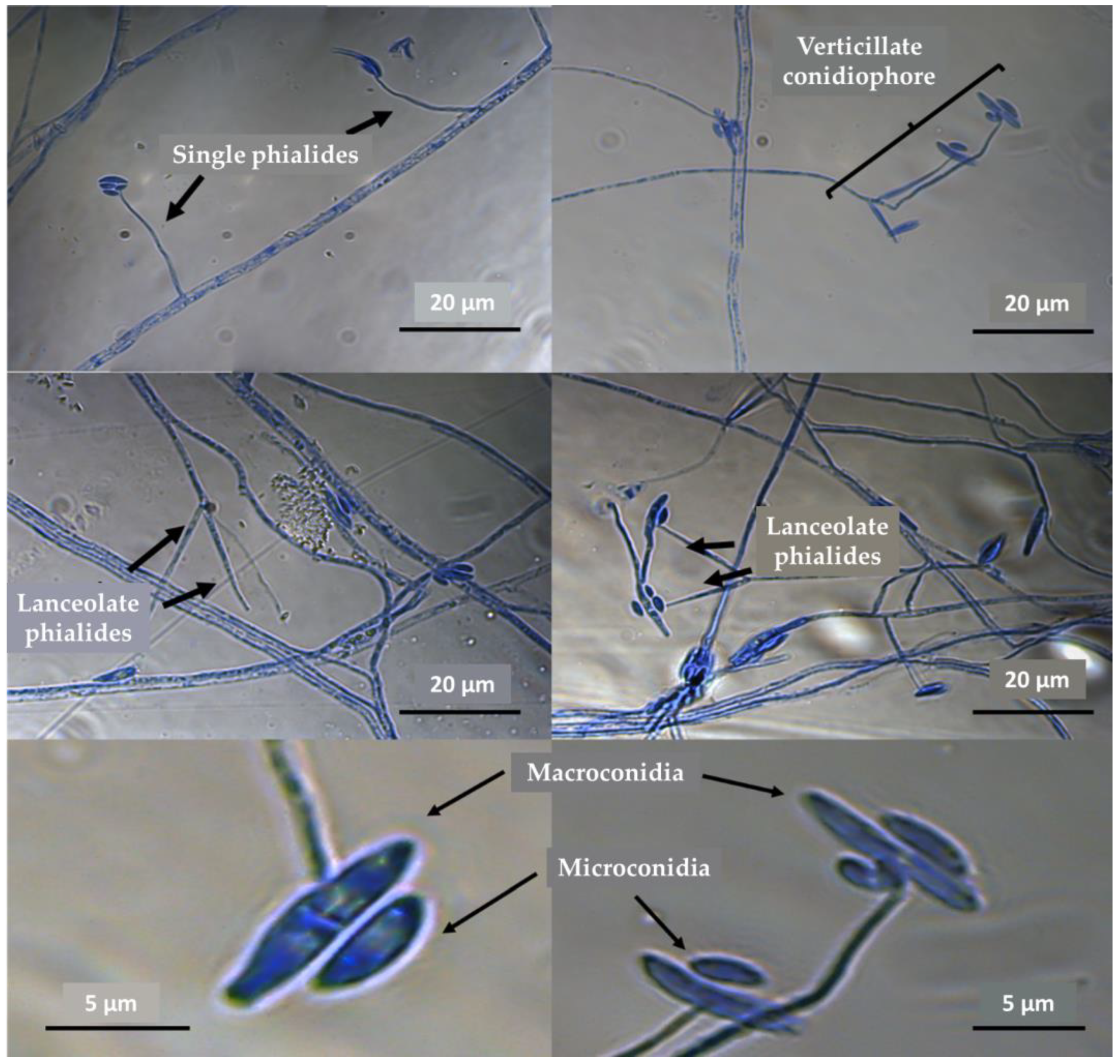





| Characteristic | n | Length (µm) | Width (µm) |
|---|---|---|---|
| Macroconidia | 50 | 9.22 (7–14) | 2.11 (1.5–2.6) |
| Microconidia | 50 | 4.46 (3–7) | 1.81 (1.2–2.4) |
| Phialides | 50 | 26.05 (14–45) | 1.52 (1–2.5) |
| Strain | Query Cover % | Similarity % | Gen Bank Accession Number |
|---|---|---|---|
| ITS | |||
| Flavocillium subprimulinum | 98 | 99.3 | MK579178.1 |
| Lecanicillium sp. | 98 | 99.3 | KX496884.1 |
| F. bifurcatum | 96 | 100 | NR_173888.1 |
| F. bifurcatum | 96 | 100 | MN576834.1 |
| F. bifurcatum | 96 | 100 | MN576833.1 |
| TEF1-α | |||
| F. subprimulinum | 98 | 99.3 | MG585321.1 |
| F. subprimulinum | 98 | 99.3 | MG585317.1 |
| F. bifurcatum | 99 | 99.0 | MN576951.1 |
| Lecanicillium sp. | 98 | 99.0 | KM283809.1 |
| Lecanicillium sp. | 98 | 98.8 | KM283824.1 |
| Concentration mg/mL | Mortality Percentage (Mean ± SE) | ||
|---|---|---|---|
| PDB | SPDB | CzDoxB | |
| 0 | 2.41 ± 1.48 a | 2.17 ± 1.69 a | 3.72 ± 2.58 a * |
| 25 | 29.89 ± 6.32 b | 16.07 ± 11.79 c | 58.44 ± 8.10 a |
| 50 | 74.95 ± 9.92 a | 21.01 ± 5.27 b | 77.99 ± 8.46 a |
| 100 | 94.43 ± 2.42 a | 64.00 ± 8.97 b | 95.82 ± 3.50 a |
| Characteristics | Flavocillium bifurcatum (1) | F. primulinum (1) | F. subprimulinum (1,2) | F. subprimulinum (Present Study) |
|---|---|---|---|---|
| Isolation source | Larvae of Noctuidae (Insecta) | Soil | Soil | Agricultural soil |
| Macroconidia characteristics | Cymbiform | Cylindrical to ellipsoidal Single-celled | Obovoidal to ellipsoidal, elongated, straight or slightly curved, non-septate, single, or usually aggregated at the apex of phialides | Cylindrical to ellipsoidal, slightly curved, single or in groups, one for each phialide. Aseptate or occasionally one-septate. |
| Measurements | 5.5–9.2 × 1.3–2.7 μm | 3–9.5 × 1–2.5 μm | 4–15 × 2–6 μm | 7–14 × 1.5–2.6 μm |
| Microconidia characteristics | Ellipsoidal to reniform | Oval to ellipsoidal, aggregated in apical heads. | Oval to ellipsoidal | Oval to ellipsoidal or globose, aggregated at the apex of phialides, 1 or up to 4 in each phialide |
| Measurements | 2.1–4.2 × 0.9–1.5 μm | NA | NA | 3–7 × 1.2–2.4 μm |
| Colony | White to yellowish, cottony with a raised mycelial density at the centrum, generating several concentric rings at the edge, reverse pale yellow to brown | White to yellowish | White, usually raised dome-shaped mycelium, dense with a sunken zone at the centrum, verrucose around the margin. Slow growth in PDA | Slow growth in PDA, fast growth in liquid cultures such as Czapek Dox broth or sweet potato dextrose broth. White, circular, cottony, slightly yellowish reverse |
| Hyphae | Hyaline, septate, branched, smooth-walled, 1–2.3 µm, conidiophore measuring 50–64.2 × 0.9–1.8 µm | Hyaline, septate | Hyaline, branched and septate, from 1–3 µm, conidiophore measuring 19–32 × 1.5–3.5 µm (24 × 2.5 µm). Phialides solitary or up to three at the node | Hyaline, branched, 1–3 µm diameter, conidiophores with one or up to three phialides. |
| Phialides | Lanceolate, single, or in whorls of two to five from 18.1–44.5 µm × 1.1–24 µm in size | Produced on prostrate aerial hyphae, solitary or in whorls of 2–5 that taper toward the apex | Lanceolate, occurring directly from the prostrate hyphae, solitary or two to three phialides, gradually attenuated toward the apex | Lanceolate, arising from aerial hyphae, wide at the base, narrowed at the apex. Measuring 14–45 × 1–2.5 µm |
| Fungus | Target | Activity | Reference |
|---|---|---|---|
| Purpureocillium lilacinum | Meloidogyne spp. Heterodera rostochiensis Apelenchoides spp. Neoplectana spp. | Nematocidal | [35] |
| P. lilacinum Trichodermalongibrachiatum | Meloidogyne sp. Heterodera sp. Radopholus sp. Pratylenchus sp. | Nematocidal | [36] |
| Verticillium leptobactrum | Meloidogyne incognita | Nematocidal | [37] |
| Arthrobotrys dactyloides A. oligospora Dactylella brochophaga | Escherichia coli Staphylococus aureus | Antibacterial | [38] |
| T. harzarium | Meloidogyne spp. | Ovicidal and nematocidal | [39] |
| A. oligospora | Caenorhabditis elegans | Nematocidal | [40] |
| A. musiformis | Gastrointestinal nematodes | Nematocidal | [41] |
| T. hamatum | Acidovorax avenae Alternaria radicina M. incognita eggs | Antibacterial Antifungal Nematocidal | [42] |
| A. musiformis | Haemonchus contortus | Nematocidal | [43] |
| T. asperellum | Meloidogyne incognita | Nematocidal | [44] |
| Fungus | Protein | Molecular Weight (kDa) | Target | Reference |
|---|---|---|---|---|
| Trichoderma pseudokoningii | Serine protease SprT | 31 | Meloidogyne incognita | [49] |
| Verticcillium chlamydosporium | Chymoelastase-like protease VCP1 | 33 | Meloidogyne incognita | [50] |
| V. suchlasporium V. chlamydosporium | Endochitinase CHI43 Serine protease P32 | 43 32 | Globodera pallida | [51] |
| Lecanicillium sp. | Endochitinase Choline dehydrogenase | 37 68 | Meloidogyne javanica eggs | [52] |
| Arthrobotrys conoides | Glucoamylase GAA | 83–87 | NA | [53] |
| Pochonia chlamydosporia and P. rubescens | Serine protease P32 | 32 | NA | [54] |
| P. lilacinum L. psalliotae | Ver 112 PL 646 | 32 | C. elegans | [55] |
| Dactylella shizishanna | Serine protease | 35 | Panagrellus redivivus | [56] |
| Monacrosporium sinense | Proteases Ms1, Ms2 and Ms3 | 37 33 11 | P. redivivus | [57] |
| Hirsutella rhossiliensis | Alcaline protease Hasp | 33 | Heterodera glycines | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Anzúrez, G.; Mendoza-de Gives, P.; Olmedo-Juárez, A.; López-Arellano, M.E.; Bautista-García, G.A.; Ocampo-Gutiérrez, A.Y.; von Son-de Fernex, E.; Alonso-Díaz, M.Á.; Delgado-Núñez, E.J.; Paz-Silva, A. First Record of Flavocillium subprimulinum (Cordycipitaceae, Hypocreales) in Mexico: Morphological and Molecular Characterisation, Nematocidal Activity of Its Liquid Culture Filtrates against Haemonchus contortus and Protease Activity. J. Fungi 2024, 10, 56. https://doi.org/10.3390/jof10010056
Pérez-Anzúrez G, Mendoza-de Gives P, Olmedo-Juárez A, López-Arellano ME, Bautista-García GA, Ocampo-Gutiérrez AY, von Son-de Fernex E, Alonso-Díaz MÁ, Delgado-Núñez EJ, Paz-Silva A. First Record of Flavocillium subprimulinum (Cordycipitaceae, Hypocreales) in Mexico: Morphological and Molecular Characterisation, Nematocidal Activity of Its Liquid Culture Filtrates against Haemonchus contortus and Protease Activity. Journal of Fungi. 2024; 10(1):56. https://doi.org/10.3390/jof10010056
Chicago/Turabian StylePérez-Anzúrez, Gustavo, Pedro Mendoza-de Gives, Agustín Olmedo-Juárez, María Eugenia López-Arellano, Génesis Andrea Bautista-García, Ana Yuridia Ocampo-Gutiérrez, Elke von Son-de Fernex, Miguel Ángel Alonso-Díaz, Edgar Jesús Delgado-Núñez, and Adolfo Paz-Silva. 2024. "First Record of Flavocillium subprimulinum (Cordycipitaceae, Hypocreales) in Mexico: Morphological and Molecular Characterisation, Nematocidal Activity of Its Liquid Culture Filtrates against Haemonchus contortus and Protease Activity" Journal of Fungi 10, no. 1: 56. https://doi.org/10.3390/jof10010056
APA StylePérez-Anzúrez, G., Mendoza-de Gives, P., Olmedo-Juárez, A., López-Arellano, M. E., Bautista-García, G. A., Ocampo-Gutiérrez, A. Y., von Son-de Fernex, E., Alonso-Díaz, M. Á., Delgado-Núñez, E. J., & Paz-Silva, A. (2024). First Record of Flavocillium subprimulinum (Cordycipitaceae, Hypocreales) in Mexico: Morphological and Molecular Characterisation, Nematocidal Activity of Its Liquid Culture Filtrates against Haemonchus contortus and Protease Activity. Journal of Fungi, 10(1), 56. https://doi.org/10.3390/jof10010056