Why Are There So Few Basidiomycota and Basal Fungi as Endophytes? A Review
Abstract
1. Introduction
1.1. Diversity of Fungi
1.2. What Are Endophytes?
1.3. Mutualistic Nature of Plants and Endophytes
1.4. Objectives of This Review
- (1)
- To determine the percentage occurrence of Basidiomycota and basal fungi as endophytes as documented in selected published papers and to compare this with that of endophytic Ascomycota.
- (2)
- To compare the diversity of fungi using culture-dependent (CD) and culture-independent (CID) methods based on a literature search.
- (3)
- To discuss factors affecting the diversity and occurrence of endophytic Basidiomycota and basal fungi.
- (4)
- To recommend procedures and methods to detect a wider range of endophytic Basidiomycota and basal fungi.
2. Diversity of Endophytes
2.1. Ascomycetous Endophytes
| Host | Plant Parts | No. of Ascomycetes | Total Number of Isolates | % Occurrence | Reference |
|---|---|---|---|---|---|
| Colobanthus quitensis | Leaves of angiosperm | 6 | 26 | 23% | [29] |
| Pterocladiella capillacea | Red alga | 2600 | 3187 | 81.58% | [43] |
| Magnolia candolli & M. garrettii | Leaves | 54 | 56 | 96.5% | [30] |
| Chloranthus japonicus | Leaves, roots, and stem | 317 | 325 | 97.5% | [31] |
| Zostera marina | Leaf of seagrass | 103 | 110 | 93.6% | [32] |
| Phragmites australis, Suaeda glauca & Limonium tetragonum | Roots | 153 | 156 | 98% | [33] |
| Nothofagus pumilio & N. dombeyi | Sapwood tissue | ND | 210 | 46% | [27] |
| Anacamptis morio | Roots of orchids | [44] | |||
| Myrtus communis | Leaves of true myrtle | 7 OTUs | 44 OTUs | 16% | [45] |
| Stipa krylovii | Roots | 110 | 135 | 81.5% | [46] |
| Nicotiana benthamiana, N. occidentalis & N. simulans | Leaves, stems, and roots | ND | 300 | 97.9% | [34] |
| 63 Species of native plants | Stems and leaves | 341 | 349 | 97.7% | [35] |
| Sophora tonkinensis | Phloem and xylem of roots of medicinal plant | 36 | 47 | 76.6% | [47] |
| Elaeis guineensis | Leaves, petioles, rachis, and roots | ND | 376 | ND | [48] |
| Vitis vinifera | Leaves | 239 | 240 | 99.6% | [49] |
| Hevea brasiliensis & H. guianensis | Sapwood and leaves of rubber tree | ND | 2500 | ND | [50] |
| Nothapodytes nimmoniana | Stem | 44 | 45 | 98% | [36] |
| Solanum cernuum | Leaves and stems | 33 | 55 | 60% | [38] |
| Populus tremula | Leaves of European aspen | 93 | 96 | 97% | [37] |
| Holcus lanatus | Leaves and roots | 337 | 348 | 97% | [51] |
| Elaeis guineensis | Petioles, rachides, vein, and intervein of leaves | 320 | 340 | 94.1% | [3] |
| Pinus sylvestris | Sapwood tissue | 53 | 143 | 37% | [28] |
| Theobroma gileri | Stem and pod tissues | 16 | 31 | 52% | [52] |
2.2. Basidiomycetous Endophytes
| Host | Plant Parts | Genus/Species | No. of Basidiomycetes | Total Number of Isolates | % Occurrence | Reference |
|---|---|---|---|---|---|---|
| Colobanthus quitensis | Leaves of angiosperm | Lenzites sp. Leucosporidium sp. Peniophora sp. Phlebia sp. Sistotrema sp. Trametes sp. | 20 | 26 | 77% | [29] |
| Halophila stipulacea | Decaying leaves of seagrass | Antrodiopsis sp. Malassezia sp. | ND | 296 OTUs | 37.2–51.6% | [40] |
| Pterocladiella capillacea | Red alga | Apiotrichum laibachii Bjerkandera adusta Cerrena sp. Chondrostereum sp. Grammothele fuligo Pseudozyma hubeiensis Rhodosporidium fluviale Rhodotorula mucilaginosa Tritirachium oryzae | 585 | 3187 | 18.36% | [43] |
| Magnolia candolli & M. garrettii | Leaves | Coprinellus magnolia Phanerina mellea | 2 | 56 | 3.5% | [30] |
| Chloranthus japonicus | Leaves, roots, and stems | Ceriporia sp. Thanatephorus sp. | 7 | 325 | 2% | [31] |
| Zostera marina | Leaf of seagrass | Naganishia sp. Pseudozyma sp. Rhodotorula sp. | 4 | 110 | 3.6% | [32] |
| Phragmites australis, Suaeda glauca & Limonium tetragonum | Roots | Meira sp. Pseudozyma sp. | 3 | 156 | 1.9% | [33] |
| Nothofagus pumilio & N. dombeyi | Sapwood tissue | Armillaria sparrei Aurantiporus albidus Coprinellus sp. Fistulina antarctica, Hypholoma frowardii Laetiporus portentosus Obba valdiviana Pholiota baeosperma Postia pelliculosa Pseudoinonotus crustosus Sistotrema brinkmanni | ND | 210 | 43% | [27] |
| Anacamptis morio | Roots of orchids | Ceratobasidium sp. Tulasnella sp. | 7 | 37 | 19% | [44] |
| Myrtus communis | Leaves of true myrtle | Aurantiporus sp. Botryobasidium sp. Calocera sp. Ceratobasidium sp. Dacrymyces sp. Filobasidium sp. Flagelloscypha sp. Ganoderma sp. Gloeoporus sp. Gymnopilus sp. Hyphoderma sp. Hyphodontia sp. Hymenochaete sp. Hymenochaetaceae sp. Hymenochaetales sp. Malassezia sp. Naganishia sp. Phragmidium sp. Physisporinus sp. Polyporaceae sp. Pterulaceae sp. Pycnoporus sp. Rhodotorula sp. Sporobolomyces sp. Sympodiomycopsis sp. Thelephorales sp. Trametes sp. Tricholomataceae sp. Tyromyces sp. | 37 | 44 | 84% | [45] |
| Stipa krylovii | Roots | Hymenochaete sp. Tricholomataceae sp. Unknown fungi | 25 | 135 | 18.5% | [46] |
| Nicotiana benthamiana, N. occidentalis & N. simulans | Leaves, stems, and roots | ND | ND | 300 | 2.1% | [34] |
| 63 Species of native plants | Stems and leaves | Coprinopsis episcopalis Coprinus cinereus Cryptococcus sp. Filobasidium chernovii Ustilago sp. | 8 | 8 | 2.3% | [35] |
| Sophora tonkinensis | Phloem and xylem of roots of medicinal plant | Fomitopsis sp. Exobasidiomycetidae sp. Schizophyllum commune Trichosporon asahii | 4 | 47 | 8.5% | [47] |
| Elaeis guineensis | Leaves, petioles, rachis, and roots | Ganoderma orbiforme Neonothopanus nambi Schizophyllum commune | 10 | 376 | 2.7% | [48] |
| Vitis vinifera | Leaves | Athelia sp. | 1 | 240 | 0.4% | [49] |
| Hevea brasiliensis & H. guianensis | Sapwood and leaves of rubber tree | Bjerkandera sp. Ceriporia sp. Coprinellus sp. Peniophora sp. Phanerochaete sp. Phlebia sp. Rigidoporus sp. Stereum sp. Tinctoporellus sp. Trametes sp. | 310 | 2500 | 12.4% | [50] |
| Nothapodytes nimmoniana | Stem | Irpex lacteus | 1 | 45 | 2% | [36] |
| Solanum cernuum | Leaves and stems | Basidiomycota sp. Coprinellus radians Coprinaceae sp. Flavodon sp. Hohenbuehelia sp. Kwoniella mangroviensis Meruliaceae sp. Oudemansiella sp. Oudemansiella canarii Peniophora sp. Phanerochaete sordida Phanerochaete subserialis Phlebiopsis sp. Polyporales sp. Schizophyllum umbrinum | 21 | 55 | 38% | [38] |
| Populus tremula | Leaves of European aspen | Agaricomycetes sp. Sporidiobolaceae sp. | 3 | 96 | 3% | [37] |
| Holcus lanatus | Leaves and roots | Agrocybe pediades Ceratobasidium sp. Coprinellus disseminates Coprinus micaceus Cryptococcus podzolicus Rhodotorula slooffiae | 11 | 348 | 3% | [51] |
| Elaeis guineensis | Petioles, rachides, vein, and intervein of leaves | Fomitopsis meliae Fomitopsis ostreiformis Fomitopsis pinicola Perenniporia sp. Pycnoporus sanguineus Schizophyllum commune Trametes lactinea | 20 | 340 | 5.9% | [3] |
| Pinus sylvestris | Sapwood tissue | Bjerkandera adusta Heterobasidion annosum Peniophora sp. Schizophyllum commune Sistotrema coroniferum Thanatephorus cucumeris Trametes versicolor | 17 | 143 | 12% | [28] |
| Theobroma gileri | Stem and pod tissues | Coprinellus sp. Ganoderma sp. Lacnocladiaceae sp. Lentinus sp. Melanotus sp. Meripilus sp. Piptoporus sp. Polyporaceae sp. Pycnoporus sp. Schizophyllum sp. Scopuloides sp. Wrightoporia sp. | 15 | 31 | 48% | [52] |
2.3. Basal Fungi as Endophytes
| Host | Plant Parts | Phylum | Genus/Species | No. of Isolates | Total Number of Isolates | % Occurrence | Reference |
|---|---|---|---|---|---|---|---|
| Opuntiaficus-indica | Cladodes of cactus | Mucoromycota | Rhizopus oryzae | 1 | ND | ND | [63] |
| Ziziphus spina | Leaves | Mucoromycota | Mucor sp. Rhizopus sp. | 6 | 26 | 23% | [65] |
| Halophila stipulacea | Decaying leaves of seagrass | Mucoromycota | Mucor sp. | ND | ND | 12.56% | [40] |
| Chytridiomycota | Entophlyctis sp. Geranomyces sp. | 5.42% | |||||
| Mortierellomycota | Mortierella sp. | 11.58% | |||||
| Neocallimastigomycota | Neocallimastix sp. Anaeromyces sp. | 13.31 | |||||
| Entomophthoromycota | Unknown fungi | ND | |||||
| Kickxellomycota | Unknown fungi | ND | |||||
| Ecklonia radiata | Kelp | Mucoromycota | Mucor circinelloides | 2 | 11 | 18% | [58] |
| Pterocladiella capillacea | Leave of red alga | Mucoromycota | Mucor irregularis | 2 | 3187 | 0.06% | [43] |
| Zostera marina | Leave of seagrass | Mucoromycota | Absidia cylindrospora | 1 | 120 | 0.9% | [32] |
| Nothofagus pumilio & N. dombeyi | Sapwood | Mucoromycota | Umbelopsis vinacea U. changbaiensis U. ramanniana U. nana/dimorpha | 10 | 88 | 11% | [27] |
| Astragalus membranaceus | Roots | Mucoromycota | Rhizopus sp. | 1 | ND | - | [64] |
| Anacamptis morio | Roots | Mortierellomycota | Mortierella sp. | 1 | 37 | 3% | [44] |
| Hedychium spicatum | Rhizome and leaves | Mucoromycota | Absidia sp. Mucor hiemalis | 2 | 28 | 7% | [61] |
| Sophora tonkinensis | Phloem and xylem of roots of a medicinal plant | Mortierellomycota | Mortierella alpina | 1 | 42 | 2% | [47] |
| Mucoromycota | Mucor circinelloides | 1 | 2% | ||||
| Stellera chamaejasme | Leaves, stems, and roots of a medicinal plant | Mortierellomycota | Mortierella spp. | 12 | 145 | 8% | [60] |
| Mucoromycota | Mucor sp. Rhizopus sp. | 2 | 1.4 | ||||
| Arabidopsis thaliana & Microthlaspi perfoliatum | Roots | Mucoromycota | Absidia cylindrospora | 1 | 100 | 1% | [59] |
| Mortierellomycota | Mortierellales sp. | 1 | 1% | ||||
| Stellera chamaejasme | Leaves, stems, and roots of a medicinal plant | Mucoromycota | Mucor racemosus M. hiemalis M. circinelloides | 34 | 714 | 4.8% | [62] |
| Isoetes echinospora Isoetes lacustris Littorella uniflora Lobelia dortmanna Subularia aquatica | Roots of submerged aquatic plants | Mucoromycota | OUT28 | 2 | 234 OTUs | 0.08% | [57] |
| Chytridiomycota | OTU34, 35 & 36 OTU29, 30 & 27 | 6 | 2.6% | ||||
| Solanum cernuum | Leaves and stems | Mucoromycota | Mucor sp. | 1 | 55 | 1.8% | [38] |
| Pinus sylvestris | Sapwood tissue | Mucoromycota | Mucor hiemalis Mucor plumbeus Rhizopus stolonifer Umbelopsis isabellina Umbelopsis vinacea | 8 | 143 | 6% | [28] |
| Mortierellomycota | Mortierella globalpina Mortierella lignicola | ||||||
| Holcus lanatus | Leaves | Mortierellomycota | Mortierella sp. | 1 | 214 | 0.5% | [51] |
2.4. Frequency of Endophytic Species of Basidiomycota and Basal Fungi
2.4.1. Basidiomycota
2.4.2. The Basal Fungi
3. Comparison of Culture-Dependent (CD) and Culture-Independent (CID) Methods
| Source | Culture-Dependent Method | Culture-Independent Method | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Isolates | Phyla | Name | No. of Genera (Isolate) | % Occurrence | No. of OTUs | Phyla | Name | % of Occurrence | ||
| Endophyte of aquatic plants | 1689 | 3 | Ascomycota | 123 (1584) | 93.8% | 1074 | 6 | Ascomycota | 43.48% | [68] |
| Basidiomycota | 29 (92) | 5.4% | Basidiomycota | 15.36% | ||||||
| Zygomycota | 2 (13) | 0.8% | Zygomycota | 1.49% | ||||||
| Chytridiomycota | 1.21% | |||||||||
| Glomeromycota | 0.02% | |||||||||
| Rozellomycota | 0.01% | |||||||||
| Unknown fungi | 38.17% | |||||||||
| Endophytes of Elymus repens | 66 | 1 | Ascomycota | 9 (27) | 40.9% | 48 | 4 | Ascomycota | 90% | [69] |
| Unidentified fungi | Unknown (39) | 59.1% | Basidiomycota | 2% | ||||||
| Glomeromycota | 2% | |||||||||
| Mortierellomycota | 2% | |||||||||
| Unknown fungi | 4% | |||||||||
| Endophytes of Vitis vinifera | 94 | 1 | Ascomycota | 19 (94) | 100% | 59 | 3 | Ascomycota | 93.6% | [70] |
| Basidiomycota | 4.2% | |||||||||
| Zygomycota | 2.1% | |||||||||
| Endophytes of Acanthus ilicifolius | 203 | 2 | Ascomycota | 30 (200) | 97.04% | 111 | 2 | Ascomycota | 65.09% | [14] |
| Basidiomycota | 2 (3) | 2.96% | Basidiomycota | 38.87% | ||||||
| Unknown fungal taxa | 4.05% | |||||||||
| Deep-sea sediment | 19 | 2 | Ascomycota | 11 (14) | 73.7% | 42 | 2 | Ascomycota | 59.5% | [71] |
| Basidiomycota | 2 (5) | 26.3 | Basidiomycota | 40.5% | ||||||
4. Factors Affecting the Occurrence of Basidiomycota and Basal Fungal Endophytes
4.1. Isolation Procedure
4.2. Isolation Media
4.3. Period of Incubation
4.4. Endophytic Yeasts—The Forgotten Bioresource
4.5. Identification
4.6. Fungus–Host Interaction
5. General Discussion
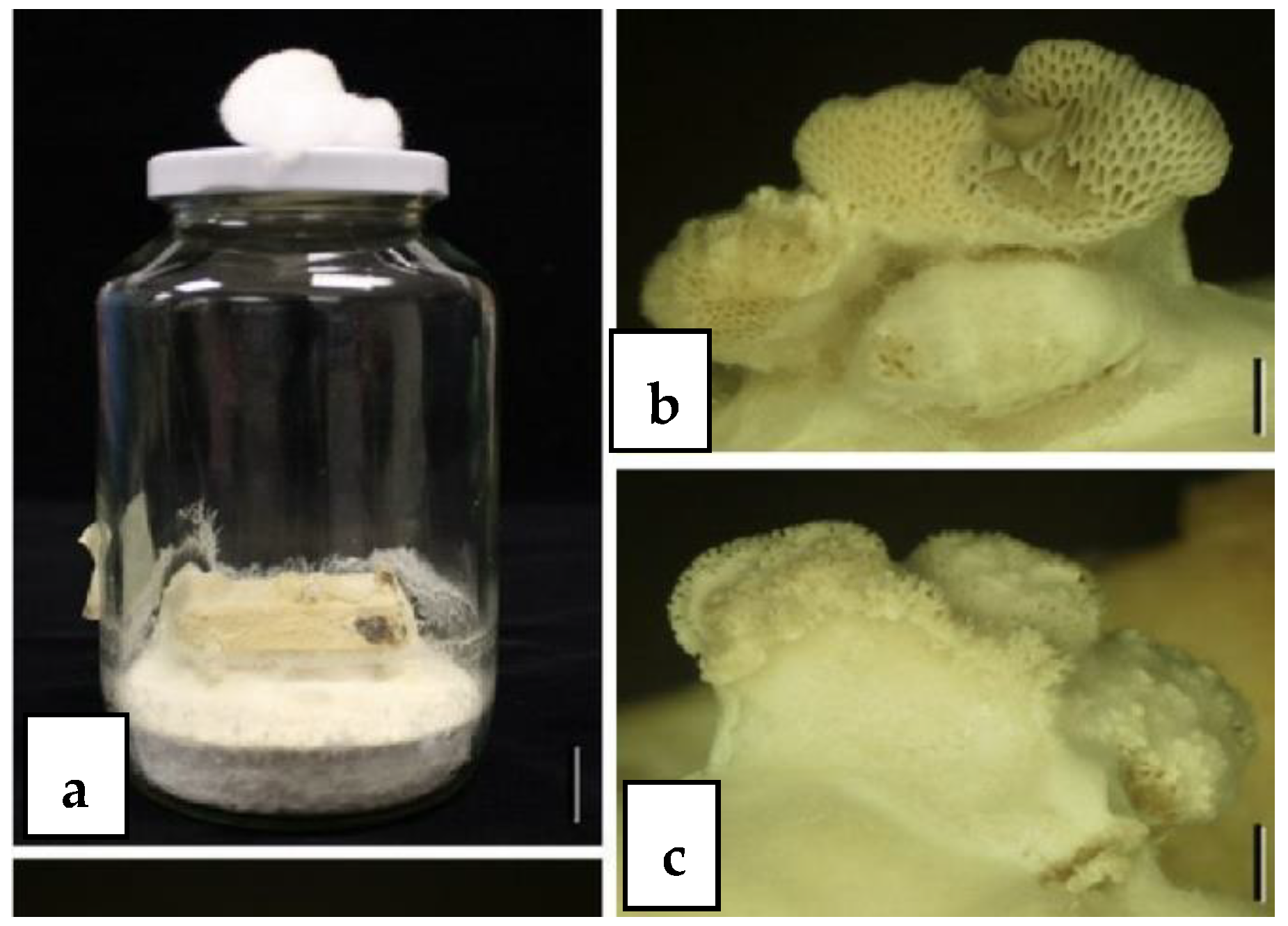
5.1. Basidiomycota as Hidden Endophytes
5.2. The Low Occurrence of Chytridiomycota as Endophytes
5.3. Other Basal Fungi as Endophytes
5.3.1. The Mucoromycota
5.3.2. Mortierellomycota
5.4. Role of Endophytes in the Senescence of Host Plants
5.5. Next-Generation Study
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu-Xu, L.; Vicedo, B.; García-Agustín, P.; Llorens, E. Advances in endophytic fungi research: A data analysis of 25 years of achievements and challenges. J. Plant Interact. 2022, 17, 244–266. [Google Scholar] [CrossRef]
- Adhikari, P.; Joshi, K.; Pandey, A. Taxus associated fungal endophytes: Anticancerous to other biological activities. Fungal Biol. Rev. 2023, 45, 100308. [Google Scholar] [CrossRef]
- Pinruan, U.; Rungjindamai, N.; Choeyklin, R.; Lumyong, S.; Hyde, K.D.; Jones, E.B.G. Occurrence and diversity of basidiomycetous endophytes from the oil palm, Elaeis guineensis in Thailand. Fungal Divers. 2010, 41, 71–88. [Google Scholar] [CrossRef]
- Rungjindamai, N.; Pinruan, U.; Choeyklin, R.; Hattori, T.; Jones, E.B.G. Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis, in Thailand. Fungal Divers. 2008, 33, 139–161. [Google Scholar]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, 163–173. [Google Scholar]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Hernández Robles, D.R.; Plata Corredor, C.A.; Stjernegaard Jeppesen, T.; Örn, A.; Vandepitte, L.; Hobern, D.; et al. Catalogue of Life Checklist (Version 2023-08-17). Catalogue of Life. Available online: https://www.catalogueoflife.org (accessed on 1 August 2023).
- Kirk, P. Species Fungorum Plus. Available online: http://www.speciesfungorum.org (accessed on 1 August 2023).
- Kumar, A.; Radhakrishnan, E. Microbial Endophytes: Functional Biology and Applications; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Sun, X.; Guo, L.-D. Endophytic fungal diversity: Review of traditional and molecular techniques. Mycology 2012, 3, 65–76. [Google Scholar]
- Sakayaroj, J.; Preedanon, S.; Supaphon, O.; Jones, E.B.G.; Phongpaichit, S. Phylogenetic diversity of endophyte assemblages associated with the tropical seagrass Enhalus acoroides in Thailand. Fungal Divers. 2010, 42, 27–45. [Google Scholar] [CrossRef]
- Arnold, A.E.; Lutzoni, F. Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef]
- Chi, W.C.; Chen, W.; He, C.C.; Guo, S.Y.; Cha, H.J.; Tsang, L.M.; Ho, T.W.; Pang, K.L. A highly diverse fungal community associated with leaves of the mangrove plant Acanthus ilicifolius var. xiamenensis revealed by isolation and metabarcoding analyses. PeerJ 2019, 7, e7293. [Google Scholar] [CrossRef] [PubMed]
- de Bary, A. Morphologie und Physiologie der Pilze, Flechten und Myxomyceten; Engelmann: Leipzig, Germany, 1866; Volume 1. [Google Scholar]
- Petrini, O. Fungal Endophytes of Tree Leaves; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar]
- Gakuubi, M.M.; Munusamy, M.; Liang, Z.-X.; Ng, S.B. Fungal endophytes: A promising frontier for discovery of novel bioactive compounds. J. Fungi 2021, 7, 786. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Alam, B.; Lǐ, J.; Gě, Q.; Khan, M.A.; Gōng, J.; Mehmood, S.; Yuán, Y.; Gǒng, W. Endophytic fungi: From symbiosis to secondary metabolite communications or vice versa? Front. Plant Sci. 2021, 12, 791033. [Google Scholar] [CrossRef] [PubMed]
- Bhunjun, C.S.; Phukhamsakda, C.; Hyde, K.D.; McKenzie, E.H.C.; Saxena, R.K.; Li, Q. Do all fungi have ancestors with endophytic lifestyles? Fungal Divers. 2023. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.J.; Caradus, J.R.; Johnson, L.J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 2016, 92, fiw194. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J. Characterization of Dark Septate Endophytic Fungi and Improve the Performance of Liquorice under Organic Residue Treatment. Front. Microbiol. 2019, 10, 1364. [Google Scholar] [CrossRef]
- Card, S.; Johnson, L.; Teasdale, S.; Caradus, J. Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiol. Ecol. 2016, 92, fiw114. [Google Scholar] [CrossRef]
- Domka, A.M.; Rozpaądek, P.; Turnau, K. Are fungal endophytes merely mycorrhizal copycats? The role of fungal endophytes in the adaptation of plants to metal toxicity. Front. Microbiol. 2019, 10, 371. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Niskanen, T.; Suwannarach, N.; Wannathes, N.; Chen, Y.J.; McKenzie, E.H.C.; Maharachchikumbura, S.S.N.; Buyck, B.; Zhao, C.L.; Fan, Y.G.; et al. The numbers of fungi: Are the most speciose genera truly diverse? Fungal Divers. 2022, 114, 387–462. [Google Scholar] [CrossRef]
- Molina, L.; Rajchenberg, M.; de Errasti, A.; Aime, M.C.; Pildain, M.B. Sapwood-inhabiting mycobiota and Nothofagus tree mortality in Patagonia: Diversity patterns according to tree species, plant compartment and health condition. For. Ecol. Manag. 2020, 462, 117997. [Google Scholar] [CrossRef]
- Giordano, L.; Gonthier, P.; Varese, G.C.; Miserere, L.; Nicolotti, G. Mycobiota inhabiting sapwood of healthy and declining Scots pine (Pinus sylvestris L.) trees in the Alps. Fungal Divers. 2009, 38, 69–83. [Google Scholar]
- Bertini, L.; Perazzolli, M.; Proietti, S.; Capaldi, G.; Savatin, D.V.; Bigini, V.; Longa, C.M.O.; Basaglia, M.; Favaro, L.; Casella, S.; et al. Biodiversity and bioprospecting of fungal endophytes from the Antarctic plant Colobanthus quitensis. J. Fungi 2022, 8, 979. [Google Scholar] [CrossRef]
- de Silva, N.I.; Maharachchikumbura, S.S.N.; Thambugala, K.M.; Bhat, D.J.; Karunarathna, S.C.; Tennakoon, D.S.; Phookamsak, R.; Jayawardena, R.S.; Lumyong, S.; Hyde, K.D. Morpho-molecular taxonomic studies reveal a high number of endophytic fungi from Magnolia candolli and M. garrettii in China and Thailand. Mycosphere 2021, 12, 163–237. [Google Scholar] [CrossRef]
- An, C.; Ma, S.; Shi, X.; Xue, W.; Liu, C.; Ding, H. Diversity and Antimicrobial Activity of Endophytic Fungi Isolated from Chloranthus japonicus Sieb in Qinling Mountains, China. Int. J. Mol. Sci. 2020, 21, 5958. [Google Scholar] [CrossRef]
- Ettinger, C.L.; Eisen, J.A. Fungi, bacteria and oomycota opportunistically isolated from the seagrass, Zostera marina. PLoS ONE 2020, 15, e0236135. [Google Scholar] [CrossRef]
- Khalmuratova, I.; Choi, D.H.; Woo, J.R.; Jeong, M.J.; Oh, Y.; Kim, Y.G.; Lee, I.J.; Choo, Y.S.; Kim, J.G. Diversity and plant growth-promoting effects of fungal endophytes isolated from salt-tolerant plants. J. Microbiol. Biotechnol. 2020, 30, 1680–1687. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Li, H.; Sivasithamparam, K.; Jones, M.G.K.; Wylie, S.J. Host specificity of endophytic mycobiota of wild Nicotiana plants from arid regions of Northern Australia. Microb. Ecol. 2018, 75, 74–87. [Google Scholar] [CrossRef]
- González-Menéndez, V.; Crespo, G.; De Pedro, N.; Diaz, C.; Martín, J.; Serrano, R.; Mackenzie, T.A.; Justicia, C.; González-Tejero, M.R.; Casares, M.; et al. Fungal endophytes from arid areas of Andalusia: High potential sources for antifungal and antitumoral agents. Sci. Rep. 2018, 8, 9729. [Google Scholar] [CrossRef]
- Shweta, S.; Gurumurthy, B.R.; Vasanthakumari, M.M.; Ravikanth, G.; Dayanandan, S.; Storms, R.; Shivanna, M.B.; Uma Shaanker, R. Endophyte fungal diversity in Nothapodytes nimmoniana along its distributional gradient in the Western Ghats, India: Are camptothecine (anticancer alkaloid) producing endophytes restricted to specific clades? Curr. Sci. 2015, 109, 127–138. [Google Scholar]
- Albrectsen, B.R.; Björkén, L.; Varad, A.; Hagner, Å.; Wedin, M.; Karlsson, J.; Jansson, S. Endophytic fungi in European aspen (Populus tremula) leaves-Diversity, detection, and a suggested correlation with herbivory resistance. Fungal Divers. 2010, 41, 17–28. [Google Scholar] [CrossRef]
- Vieira, M.L.A.; Hughes, A.F.S.; Gil, V.B.; Vaz, A.B.M.; Alves, T.M.A.; Zani, C.L.; Rosa, C.A.; Rosa, L.H. Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional brazilian medicinal plant solanum cernuum vell. (Solanaceae). Can. J. Microbiol. 2012, 58, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E. Understanding the diversity of foliar endophytic fungi: Progress, challenges, and frontiers. Fungal Biol. Rev. 2007, 21, 51–66. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.A.; Bahkali, A.H.; Elgorban, A.M.; Jones, E.B.G. High-throughput amplicon sequencing of fungi and microbial eukaryotes associated with the seagrass Halophila stipulacea (Forssk.) Asch. from Al-Leith mangroves, Saudi Arabia. Mycol. Prog. 2021, 20, 1365–1381. [Google Scholar] [CrossRef]
- He, D.; Wu, F.; Ma, W.; Zhang, Y.; Gu, J.-D.; Duan, Y.; Xu, R.; Feng, H.; Wang, W.; Li, S.-W. Insights into the bacterial and fungal communities and microbiome that causes a microbe outbreak on ancient wall paintings in the Maijishan Grottoes. Int. Biodeterior. Biodegrad. 2021, 163, 105250. [Google Scholar] [CrossRef]
- Menkis, A.; Redr, D.; Bengtsson, V.; Hedin, J.; Niklasson, M.; Nordén, B.; Dahlberg, A. Endophytes dominate fungal communities in six-year-old veteranisation wounds in living oak trunks. Fungal Ecol. 2022, 59, 101020. [Google Scholar] [CrossRef]
- Cha, H.-J.; Chiang, M.W.L.; Guo, S.-Y.; Lin, S.-M.; Pang, K.-L. Culturable fungal community of Pterocladiella capillacea in Keelung, Taiwan: Effects of surface sterilization method and isolation medium. J. Fungi 2021, 7, 651. [Google Scholar] [CrossRef]
- Herrera-Rus, I.; Pastor, J.E.; Juan, R. Fungal colonization associated with phenological stages of a photosynthetic terrestrial temperate orchid from the Southern Iberian Peninsula. J. Plant Res. 2020, 133, 807–825. [Google Scholar] [CrossRef]
- Vaz, A.B.M.; Fonseca, P.L.C.; Silva, F.F.; Quintanilha-Peixoto, G.; Sampedro, I.; Siles, J.A.; Carmo, A.; Kato, R.B.; Azevedo, V.; Badotti, F.; et al. Foliar mycoendophytome of an endemic plant of the Mediterranean biome (Myrtus communis) reveals the dominance of basidiomycete woody saprotrophs. PeerJ 2020, 8, e10487. [Google Scholar] [CrossRef]
- Knapp, D.G.; Imrefi, I.; Boldpurev, E.; Csíkos, S.; Akhmetova, G.; Berek-Nagy, P.J.; Otgonsuren, B.; Kovács, G.M. Root-colonizing endophytic fungi of the dominant grass Stipa krylovii from a Mongolian steppe grassland. Front. Microbiol. 2019, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Q.; Lan, F.; Qiao, Y.M.; Wei, J.G.; Huang, R.S.; Li, L.B. Endophytic fungi harbored in the root of Sophora tonkinensis Gapnep: Diversity and biocontrol potential against phytopathogens. MicrobiologyOpen 2017, 6, e00437. [Google Scholar] [CrossRef] [PubMed]
- Naidu, Y.; Idris, A.S.; Madihah, A.Z.; Kamarudin, N. In vitro Antagonistic Interactions Between Endophytic Basidiomycetes of Oil Palm (Elaeis guineensis) and Ganoderma boninense. J. Phytopathol. 2016, 164, 779–790. [Google Scholar] [CrossRef]
- Varanda, C.M.R.; Oliveira, M.; Materatski, P.; Landum, M.; Clara, M.I.E.; Félix, M.D.R. Fungal endophytic communities associated to the phyllosphere of grapevine cultivars under different types of management. Fungal Biol. 2016, 120, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Gazis, R.; Skaltsas, D.; Chaverri, P.; Hibbett, D. Unexpected diversity of basidiomycetous endophytes in sapwood and leaves of Hevea. Mycologia 2015, 107, 284–297. [Google Scholar] [CrossRef]
- Márquez, S.S.; Bills, G.F.; Acuña, L.D.; Zabalgogeazcoa, I. Endophytic mycobiota of leaves and roots of the grass holcus lanatus. Fungal Divers. 2010, 41, 115–123. [Google Scholar] [CrossRef]
- Thomas, S.E.; Crozier, J.; Catherine Aime, M.; Evans, H.C.; Holmes, K.A. Molecular characterisation of fungal endophytic morphospecies associated with the indigenous forest tree, Theobroma gileri, in Ecuador. Mycol. Res. 2008, 112, 852–860. [Google Scholar] [CrossRef]
- He, M.-Q.; Zhao, R.-L.; Liu, D.-M.; Denchev, T.T.; Begerow, D.; Yurkov, A.; Kemler, M.; Millanes, A.M.; Wedin, M.; McTaggart, A.R.; et al. Species diversity of Basidiomycota. Fungal Divers. 2022, 114, 281–325. [Google Scholar] [CrossRef]
- Rytioja, J.; Hildén, K.; Yuzon, J.; Hatakka, A.; de Vries, R.P.; Mäkelä, M.R. Plant-polysaccharide-degrading enzymes from Basidiomycetes. Microbiol. Mol. Biol. Rev. 2014, 78, 614–649. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Suetrong, S.; Sakayaroj, J.; Bahkali, A.H.; Abdel-Wahab, M.A.; Boekhout, T.; Pang, K.-L. Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers. 2015, 73, 1–72. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Oshita, Y.; Yasuda, M.; Kanasugi, M.; Matsuura, E.; Xu, Q.; Okazaki, S. Host specificity of endophytic fungi from stem tissue of nature farming tomato (Solanum lycopersicum Mill.) in Japan. Agronomy 2020, 10, 1019. [Google Scholar] [CrossRef]
- Kohout, P.; Sykorova, Z.; Ctvrtlikova, M.; Rydlova, J.; Suda, J.; Vohnik, M.; Sudova, R. Surprising spectra of root-associated fungi in submerged aquatic plants. FEMS Microbiol. Ecol. 2012, 80, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.K.; Rose, A.L.; Grossart, H.P.; Rojas-Jimenez, K.; Barroso Prescott, S.K.; Oakes, J.M. Oxic and anoxic organic polymer degradation potential of endophytic fungi from the marine macroalga, Ecklonia radiata. Front. Microbiol. 2021, 12, 726138. [Google Scholar] [CrossRef] [PubMed]
- Keim, J.; Mishra, B.; Sharma, R.; Ploch, S.; Thines, M. Root-associated fungi of Arabidopsis thaliana and Microthlaspi perfoliatum. Fungal Divers. 2014, 66, 99–111. [Google Scholar] [CrossRef]
- Jin, H.; Yang, X.; Lu, D.; Li, C.; Yan, Z.; Li, X.; Zeng, L.; Qin, B. Phylogenic diversity and tissue specificity of fungal endophytes associated with the pharmaceutical plant, Stellera chamaejasme L. revealed by a cultivation-independent approach. Antonie Van Leeuwenhoek 2015, 108, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.; Dkhar, M.S.; Kayang, H.; Kumar, M.; Dubey, N.K.; Raghuwanshi, R. Diversity of endophytic fungi associated with Hedychium spicatum ham ex sm. And their antifungal activity against the phytopathogen Alternaria solani. Stud. Fungi 2020, 5, 84–93. [Google Scholar] [CrossRef]
- Jin, H.; Yan, Z.; Liu, Q.; Yang, X.; Chen, J.; Qin, B. Diversity and dynamics of fungal endophytes in leaves, stems and roots of Stellera chamaejasme L. in northwestern China. Antonie Van Leeuwenhoek 2013, 104, 949–963. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Ali, S.S.; Nouh, H.S. Exploring the potential of Rhizopus oryzae AUMC14899 as a novel endophytic fungus for the production of l-tyrosine and its biomedical applications. Microb. Cell Factories 2023, 22, 31. [Google Scholar] [CrossRef]
- Zhang, H.; An, Z.; Zhou, F. Secondary metabolites from Rhizopus sp., an endophytic fungus in Astragalus membranaceus and preliminary evaluation of inhibition of plant pathogen activity. Chem. Nat. Compd. 2020, 56, 366–369. [Google Scholar] [CrossRef]
- Hawar, S.N. Extracellular enzyme of endophytic fungi isolated from Ziziphus spina leaves as medicinal plant. Int. J. Biomater. 2022, 2022, 2135927. [Google Scholar] [CrossRef]
- Boekhout, T.; Guého-Kellermann, E.; Mayser, P.; Velegraki, A. Malassezia and the Skin: Science and Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Zheng, H.; Qiao, M.; Xu, J.; Yu, Z. Culture-based and culture-independent assessments of endophytic fungal diversity in aquatic plants in Southwest China. Front. Fungal Biol. 2021, 2, 692549. [Google Scholar] [CrossRef] [PubMed]
- Høyer, A.K.; Hodkinson, T.R. Hidden fungi: Combining culture-dependent and -independent DNA barcoding reveals inter-plant variation in species richness of endophytic root fungi in Elymus repens. J. Fungi 2021, 7, 466. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Purahong, W.; Wubet, T.; Hyde, K.D.; Zhang, W.; Xu, H.; Zhang, G.; Fu, C.; Liu, M.; Xing, Q.; et al. Direct comparison of culture-dependent and culture-independent molecular approaches reveal the diversity of fungal endophytic communities in stems of grapevine (Vitis vinifera). Fungal Divers. 2018, 90, 85–107. [Google Scholar] [CrossRef]
- Singh, P.; Raghukumar, C.; Meena, R.M.; Verma, P.; Shouche, Y. Fungal diversity in deep-sea sediments revealed by culture-dependent and culture-independent approaches. Fungal Ecol. 2012, 5, 543–553. [Google Scholar] [CrossRef]
- dos Reis, J.B.A.; Lorenzi, A.S.; do Vale, H.M.M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022, 204, 675. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Van Staden, J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020, 39, 107462. [Google Scholar] [CrossRef]
- Kandasamy, G.D.; Kathirvel, P. Insights into bacterial endophytic diversity and isolation with a focus on their potential applications–A review. Microbiol. Res. 2023, 266, 127256. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Z.; Xie, H.; Feng, X.; Wang, Y.; Xu, P. Overhauling the effect of surface sterilization on analysis of endophytes in tea plants. Front. Plant Sci. 2022, 13, 849658. [Google Scholar] [CrossRef]
- Sahu, P.; Tilgam, J.; Mishra, S.; Baba, S.; Gupta, A.; Krishnappa, J.; Verma, S.K.; Kharwar, R. Surface sterilization for isolation of endophytes: Ensuring what (not) to grow. J. Basic Microbiol. 2022, 62, 647–668. [Google Scholar] [CrossRef]
- Samson, R.; Houbraken, J.; Thrane, U.; Frisvad, J.; Andersen, B. Food and Indoor Fungi; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010. [Google Scholar]
- Ibrahim, M.; Oyebanji, E.; Fowora, M.; Aiyeolemi, A.; Orabuchi, C.; Akinnawo, B.; Adekunle, A.A. Extracts of endophytic fungi from leaves of selected Nigerian ethnomedicinal plants exhibited antioxidant activity. BMC Complement. Med. Ther. 2021, 21, 98. [Google Scholar] [CrossRef]
- Boddy, L. Effect of temperature and water potential on growth rate of wood-rotting basidiomycetes. Trans. Br. Mycol. Soc. 1983, 80, 141–149. [Google Scholar] [CrossRef]
- Lu, Z.W.; Liu, H.Y.; Wang, C.L.; Chen, X.; Huang, Y.X.; Zhang, M.M.; Huang, Q.L.; Zhang, G.F. Isolation of endophytic fungi from Cotoneaster multiflorus and screening of drought-tolerant fungi and evaluation of their growth-promoting effects. Front. Microbiol. 2023, 14, 1267404. [Google Scholar] [CrossRef] [PubMed]
- Summerbell, R.C. The benomyl test as a fundamental diagnostic method for medical mycology. J. Clin. Microbiol. 1993, 31, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Worrall, J. Media for selective isolation of Hymenomycetes. Mycologia 1991, 83, 296–302. [Google Scholar] [CrossRef]
- Hoff, J.A.; Klopfenstein, N.B.; Tonn, J.R.; McDonald, G.I.; Zambino, P.J.; Rogers, J.D.; Peever, T.L.; Carris, L.M. Roles of woody root-associated fungi in forest ecosystem processes: Recent advances in fungal identification. In USDA Forest Service-Research Paper RMRS-RP; 2004; pp. 1–8. Available online: https://www.fs.usda.gov/rm/pubs/rmrs_rp047.pdf (accessed on 1 August 2023).
- Mainfeld, D. Endophytische Pilze der Fichte (Picea abies (L.) Karst.)-Neue Aspekte zur biologischen Kontrolle von Heterbasidion annosum (Fr.) Bref.; Eberhard-Karls-Universitat: Tubingen, Germany, 1998. [Google Scholar]
- Gorke, C. Mycozonozen von Wurzeln und Stamm von Jungbaumen unterschiedlicher Bestandsbergrundungen. Bibl. Mycol. 1998, 173, 1–462. [Google Scholar]
- Sieber, T. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Zhang, T.; Yao, Y.-F. Endophytic fungal communities associated with vascular plants in the high Arctic zone are highly diverse and host-plant specific. PLoS ONE 2015, 10, e0130051. [Google Scholar] [CrossRef]
- Geisen, S.; Kostenko, O.; Cnossen, M.C.; ten Hooven, F.C.; Vreš, B.; van der Putten, W.H. Seed and root endophytic fungi in a range expanding and a related plant species. Front. Microbiol. 2017, 8, 1645. [Google Scholar] [CrossRef]
- Hagh Doust, N.; Safaie, N.; Schmitt, I.; Otte, J.; Bálint, M. Culture-based methods using low-temperature incubation revealed cold-adapted fungal endophytes from semiarid forests. For. Pathol. 2019, 49, e12515. [Google Scholar] [CrossRef]
- Baum, S.; Sieber, T.; Schwarze, F.; Fink, S. Latent infections of Fomes fomentarius in the xylem of European beech (Fagus sylvatica). Mycol. Prog. 2003, 2, 141–148. [Google Scholar] [CrossRef]
- Islam, F.; Salam, M.A.; Rahman, M.A.; Paul, S.I.; Das, T.R.; Rahman, M.M.; Shaha, D.C.; Gupta, D.R.; Alam, M.S.; Islam, T. Plant endophytic yeasts Pichia fermentans and Meyerozyma caribbica improve growth, biochemical composition, haematological parameters and morphology of internal organs of premature Barbonymus gonionotus. Aquac. Rep. 2021, 19, 100575. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, W.; Chen, Y.; Wang, L.; Zhang, C.; Deng, W.; Zhang, L.; Liu, G.; Shen, C.; Lou, K.; et al. Isolation and characterization of yeast for the production of rice wine with low fusel alcohol content. PLoS ONE 2021, 16, e0260024. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.; Farhat, H.; Urooj, F.; Rahman, A.; Irfan, M.; Ehteshamul-Haque, S. Characterization of endophytic yeast and its suppressive effect on root rotting fungi of tomato under neem cake soil amendment. Egypt. J. Biol. Pest Control. 2021, 31, 152. [Google Scholar] [CrossRef]
- Isaeva, O.V.; Glushakova, A.M.; Garbuz, S.A.; Kachalkin, A.V.; Chernov, I. Endophytic yeast fungi in plant storage tissues. Biol. Bull. 2010, 37, 26–34. [Google Scholar] [CrossRef]
- Taylor, J.E.; Hyde, K.D.; Jones, E.B.G. Endophytic fungi associated with the temperate palm, Trachycarpus fortunei, within and outside its natural geographic range. New Phytol. 1999, 142, 335–346. [Google Scholar] [CrossRef]
- Fisher, P.J.; Petrini, O.; Petrini, L.E.; Sutton, B.C. Fungal endophytes from the leaves and twigs of Quercus ilex L. from England, Majorca and Switzerland. New Phytol. 1994, 127, 133–137. [Google Scholar] [CrossRef]
- Gnavi, G.; Ercole, E.; Panno, L.; Vizzini, A.; Varese, G. Dothideomycetes and Leotiomycetes sterile mycelia isolated from the Italian seagrass Posidonia oceanica based on rDNA data. SpringerPlus 2014, 3, 508. [Google Scholar] [CrossRef]
- Mattoo, A.J.; Nonzom, S. Investigating diverse methods for inducing sporulation in endophytic fungi. Stud. Fungi 2022, 7, 16. [Google Scholar] [CrossRef]
- Tanney, J.B.; Seifert, K.A. Mollisiaceae: An overlooked lineage of diverse endophytes. Stud. Mycol. 2020, 95, 293–380. [Google Scholar] [CrossRef]
- Ibrahim, A.; Tanney, J.B.; Fei, F.; Seifert, K.A.; Cutler, G.C.; Capretta, A.; Miller, J.D.; Sumarah, M.W. Metabolomic-guided discovery of cyclic nonribosomal peptides from Xylaria ellisii sp. nov., a leaf and stem endophyte of Vaccinium angustifolium. Sci. Rep. 2020, 10, 4599. [Google Scholar] [CrossRef]
- Truong, C.; Mujic, A.B.; Healy, R.; Kuhar, F.; Furci, G.; Torres, D.; Niskanen, T.; Sandoval-Leiva, P.A.; Fernández, N.; Escobar, J.M.; et al. How to know the fungi: Combining field inventories and DNA-barcoding to document fungal diversity. New Phytol. 2017, 214, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-Y.; Qi, Y.-L.; Cai, L. Induction of sporulation in plant pathogenic fungi. Mycology 2012, 3, 195–200. [Google Scholar]
- Adnan, M.; Patel, M.; Reddy, M.N.; Alshammari, E. Formulation, evaluation and bioactive potential of Xylaria primorskensis terpenoid nanoparticles from its major compound xylaranic acid. Sci. Rep. 2018, 8, 1740. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Morin, N.R.; Bills, G.F.; Dombrowski, A.W.; Salituro, G.M.; Smith, S.K.; Zhao, A.; MacNeil, D.J. Novel sesquiterpenoids from the fermentation of Xylaria persicaria are selective ligands for the NPY Y5 receptor. J. Org. Chem. 2002, 67, 5001–5004. [Google Scholar] [CrossRef]
- Coval, S.J.; Puar, M.S.; Phife, D.W.; Terracciano, J.S.; Patel, M. SCH57404, an antifungal agent possessing the rare sodaricin skeleton and a tricyclic sugar moiety. J. Antibiot. 1995, 48, 1171–1172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deyrup, S.T.; Gloer, J.B.; O’Donnell, K.; Wicklow, D.T. Kolokosides A−D: Triterpenoid glycosides from a Hawaiian isolate of Xylaria sp. J. Nat. Prod. 2007, 70, 378–382. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Li, Y.-Y.; Huang, Y.-J.; Su, W.-J.; Shen, Y.-M. Three new sesquiterpenoids from Xylaria sp. NCY2. Helv. Chim. Acta 2008, 91, 46–52. [Google Scholar] [CrossRef]
- Isaka, M.; Yangchum, A.; Auncharoen, P.; Srichomthong, K.; Srikitikulchai, P. Ring B aromatic norpimarane glucoside from a Xylaria sp. J. Nat. Prod. 2011, 74, 300–302. [Google Scholar] [CrossRef]
- Davis, R.A.; Pierens, G.K. 1H and 13C NMR assignments for two new xanthones from the endophytic fungus Xylaria sp. FRR 5657. Magn. Reson. Chem. 2006, 44, 966–968. [Google Scholar] [CrossRef]
- Healy, P.C.; Hocking, A.; Tran-Dinh, N.; Pitt, J.I.; Shivas, R.G.; Mitchell, J.K.; Kotiw, M.; Davis, R.A. Xanthones from a microfungus of the genus Xylaria. Phytochemistry 2004, 65, 2373–2378. [Google Scholar] [CrossRef]
- Jayasuriya, H.; Herath, K.B.; Ondeyka, J.G.; Polishook, J.D.; Bills, G.F.; Dombrowski, A.W.; Springer, M.S.; Siciliano, S.; Malkowitz, L.; Sanchez, M.; et al. Isolation and structure of antagonists of chemokine receptor (CCR5). J. Nat. Prod. 2004, 67, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; She, Z.; Lin, Y.; Vrijmoed, L.L.; Lin, W. Cyclic peptides from an endophytic fungus obtained from a mangrove leaf (Kandelia candel). J. Nat. Prod. 2007, 70, 1696–1699. [Google Scholar] [CrossRef] [PubMed]
- Boonphong, S.; Kittakoop, P.; Isaka, M.; Pittayakhajonwut, D.; Tanticharoen, M.; Thebtaranonth, Y. Multiplolides A and B, new antifungal 10-membered lactones from Xylaria multiplex. J. Nat. Prod. 2001, 64, 965–967. [Google Scholar] [CrossRef] [PubMed]
- O’Hagana, D.; Rogers, S.V.; Duffin, G.R.; Edwards, R.L. Biosynthesis of the fungal polyketide, cubensic acid from Xylaria cubensis. Tetrahedron Lett. 1992, 33, 5585–5588. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L.L.P.; Jones, E.B.G.; Krohn, K.; Steingröver, K.; et al. Five unique compounds: Xyloketals from mangrove fungus Xylaria sp. from the South China sea coast. J. Org. Chem. 2001, 66, 6252–6256. [Google Scholar] [CrossRef] [PubMed]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants 2022, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Muthu Narayanan, M.; Ahmad, N.; Shivanand, P.; Metali, F. The role of endophytes in combating fungal- and bacterial-induced stress in plants. Molecules 2022, 27, 6549. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Sayed, S.R.M.; Rady, A.M. Antagonistic activity of Trichoderma harzianum and Trichoderma viride strains against some fusarial pathogens causing stalk rot disease of maize, in vitro. J. King Saud Univ.-Sci. 2021, 33, 101363. [Google Scholar] [CrossRef]
- Li, Y.; Duan, T.; Nan, Z.; Li, Y. Arbuscular mycorrhizal fungus alleviates alfalfa leaf spots caused by Phoma medicaginis revealed by RNA-seq analysis. J. Appl. Microbiol. 2021, 130, 547–560. [Google Scholar] [CrossRef]
- Duckett, J.G.; Russell, J.; Ligrone, R. Basidiomycetous endophytes in jungermannialean (leafy) liverworts have novel cytology and species-specific host ranges: A cytological and experimental study. Can. J. Bot. 2006, 84, 1075–1093. [Google Scholar] [CrossRef]
- Xie, L.; Bi, Y.; Ma, S.; Shang, J.; Hu, Q.; Christie, P. Combined inoculation with dark septate endophytes and arbuscular mycorrhizal fungi: Synergistic or competitive growth effects on maize? BMC Plant Biol. 2021, 21, 498. [Google Scholar] [CrossRef] [PubMed]
- Hassett, B.T.; Vonnahme, T.R.; Peng, X.; Jones, E.B.G.; Heuzé, C. Global diversity and geography of planktonic marine fungi. Bot. Mar. 2020, 63, 121–139. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Boguś, M.I. Fungi of entomopathogenic potential in Chytridiomycota and Blastocladiomycota, and in fungal allies of the Oomycota and Microsporidia. IMA Fungus 2021, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Van den Wyngaert, S.; Ganzert, L.; Seto, K.; Rojas-Jimenez, K.; Agha, R.; Berger, S.A.; Woodhouse, J.; Padisak, J.; Wurzbacher, C.; Kagami, M.; et al. Seasonality of parasitic and saprotrophic zoosporic fungi: Linking sequence data to ecological traits. ISME J. 2022, 16, 2242–2254. [Google Scholar] [CrossRef] [PubMed]
- Calabon, M.S.; Jones, E.B.G.; Pang, K.-L.; Abdel-Wahab, M.A.; Jin, J.; Devadatha, B.; Sadaba, R.B.; Apurillo, C.C.; Hyde, K.D. Updates on the classification and numbers of marine fungi. Bot. Mar. 2023, 66, 213–238. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Pang, K.-L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J.; et al. An online resource for marine fungi. Fungal Divers. 2019, 96, 347–433. [Google Scholar] [CrossRef]
- Calabon, M.; Hyde, K.; Jones, E.; Luo, Z.-L.; Dong, W.; Hurdeal, V.; Gentekaki, E.; Rossi, W.; Leonardi, M.; Thiyagaraja, V.; et al. Freshwater fungal numbers. Fungal Divers. 2022, 114, 3–235. [Google Scholar] [CrossRef]
- Abdelrazek, S.; Choudhari, S.; Thimmapuram, J.; Simon, P.; Colley, M.; Mengiste, T.; Hoagland, L. Changes in the core endophytic mycobiome of carrot taproots in response to crop management and genotype. Sci. Rep. 2020, 10, 13685. [Google Scholar] [CrossRef]
- Nagano, Y.; Nagahama, T. Fungal diversity in deep-sea extreme environments. Fungal Ecol. 2012, 5, 463–471. [Google Scholar] [CrossRef]
- Hassett, B.T.; Ducluzeau, A.L.; Collins, R.E.; Gradinger, R. Spatial distribution of aquatic marine fungi across the western Arctic and sub-arctic. Environ. Microbiol. 2017, 19, 475–484. [Google Scholar] [CrossRef]
- Comeau, A.M.; Vincent, W.F.; Bernier, L.; Lovejoy, C. Novel chytrid lineages dominate fungal sequences in diverse marine and freshwater habitats. Sci. Rep. 2016, 6, 30120. [Google Scholar] [CrossRef] [PubMed]
- Picard, K.T. Coastal marine habitats harbor novel early-diverging fungal diversity. Fungal Ecol. 2017, 25, 1–13. [Google Scholar] [CrossRef]
- Hassett, B.T.; Borrego, E.J.; Vonnahme, T.R.; Rama, T.; Kolomiets, M.V.; Gradinger, R. Arctic marine fungi: Biomass, functional genes, and putative ecological roles. ISME J. 2019, 13, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Supaphon, P.; Phongpaichit, S.; Sakayaroj, J.; Rukachaisirikul, V.; Kobmoo, N.; Spatafora, J. Phylogenetic community structure of fungal endophytes in seagrass species. Bot. Mar. 2017, 60, 489–501. [Google Scholar] [CrossRef]
- Sandberg, D.C.; Battista, L.J.; Arnold, A.E. Fungal endophytes of aquatic macrophytes: Diverse host-generalists characterized by tissue preferences and geographic structure. Microb. Ecol. 2014, 67, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhao, C.A.; Liu, C.J.; Xu, X.F. Endophytic fungi diversity of aquatic/riparian plants and their antifungal activity in vitro. J. Microbiol. 2010, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Al-Ajam, M.R.; Bizri, A.R.; Mokhbat, J.; Weedon, J.; Lutwick, L. Mucormycosis in the Eastern Mediterranean: A seasonal disease. Epidemiol. Infect. 2006, 134, 341–346. [Google Scholar] [CrossRef]
- Deng, Z.; Cao, L.; Huang, H.; Jiang, X.; Wang, W.; Shi, Y.; Zhang, R. Characterization of Cd- and Pb-resistant fungal endophyte Mucor sp. CBRF59 isolated from rapes (Brassica chinensis) in a metal-contaminated soil. J. Hazard. Mater. 2011, 185, 717–724. [Google Scholar] [CrossRef]
- Grządziel, J.; Gałązka, A. Fungal biodiversity of the most common types of Polish soil in a long-term microplot experiment. Front. Microbiol. 2019, 10, 6. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Zhong, F.; Fan, X.; Ji, W.; Hai, Z.; Hu, N.; Li, X.; Liu, G.; Yu, C.; Chen, Y.; Lian, B.; et al. Soil fungal community composition and diversity of culturable endophytic fungi from plant roots in the reclaimed area of the eastern coast of China. J. Fungi 2022, 8, 124. [Google Scholar] [CrossRef]
- Howard, N.; Pressel, S.; Kaye, R.S.; Daniell, T.J.; Field, K.J. The potential role of Mucoromycotina ‘fine root endophytes’ in plant nitrogen nutrition. Physiol. Plant 2022, 174, e13715. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Miao, Y.F.; Chen, L.; Zhou, J.; Yang, Z.P.; Dong, X.F.; Zhang, H.B. Tissue-specific and geographical variation in endophytic fungi of Ageratina adenophora and fungal associations with the environment. Front. Microbiol. 2019, 10, 2919. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Shin, K.C.; Lee, J.K. Fungal community analyses of endophytic fungi from two oak species, Quercus mongolica and Quercus serrata, in Korea. Mycobiology 2021, 49, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Wolińska, A.; Podlewski, J.; Słomczewski, A.; Grządziel, J.; Gałązka, A.; Kuźniar, A. Fungal indicators of sensitivity and resistance to long-term maize monoculture: A culture-independent approach. Front. Microbiol. 2022, 12, 799378. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L.; Griffith, G.S. Role of endophytes and latent invasion in the development of decay communities in sapwood of angiospermous trees. Sydowia 2011, 41, 41–73. [Google Scholar]
- Duong, L.; McKenzie, E.; Lumyong, S.; Hyde, K. Fungal succession on dead leaves of Castanopsis diversifolia in Doi Suthep-Pui National Park. Fungal Divers. 2008, 30, 23–36. [Google Scholar]
- Zhou, J.; Li, X.; Huang, P.-W.; Dai, C.-C. Endophytism or saprophytism: Decoding the lifestyle transition of the generalist fungus Phomopsis liquidambari. Microbiol. Res. 2018, 206, 99–112. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Hui, S.; Choi, J.; Asiegbu, F.O.; Valkonen, J.P.T.; Lee, Y.-H. Secret lifestyles of Neurospora crassa. Sci. Rep. 2014, 4, 5135. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Wenndt, A.J.; Evans, S.E.; van Diepeningen, A.D.; Logan, J.R.; Jacobson, P.J.; Seely, M.K.; Jacobson, K.M. Why plants harbor complex endophytic fungal communities: Insights from perennial bunchgrass Stipagrostis sabulicola in the Namib Sand Sea. Front. Microbiol. 2021, 12, 691584. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, F.W.; Engels, J.; Mattheck, C. Fungal Strategies of Wood Decay in Trees; Springer Science & Business Media: Berlin, Germany, 2000. [Google Scholar]
- Thormann, M.N.; Currah, R.S.; Bayley, S.E. The relative ability of fungi from Sphagnum fuscum to decompose selected carbon substrates. Can. J. Microbiol. 2002, 48, 204–211. [Google Scholar] [CrossRef]
- Oses, R.; Valenzuela, S.; Freer, J.; Baeza, J.; Rodríguez, J. Evaluation of fungal endophytes for lignocellulolytic enzyme production and wood biodegradation. Int. Biodeterior. Biodegrad. 2006, 57, 129–135. [Google Scholar] [CrossRef]
- Sieber, T.N. Fungal root endophytes. In The Hidden Half; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Dekker: New York, NY, USA, 2002; pp. 887–917. [Google Scholar]
- Eaton, R. A breakthrough for wood decay fungi. New Phytol. 2009, 146, 3–4. [Google Scholar] [CrossRef]
- Krause, K.; Jung, E.M.; Lindner, J.; Hardiman, I.; Poetschner, J.; Madhavan, S.; Matthäus, C.; Kai, M.; Menezes, R.C.; Popp, J.; et al. Response of the wood-decay fungus Schizophyllum commune to co-occurring microorganisms. PLoS ONE 2020, 15, e0232145. [Google Scholar] [CrossRef]
- Yusran, Y.; Erniwati, E.; Khumaidi, A.; Pitopang, R.; Jati, I.R.A.P. Diversity of substrate type, ethnomycology, mineral composition, proximate, and phytochemical compounds of the Schizopyllum commune Fr. in the area along Palu-Koro Fault, Central Sulawesi, Indonesia. Saudi J. Biol. Sci. 2023, 30, 103593. [Google Scholar] [CrossRef] [PubMed]
- Lahbib, A.; Chattaoui, M.; Aydi, N.; Zaghouani, H.; Beldi, O.; Daami-Remadi, M.; Nasraoui, B. First report of Schizophyllum commune associated with apple wood rot in Tunisia. New Dis. Rep. 2016, 34, 26. [Google Scholar] [CrossRef]
- Rezgui, A.; Vallance, J.; Ben Ghnaya-Chakroun, A.; Bruez, E.; Dridi, M.; Demasse, R.D.; Rey, P.; Sadfi-Zouaoui, N. Study of Lasidiodiplodia pseudotheobromae, Neofusicoccum parvum and Schizophyllum commune, three pathogenic fungi associated with the Grapevine Trunk Diseases in the North of Tunisia. Eur. J. Plant Pathol. 2018, 152, 127–142. [Google Scholar] [CrossRef]
- Robles, C.A.; Ceriani-Nakamurakare, E.D.; Pereira, S.; Carmarán, C.C. Relationships between endophytic and pathogenic strains of Inonotus (Basidiomycota) and Daldinia (Ascomycota) from urban trees. Mycol. Prog. 2019, 18, 1155–1171. [Google Scholar] [CrossRef]
- Promputtha, I.; Lumyong, S.; Dhanasekaran, V.; McKenzie, E.H.; Hyde, K.D.; Jeewon, R. A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microb. Ecol. 2007, 53, 579–590. [Google Scholar] [CrossRef]
- Bouhajja, E.; Agathos, S.N.; George, I.F. Metagenomics: Probing pollutant fate in natural and engineered ecosystems. Biotechnol. Adv. 2016, 34, 1413–1426. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Muller, D.; Babalola, O.O. A metagenomic lens into endosphere microbial communities, promises, and discoveries. Lett. Appl. Microbiol. 2022, 76, ovac030. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Al-Zahrani, H.S.; Almaghrabi, O.A.; Abdelmoneim, T.S.; Fuller, M.P. Comparative metagenomics approaches to characterize the soil fungal communities of western coastal region, Saudi Arabia. PLoS ONE 2017, 12, e0185096. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.J.; Kim, H.; Lee, J.H.; Yoon, H.; Kim, J.G. Metagenomic analysis of soil fungal communities on Ulleungdo and Dokdo Islands. J. Gen. Appl. Microbiol. 2015, 61, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Popescu, S.C.; Tomaso-Peterson, M.; Wilkerson, T.; Bronzato-Badial, A.; Wesser, U.; Popescu, G.V. Metagenomic analyses of the soybean root mycobiome and microbiome reveal signatures of the healthy and diseased plants affected by taproot decline. Microorganisms 2022, 10, 856. [Google Scholar] [CrossRef]
- Choi, B.Y.; Lee, S.; Kim, J.; Park, H.; Kim, J.-H.; Kim, M.; Park, S.-J.; Kim, K.-T.; Ryu, H.; Shim, D. Comparison of endophytic and epiphytic microbial communities in surviving and dead Korean fir (Abies koreana) Using metagenomic sequencing. Forests 2022, 13, 1932. [Google Scholar] [CrossRef]
- Das, A.; Rahman, M.I.; Ferdous, A.S.; Amin, A.; Rahman, M.M.; Nahar, N.; Uddin, M.A.; Islam, M.R.; Khan, H. An endophytic Basidiomycete, Grammothele lineata, isolated from Corchorus olitorius, produces paclitaxel that shows cytotoxicity. PLoS ONE 2017, 12, e0178612. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungjindamai, N.; Jones, E.B.G. Why Are There So Few Basidiomycota and Basal Fungi as Endophytes? A Review. J. Fungi 2024, 10, 67. https://doi.org/10.3390/jof10010067
Rungjindamai N, Jones EBG. Why Are There So Few Basidiomycota and Basal Fungi as Endophytes? A Review. Journal of Fungi. 2024; 10(1):67. https://doi.org/10.3390/jof10010067
Chicago/Turabian StyleRungjindamai, Nattawut, and E. B. Gareth Jones. 2024. "Why Are There So Few Basidiomycota and Basal Fungi as Endophytes? A Review" Journal of Fungi 10, no. 1: 67. https://doi.org/10.3390/jof10010067
APA StyleRungjindamai, N., & Jones, E. B. G. (2024). Why Are There So Few Basidiomycota and Basal Fungi as Endophytes? A Review. Journal of Fungi, 10(1), 67. https://doi.org/10.3390/jof10010067






