Abstract
The genus Gymnopus plays a significant role in ecological systems, with certain species holding potential as food or medicinal resources. However, the species diversity of Gymnopus in China remains unclear. In recent years, more than one thousand Gymnopus specimens have been collected across China. Thus, through the integration of ecological evidence, detailed morphological studies, and phylogenetic analysis using a multiloci dataset of ITS + nLSU + tef1-ɑ, four new species—Gymnopus longistipes, Gymnopus striatipileatus, Gymnopus viridiscus, and Gymnopus spadiceus—have been differentiated from known species. Gymnopus similis has been newly documented from Jiangxi Province, China. Detailed descriptions and vivid illustrations have been provided based on the newly collected specimens, along with comparisons to closely related species. Additionally, a key to the reported species of Gymnopus s.l. from East China has been included.
1. Introduction
The genus Gymnopus (Pers.) Roussel holds high economic and ecological value [1]. For example, species belonging to the Gymnopus dryophilus (Bull.) Murrill complex are hunted and consumed as food resources in China [2]. Additionally, Gymnopus androsaceus (L.) Della Magg. & Trassin. has been developed for medicinal purposes [3]. However, the species diversity of Gymnopus in China has been greatly overlooked.
Taxonomic research on Gymnopus has been ongoing for a significant period. Initially, the genus Gymnopus was considered a tribe within Agaricus L. [4]. Later, Fries transferred these species to trib. Collybia Fr., as proposed by Fries [5]. Subsequently, Staude [6] established the genus Collybia (Fr.) Staude. Researchers followed the opinions of Fries and Staude until Singer divided the genus Collybia into nine sections in his book, The Agaricales in Modern Taxonomy [7,8,9,10], which became the mainstream view. Although some researchers proposed their own perspectives on the taxonomic systems [11,12], Singer’s classification remained widely accepted. However, with the advancement of taxonomic studies in Collybia, Halling [13] and Antonín and Noordeloos [14,15] identified problematic taxonomy within the genus Collybia. As a result, Antonín et al. [16] proposed the establishment of the genus Gymnopus, transferring the species into it and leaving only four species within Collybia.
The application of molecular studies in the genus Gymnopus has sparked debates regarding the boundaries between Gymnopus and allied genera [17,18,19]. The concept of the genus Gymnopus is changing, as evidenced by recent changes. For instance, Noordeloos and Antonín [20] combined sect. Androsacei (Kühner) Antonín & Noordel. into Gymnopus. In a more recent development, Oliveira et al. [21] elevated sect. Perforantia (Singer) R.H. Petersen to the genus rank, naming it Paragymnopus J.S. Oliveira, and transferred sect. Vestipedes (Fr.) Antonín, Halling & Noordel. to Marasmiellus Murrill (later confirmed to be a synonym of Collybiopsis (J. Schröt.) Earle [22]), thereby establishing a distinct boundary between Gymnopus and Collybiopsis. However, this also leaves the multiloci phylogeny of Gymnopus and Collybiopsis [23]. Through continued research, the now genus Gymnopus has become delimited as a group, which is characterized by a collybioid appearance, rarely tricholomatoid or marasmioid; free to adnate and usually crowded lamellae; an insititious stipe, or not; a white spore print; basidiospores ellipsoid to short-oblong, inamyloid; cheilocystidia usually present and varied; a cutis or ixocutis pileipellis with radially arranged cylindrical hyphae or interwoven more akin to a trichoderm or ixotrichoderm, made up of irregular coralloid terminal elements (“Dryophila structures”), often incrusted, diverticulate hyphal elements, mixed with broom cells and coralloid hyphae; and clamp connections present in all tissues [21,24].
Furthermore, several infrageneric taxonomic issues remain unresolved. Within the genus Gymnopus, the foetid species was previously classified in sect. Vestipedes due to their similar tomentose stipes. This classification was later elevated to section rank due to the indistinct odour [24]. However, some foetid species still reside in sect. Vestipedes. Additionally, there are species that do not align well with the sectional characteristics. For example, Gymnopus earleae Murrill can be easily distinguished from other species in sect. Levipedes by its absence or inconspicuous cheilocystidia and the non-diverticulate or coralloid pileipellis, which are considered key characteristics of this section. The dense stipe vesture, inconspicuous cheilocystidia, and presence of caulocystidia allow Gymnopus kauffmanii (Halling) Halling to be differentiated from sect. Levipedes.
To date, approximately 300 Gymnopus species have been described globally, with North America, Europe and Asia reporting the highest numbers of species, accounting for 39.14%, 18.75% and 14.14% of the total, respectively. In contrast, Oceania and Africa have reported the fewest species.
The taxonomic studies of Gymnopus in China are comparatively less advanced than those conducted abroad. The earliest recorded instance of Collybia (Gymnopus) could be track back to the book Fungi of China [25]. Subsequently, Liu et al. [26] described the first new species of Collybia from China, Collybia citrina B. Liu, Rong & H.S. Jin. Since then, several new species, previously undocumented in China and new to science, have been reported [23,27,28,29,30,31,32,33,34]. However, research on Gymnopus is uneven, with Northeast China and South China reporting the highest number of species. Nonetheless, a comprehensive taxonomic study of Gymnopus in China is still lacking.
This paper aims to increase the species diversity of Gymnopus in China based on the combined ecology, morphology, and phylogenetic evidence. Furthermore, it aims to report the new distribution area of Gymnopus similis Antonín, Ryoo, and Ka in China.
2. Materials and Methods
2.1. Sampling and Morphological Study
Specimens were collected using the random sampling method and photographed in situ. The sizes of the basidiomata were measured when fresh. Following the examination and recording of the macroscopic characteristics, the specimens were dried in an electric drier at approximately 45 °C. Then the dried specimens were stored in self-zip plastic bag with colour silica gel.
The macroscopic characteristics were documented using filed notes and photographs. Additionally, colour descriptions were conducted based on the Flora of British Fungi: Colour Identification Chart [35]. Ethanol 94% was used to rehydrate the dried specimen before microscopic examination. Subsequently, they were examined under a Zeiss Axio lab. A1 microscope at magnifications up to 1000×. During the examination, 3% potassium hydroxide (3% KOH), 1% Congo red solution, or Melzer’s reagent [36], were employed as floating agents. All measurements were taken from sections mounted in 1% Congo red. For each specimen, characteristics such as basidiospores, basidia, cheilocystidia, or width of pileipellis elements, etc., were measured 40 times from at least two different basidiomata. The measurements were recorded as length × width (L × W). Of the obtained measurements, 5% were considered outliers and were excluded from each extreme end of the range and then given in parentheses. The basidiospores’ variation in the ratio of L to W among the studied specimens was denoted as Q. Qm represented the average Q value of all the basidiospores ± the standard deviation. The examined specimens are deposited in the herbarium of Jilin Agricultural University (HMJAU).
2.2. DNA Extraction, PCR Amplification, and Sequencing
The total DNA was extracted from dried specimens or biomaterials using the NuClean Plant Genomic DNA Kit (Kangwei Century Biotechnology Company Ltd., Beijing, China), following the manufacturer’s instructions. For each specimen, about 10 mg starting material was used for DNA extraction. Phylogenetic analysis utilized sequences from the internal transcribed spacer (ITS) region, nuclear large ribosomal subunits (nLSU), and translation elongation factor 1-α (tef1-ɑ). The primer pairs ITS1-F/ITS4-B [37], LR0R/LR7 or LR5 [38,39], and tef1f/tef1r [40] or 983f/1953r were employed to amplify the ITS, nLSU, and tef1-ɑ regions, respectively. Each PCR reaction (25 μL) consisted of 12.5 μL 2 × EasyTaq PCR SuperMix (TransGen Biotech Co., Ltd., Beijing, China), 1 μL of each 10 μM primer, 2 μL DNA solution, and 8.5 μL dd H2O [23,33]. The reaction programs followed those described by Coimbra et al. [41] for ITS, Ryoo et al. [42] for nLSU, and Xu et al. [43,44] for tef1-ɑ. The PCR products were visualized under UV light after electrophoresis on 1.2% agarose gels stained with ethidium bromide. Subsequently, the purified PCR products were sent to Sangon Biotech Limited Company (Shanghai, China) for sequencing using the Sanger method through Thermo Fisher TM 3730XL Analyzer Applied Biosystems (Waltham, MA, USA). For newly described species, two or more collections were sequenced for their ITS, nLSU, and tef1-ɑ sequences. The newly obtained sequences were deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank; Table 1).

Table 1.
Voucher/specimen numbers, country, and GenBank accession numbers of the specimens included in this study. Sequences produced in this study are in bold. The sequences from type specimens are marked as T.
2.3. Data Analysis
Representatives of published sequences from closely related species to the new Chinese species (i.e., >97% similarity in BLASTn results) were included (Table 1). A combined dataset of ITS, nLSU, and tef-1ɑ sequences included sequences from this study, comprising 49 sequences obtained from the type species. Furthermore, some Collybiopsis (J. Schröt.) Earle and Marasmiellus Murrill species were selected for phylogenetic analysis due to the unclear of the boundaries between these genera and the taxonomic system changes recently. Additionally, species belonging to Marasmius Fr. were selected as outgroups [45].
In the dataset, each gene region was aligned using either Clustal X [46] or MAFFT 7.490 [47] and subsequently manually checked in BioEdit 7.0.5.3 [48]. The alignments of the ITS, nLSU, and tef1-ɑ sequences were then combined using Phylosuite 1.2.2 [49]. The partition homogeneity test (PHT) [50] was performed on the multigene dataset using PAUP 4.0b10 [51] with 1000 homogeneity replicates. The best-fit evolutionary model was estimated using ModelFinder [52]. Bayesian inference (BI) algorithms were employed to conduct the phylogenetic analysis, utilizing MrBayes 3.2.6 with a general time-reversible DNA substitution model and gamma distribution rate variation across the sites [53]. Four Markov chains were run for two independent runs, starting from random trees, until the split deviation frequency value was less than 0.01. Trees were sampled every 100 generations, with the first 25% of sampled trees discarded as burn-in. The remaining trees were used to construct a 50% majority consensus tree and calculate the Bayesian posterior probabilities (PP). Maximum likelihood (ML) analysis was performed using RaxmlGUI 2.0.10 [54] with 1000 bootstrap (BS) replicates to search for the optimal topology. The resulting trees were visualized using FigTree 1.4.3.
3. Results
3.1. Phylogenetic Analysis
In the combined dataset of ITS, nLSU, and tef1-ɑ, a total of 211 sequences were included from 121 different collections, with 115 sequences for ITS, 82 sequences for nLSU, and 14 sequences for tef1-ɑ. After trimming, the combined dataset contained 3237 characters, including gaps. Out of these sequences, 32 were newly obtained in the present study, with 12 sequences for ITS, 12 sequences for nLSU, and 8 for tef1-ɑ. The best model selected for the Bayesian inference (BI) analysis using ModelFinder in Phylosuite 1.2.2 was GTR+F+I+G4, while the ML analysis used the GTRGAMMA model. The Bayesian analysis was run for four million generations using MrBayes 3.2.6 in Phylosuite 1.2.2, resulting in an average standard deviation of split frequencies of 0.006578. The same dataset and alignment were also analyzed using the ML method in RaxmlGUI 2.0.10. Both the phylogenetic analyses yielded a congruent topology, which has been depicted in Figure 1.
The phylogeny inferred from the combined dataset showed that the Gymnopus split into four well-supported clades, representing sect. Gymnopus, sect. Levipedes, sect. Androsacei, and sect. Impudicae. Our newly achieved sequences formed four new well-supported lineages that were nested in sect. Levipedes and sect. Impudicae (Figure 1).
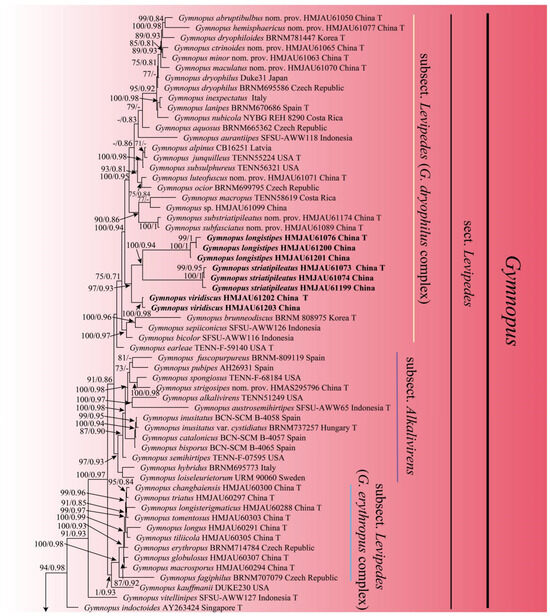
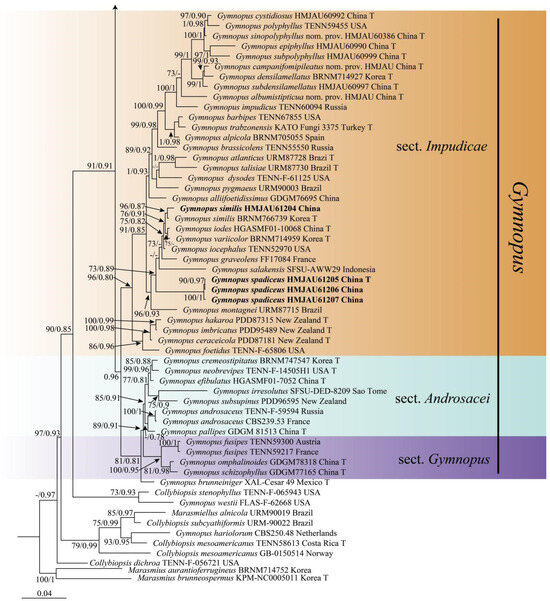
Figure 1.
Maximum likelihood analysis generated from the combined ITS, nLSU, and tef1-ɑ dataset. Bootstraps values (BS) ≥ 70% from ML analysis and Bayesian posterior probabilities (PP) ≥ 0.80 are shown on the branches. Newly sequenced collections are indicated in bold, and the type specimens are denoted by (T).
3.2. Taxonomy
3.2.1. New Species
Fungal Name: FN 571752.
Etymology: refers to the long stipe of this species.
Diagnosis [English]: This species differs from closely related species due to large basidiomata, irregular pileus that are usually reddish brown, paler at disc, smooth, yellow to yellowish stipe, branched or coralloid pileipellis, absence of cheilocystidia and caulocystidia, and bigger basidiospores.
Type: China. Jiangxi Province: Ji’an City, Jinggangshan City, Jinggangshan Mountain, 6 May 2019, Jia-Jun Hu, Bo Zhang, and Dai Dan, HMJAU 61076 (holotype HMJAU 61076).
Basidiomata medium. Pileus 5.1–6.9 cm in diameter, convex, slightly depressed occasionally, yellowish brown to sepia, smooth, hygrophanous; margin entire, wavy, sepia to dark brown, striped, hygrophanous. Context thin, fresh, odourless. Stipe 7.1–11.0 × 0.4–1.2 cm, central, cylindrical, yellowish brown to reddish brown, smooth, striped, fistulose, fibrous. Lamellae adnate to adnexed, close, yellowish brown to light brown, unequal. Occurrence in leaf-litter.
Basidiospores (5.2)5.8–6.7(7.0) × (2.7)2.8–3.1(3.2) µm [Q = (1.73)1.93–2.23(2.33), Qm = 2.05 ± 0.14], elliptic, hyaline, smooth, inamyloid, thin-walled. Basidia (18)19–25(30) × 6–7 µm, clavate, two- or four-spored, hyaline, smooth, thin-walled. Cheilocystidia and caulocystidia absent. Pileipellis a cutis, 7–15(17) µm wide, made up of irregular branched or coralloid hyphae, flattened, hyaline, smooth, thin-walled. Clamp connections present.
Habit, habitat, and distribution: Scattered to gregarious. Saprotrophic, with humicolous habitat, found in broad-leaved forests. So far, only known from Jiangxi Province, China.
Other specimens examined: China. Jiangxi Province: Ji’an City, Jinggangshan City, Jinggangshan Mountain, 10 May 2020, Bo Zhang, and Dai Dan, HMJAU 61200; Jiangxi Province: Pingxiang City, Wugong Mountain, 13 May 2023, Jia-Jun Hu, and Dai Dan, HMJAU 61201.
Note: This species is characterized by large basidiomata, irregular pileus that are usually reddish brown, paler at disc, smooth, yellow to yellowish stipe, branched or coralloid pileipellis, absence of cheilocystidia and caulocystidia.
Gymnopus longistipes shares similarities with Gymnopus aurantiipes (Corner) A.W. Wilson, Desjardin & E. Horak, such as the yellow to orange basidiomata and long stipes. However, G. longistipes differs from G. aurantiipes by the close, but not crowded lamellae, not pruinose stipe upon drying, absence of cheilocystidia, and smaller basidiospores [55]. Gymnopus longistipes is a sister to Gymnopus striatipileatus, according to our phylogenetic analysis. However, G. longistipes can be distinguished from G. striatipileatus through the wavy margin of pileus, longer stipe, and absence of cheilocystidia.

Figure 2.
Habitat of Gymnopus species from China. (A) Gymnopus longistipes; (B) Gymnopus striatipileatus; (C) Gymnopus viridiscus; (D) Gymnopus spadiceus. Bars: 1 cm (A–D).
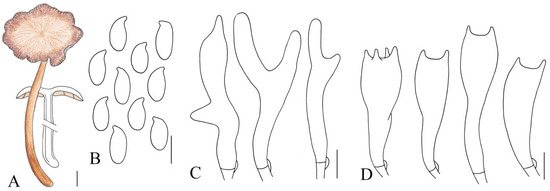
Figure 3.
Illustration of Gymnopus longistipes (HMJAU 61076) morphological features. (A) Basidiomata; (B) Basidiospores; (C) Pileipellis elements; (D) Basidia. Bars: 1 cm (A); 10 μm (C); 5 μm (B,D).
Fungal Name: FN 571371.
Etymology: refers to the striate pileus margin.
Diagnosis [English]: This species is differentiated from other species due to convex pileus that is brown at disc, light brown outwards, striped, cylindrical, brown to reddish brown stipe, irregular cheilocystidia with an umbo at apex, branched or coralloid pileipellis usually flattened, diverticulated, or with a long and slim branch, and smaller basidiospores.
Type: China. Jiangxi Province: Ji’an City, Jinggangshan City, Jinggangshan Mountain, 6 May 2019, Jia-Jun Hu, Bo Zhang, and Dai Dan, HMJAU 61073 (holotype HMJAU 61073).
Basidiomata small to medium. Pileus 1.5–5.2 cm in diameter, convex, brown to dark brown, paler outwards, yellow to light brown, smooth, glabrous, hygrophanous; margin entire, yellowish white to light brown, hygrophanous, striped. Context light brown, thin, fresh, odourless. Stipe 4.2–9.5 × 0.1–0.3 cm, central, cylindrical, yellowish white to light reddish brown, fistulose, fibrous. Lamellae adnexed, close, yellowish brown to light reddish brown, unequal. Occurrence in leaf-litter.
Basidiospores 5.0–6.0(6.1) × (2.4)2.6–3.0 µm [Q = (1.58)1.67–2.08(2.77), Qm = 1.84 ± 0.15], elliptic, hyaline, smooth, inamyloid, thin-walled. Basidia (12)14–24(26) × 4–6 µm, clavate, two- or four-spored, hyaline, smooth, thin-walled. Cheilocystidia abundant, 15–25 × 4–6(7) µm, irregularly clavate, with an umbo at apex, hyaline, smooth, thin-walled. Pileipellis a cutis, (5)6–12(13) µm wide, made up of irregular branched or coralloid hyphae, diverticulate, flatten, hyaline, smooth, thin-walled. Clamp connections present.
Habit, habitat, and distribution: Scattered to gregarious. Saprotrophic, with humicolous habitat, found in broad-leaved forests. So far, only known from Jiangxi Province, China.
Other specimen examined: China. Jiangxi Province: Ji’an City, Jinggangshan City, Jinggangshan Mountain, 3 May 2021, Bo Zhang, and Dai Dan, HMJAU 61074; Jiangxi Province: Ji’an City, Jinggangshan City, Jinggangshan Mountain, 20 May 2022, Bo Zhang, and Dai Dan, HMJAU 61199.
Note: This species is characterized by its convex pileus that is brown at the disc, turning light brown outwards, striped, cylindrical, brown to reddish brown stipe, irregular cheilocystidia with an umbo at the apex, branched or coralloid pileipellis that is usually flattened, diverticulate, or having long and slim branches, and smaller basidiospores.
This species bears a resemblance to Gymnopus erythropus (Pers.) Antonín, Halling & Noordel. due to its brown to reddish brown stipe. However, it can be differentiated from G. erythropus due to its smaller basidiospores and a pileipellis that is typically branched or coralloid, often flattened, diverticulate, or with long and slender branches [23,24,56]. Gymnopus striatipileatus differentiate from Gymnopus globulosus J.J. Hu, Y.L. Tuo, B. Zhang & Yu Li due to the habitat and coralloid pileipellis. [23].
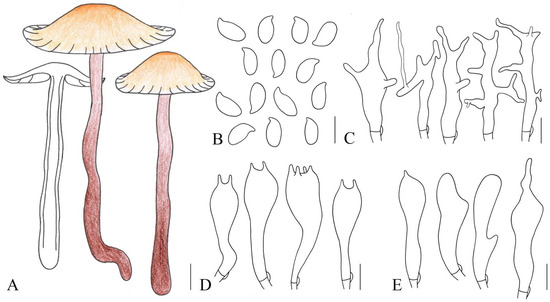
Figure 4.
Illustration of Gymnopus striatipileatus (HMJAU 61073) morphological features. (A) Basidiomata; (B) Basidiospores; (C) Pileipellis elements; (D) Basidia; (E) Cheilocystidia. Bars: 1 cm (A); 10 μm (C); 5 μm (B,D,E).
Fungal Name: FN 571913.
Etymology: refers to the green pileus of the species.
Diagnosis [English]: This species can be differentiated from other species through its arising in early spring, its green pileus, Dryophila-structured but inflated pileipellis, presence of caulocystidia, and non-finger-like cheilocystidia.
Type: China. Zhejiang Province: Hangzhou City, Lin’an City, Tianmu Mountain, Longfengjian, 119.44 E, 30.34 N, 31 May 2021, Jin-Bao Pu, HMJAU 61202 (holotype HMJAU 61202).
Basidiomata small. Pileus 1.7–1.9 cm diameter, convex to applanate, depressed when mature, grayish green at disc, with yellow tone sometimes, grayish white outwards, smooth, hygrophanous; margin entire, wavy, striped, yellowish green, grayish green, or white. Context thin, white, odourless. Stipe 2.2–3.2 × 0.2–0.3 cm, central, cylindrical, fresh-coloured to light greenish yellow, darker at base, smooth, covered with furfuraceous or tomentose, fistulose, fibrous. Lamellae closed, white to light yellow, unequal. Occurrence in soil.
Basidiospores (5.1)5.2–6.2(6.3) × (2.5)2.8–3.3 μm [Q = (1.6)1.7–2.2, Qm = 2.0 ± 0.1], elliptic, hyaline, smooth, inamyloid, thin-walled. Basidia 16–24(26) μm, clavate, four-spored, occasionally two-spored, hyaline, thin-walled. Cheilocystidia scattered, clavate, bifurcated, finger-like, or with an umbo at apex, hyaline, thin-walled. Caulocystidia (30)50–85(110) × 4–6(7) μm, clavate, or bifurcated at apex, scattered, hyaline, thin-walled. Pleurocystidia absent. Pileipellis a cutis, (4)5–10(12) µm wide, made up of irregular branched or coralloid hyphae, inflated, hyaline, smooth, thin-walled. Clamp connections present.
Habit, habitat, and distribution: Scattered to gregarious. Saprotrophic, with terrestrial habitat, found in broad-leaved and coniferous mixed forests. So far, only known from Zhejiang and Anhui Province, China.
Other specimen examined: China. Anhui Province, Lu’an City, Jinzhai County, Tianma National Nature Reserve, 115.77 E, 31.16 N, 16 April 2023, Yong-Lan Tuo, HMJAU 61203.
Note: This species is characteristic of arising in early spring, its green pileus, Dryophila-structured but inflated pileipellis, presence of caulocystidia, and non-finger-like cheilocystidia.
This species is closely related to Gymnopus aurantiipes (Corner) A.W. Wilson, Desjardin & E. Horak, Gymnopus fagiphilus Antonín, Halling & Noordel., Gymnopus indoctoides A.W. Wilson, Desjardin & E. Horak, Gymnopus inexpectatus Consiglio, Vizzini, Antonín & Contu, Gymnopus kauffmanii (Halling) Halling, Gymnopus macropus Halling, Gymnopus mucubajiensis (Dennis) Halling, Gymnopus nubicola Halling, and Gymnopus vitellinipes A.W. Wilson, Desjardin & E. Horak, due to the presence of caulocystidia. However, G. viridiscus differs from these species in terms of its small basidiomata and grayish green pileus [24].
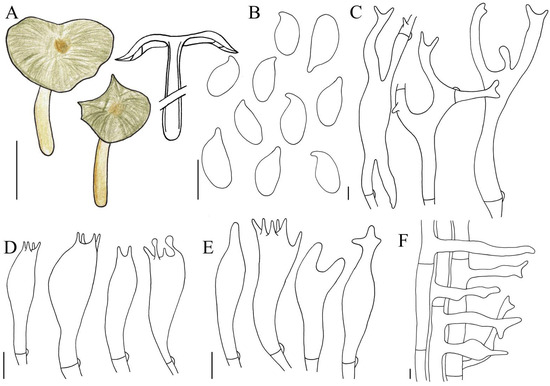
Figure 5.
Illustration of Gymnopus viridiscus (HMJAU 61202) morphological features. (A) Basidiomata; (B) Basidiospores; (C) Pileipellis elements; (D) Basidia; (E) Cheilocystidia; (F) Caulocystidia. Bars: 1 cm (A); 5 μm (B–F).
Fungal Name: FN 571914.
Etymology: refers to the tan basidiomata of this species.
Diagnosis [English]: This species differs from other species due to its small basidiomata and branched pileipellis (weakly Dryophila-structured), grows on the leave litter of Picea koraiensis Nakai.
Type: China. Jilin Province: Changchun City, Jingyue District, campus of Jilin Agriculture University, 125.42 E, 43.82 N, 30 June 202, Jia-Jun Hu, HMJAU 61205 (holotype HMJAU 61205).
Basidiomata small. Pileus 0.3–1.8 cm in diameter, hemispherical to convex, or reflex sometimes, depressed at disc occasionally, smooth, glabrous, striped to half, tan, deep colour at disc; margin entire, involute to reflex, paler than center. Context thin, with strong smell reminiscent of rotten cabbage. Stipe 1.5–4.7 × 0.1–0.4 cm, central, cylindrical or tapering downwards sometimes, tan, paler at apex, tomentose. Lamellae middle, adnate to adnexed, paler than pileus, unequal. Occurrence in leaf-litter.
Basidiospores (5.2)6.0–7.4(8.0) × (2.6)2.8–3.4 μm [Q = (1.7)1.9–2.4, Qm = 2.2 ± 0.2], elliptic, hyaline, smooth, inamyloid, thin-walled. Basidia (17)18–31 μm, clavate, two- or four-spored, hyaline, thin-walled. Cheilocystidia scattered, (16)17–31 μm, clavate, with an umbo at apex, hyaline, thin-walled. Caulocystidia abundant, (30)35–85 × 3–12 μm, clavate, branched or with an umbo at apex sometimes, hyaline, thin-walled. Pleurocystidia absent. Pileipellis a cutis, (4)5–10(12) µm wide, made up of irregular branched or coralloid hyphae (weakly Dryophila-structured), inflated, incrusted sometimes, hyaline, smooth, thin-walled. Clamp connections present.
Habit, habitat, and distribution: Gregarious. Saprotrophic, with humicolous habitat, found in leaf litter of P. koraiensis. So far, only known from Jilin Province, China.
Other specimen examined: China. Jilin Province: Changchun City, Jingyue District, campus of Jilin Agricultural University, 125.42 E, 43.82 N, 17 July 2023, Jia-Jun Hu, HMJAU 61206 (Collection no.: Hu J.J. 601); Changchun City, Jingyue District, campus of Jilin Agricultural University, 125.42 E, 43.82 N, 17 July 2023, Jia-Jun Hu, HMJAU 61207 (Collection no.: Hu J.J. 604).
Note: This species is characterized through its small basidiomata, branched pileipellis (weakly Dryophila-structured), grows in the leaf litter of Picea koraiensis Nakai.
The species Gymnopus spadiceus is often confused with Gymnopus similis Antonín, Ryoo & Ka due to their similar appearances. However, G. spadiceus can be distinguished from G. similis due to its gymnopoid appearance, smaller basidiomata, weakly Dryophila-structured pileipellis, and clavate cheilocystidia [42]. In China, Gymnopus iodes J.P. Li, Chang Tian Li, Chun Y. Deng & Yu Li can also be confused with G. spadiceus; however, G. iodes can be separated due to their marasmioid appearance, shape of cheilocystidia, and caulocystidia [31].
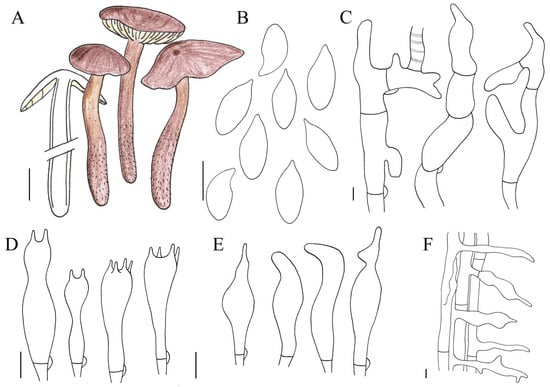
Figure 6.
Illustration of Gymnopus spadiceus (HMJAU 61205) morphological features. (A) Basidiomata; (B) Basidiospores; (C) Pileipellis elements; (D) Basidia; (E) Cheilocystidia; (F) Caulocystidia. Bars: 1 cm (A); 10 μm (F); 5 μm (B–E).
3.2.2. New Record of Jiangxi Province, China
- Gymnopus similis Antonín, Ryoo & Ka
Specimen examined: China. Jiangxi Province: Pingxiang City, Lvxi County, Wugong Mountain, Jia-Jun Hu, Zheng-Xiang Qi, and Dan Dai, 5 July 2023, HMJAU 61204.
Note: This species was originally described from South Korea, and first recorded from Zhejiang Province, China. This time, a similar specimen was collected from Jiangxi Province. A combination of morphological and molecular study confirmed it was G. similis. Before this time, it was never recorded in Jiangxi Province, China.
- Key to the reported Gymnopus s.l. and related species from East China
| 1 Basidiospores amyloid | Rhodocollybia butyracea |
| 1 Basidiospores inamyloid | 2 |
| 2 Basidiomata with foetid smell | 3 |
| 2 Basidiomata without foetid smell | 6 |
| 3 Basidiomata white, small | Gymnopus alliifoetidissimus |
| 3 Basidiomata not white, small to medium | 4 |
| 4 Basidiomata marasmioid, covered with tomentose at stipe | Gymnopus similis |
| 4 Basidiomata gymnopoid, covered with pruinose at stipe | 5 |
| 5 Basidiomata small, arose from coniferous forest | Gymnopus spadiceus |
| 5 Basidiomata small to medium, arose on the ground of broad-leaved forest | Gymnopus dysodes |
| 6 Stipe smooth | 7 |
| 6 Stipe usually covered with tomentose or pruinose | 14 |
| 7 Basidiomata marasmioid | Gymnopus androsaceus |
| 7 Basidiomata not marasmioid | 8 |
| 8 Pileipellis green in KOH | Gymnopus fuscopurpureus |
| 8 Pileipellis not green in KOH | 9 |
| 9 Stipe red | 10 |
| 9 Stipe not red, usually happens in early spring or late autumn | 11 |
| 10 Pileus red, grows on ground | Gymnopus erythropus |
| 10 Pileus yellow to lemon yellow, grows on leaves | Gymnopus subsulphureus |
| 11 Cystidia absent | Gymnopus longistipes |
| 11 Cystidia present | 12 |
| 12 Caulocystidia present and massive | 13 |
| 12 Caulocystidia absent or rare | Gymnopus dryophilus |
| 13 Basidiomata marasmioid, stipe red | Gymnopus striatipileatus |
| 13 Basidiomata gymnopoid, stipe fresh to light greenish yellow | Gymnopus viridiscus |
| 14 Stipe fusiformis, pileipellis not Dryophila-structure | Gymnopus fusipes |
| 14 Stipe usually cylindrical, pileipellis usually Dryophila-structure | 15 |
| 15 Basidiomata small, marasmioid | 16 |
| 15 Basidiomata small to medium, usually gymnopoid | 17 |
| 16 Basidiomata small, grows on leaves | Gymnopus hirtellus |
| 16 Basidiomata, medium to large, grows on ground | Collybiopsisperonata |
| 17 Cystidia absent | 18 |
| 17 Cystidia present | 19 |
| 18 Hyphae blue in KOH | Gymnopus iocephalus |
| 18 Hyphae not blue in KOH | Gymnopus castaneus |
| 19 Stipe with white mycelioid at the base | 20 |
| 19 Stipe without white mycelioid at the base | 21 |
| 20 Caulocystidia absent | Collybiopsisbiformis |
| 20 Caulocystidia present | Collybiopsisconfluens |
| 21 Pileus covered with obvious stripe | Collybiopsispolygramma |
| 21 Pileus without obvious stripe | Collybiopsisluxurians |
4. Discussion
This study presents the detailed description of four new species belonging to sect. Levipedes and sect. Impudicae, which were collected from East and Northeast China. These new species are well-supported by a combination of ecological characteristics, morphological evidence, and phylogenetic analysis. Furthermore, the newly recognized and delimited species belonging to sect. Levipedes were found to occur in early spring in broad-leaved forests.
4.1. Morphological Characteristics in Genus and Species Delimitation
In taxonomy under genus level, the type of pileipellis is commonly regarded as a significant feature. Antonín et al. [16] classified Gymnopus based on a combination of pileipellis structure, basidiomata shape, and odour, dividing the genus into four sections. The criteria for genus and species delineation have been subject to ongoing evolution. The marasmioid appearance and presence of broom cells in the pileipellis were previously considered distinguishing features of sect. Androsacei. However, this led to the recognition of the polyphyletic nature of sect. Androsacei, which was subsequently resolved through the use of Melinda’s reagent [57].
In the Gymnopus dryophilus complex, similar challenges have been encountered. Initially, it was believed that the size of the basidiospores, pileus colour, and shape of cheilocystidia were key features for distinguishing species within the G. dryophilus complex [58,59]. However, a combination of morphological analysis and molecular studies conducted by Antonín et al. [56] revealed that the colour of lamellae and shape of cheilocystidia play a significant role in species differentiation. Additionally, the foetid smell was considered a significant characteristic of sect. Impudicae. However, there are species within this section that lack a distinct smell, and some species in sect. Vestipedes that have a strong smell. Furthermore, there are species that do not always conform to the characteristics of their respective sections, and some species exhibit variations. For example, Gymnopus montagnei (Berk.) Redhead lacks clearly defined lamellae and resembles a goblet. As a result, some researchers have classified it as a member of Hypolyssus Pers. or Perona Pers., but phylogenetic analysis confirms its membership in sect. Impudicae [33]. In the case of sect. Levipedes, the pileipellis is typically composed of flattened and radially arranged hyphae (Dryophila-structure). However, in a previous study, the pileipellis of Gymnopus globulosus J.J. Hu, Y.L. Tuo, B. Zhang & Yu Li was observed to have two layers. The lower layer exhibited the typical Dryophila-structure, while the terminal hyphae formed bulbous structures, which had not been previously observed in this section [23]. These examples shed light on the taxonomic challenges faced in understanding the diversity, characteristics, and limits of the genus Gymnopus. Although the name “Gymnopus” was proposed in 1801 by Persoon, our knowledge of its species diversity and taxonomic characteristics remains limited.
4.2. Phylogenetic Relationships within Gymnopus
Phylogenetic analyses of Gymnopus s.l. within taxonomic systems have been ongoing for approximately two decades. Researchers are striving to determine the monophyletic nature of Gymnopus [16,18,19,21,57]. However, to date, the relationships within and among Gymnopus species remain contentious.
The relationships within Gymnopus require in-depth analysis. Gymnopus comprises species with collybioid basidiomata, occasionally marasmioid, white spore prints, and typically smooth, inamyloid, and hyaline basidiospores [21,24]. Recently, Hu [57] conducted a comprehensive phylogenetic analysis on Gymnopus specimens from China, proposing a novel perspective. However, this new perspective presented challenges in accurately placing all species. For instance, most sect. Impudicae species exhibit distinct smells, matte stipes, and non-flattened pileipellis, while some species lack the foetid odor, cheilocystidia, or typical gilled structures. Sect. Levipedes species are characterized by a Dryophila-like pileipellis and easily identifiable cheilocystidia. Some species did not conform to typical clustering patterns. Therefore, comprehensive and systematic studies are needed for these groups.
The relationships within the genus Gymnopus are a subject of debate and contention. Section Levipedes has been subdivided into two subsections, subsect. Levipedes Antonín & Noordel. and subsect. Alkalivirens Antonín & Noordel., based on whether the pileus turns green in potassium hydroxide solution (KOH) [60]. Additionally, due to a lack of interest, G. erythropus/G. fagiphilus have often been grouped within subsect. Levipedes. However, previous studies suggest that G. erythropus/G. fagiphilus may have a more distant relationship with other species in subsect. Levipedes. Furthermore, our previous study of red stipe specimens from Northeast China also confirmed a polyphyletic relationship between these species [23]. Therefore, an in-depth investigation of Gymnopus is warranted.
Author Contributions
Conceptualization, Y.L., B.Z. and J.-J.H.; experimental design and methodology, Y.L., B.Z., J.-J.H. and Y.-L.T.; performance of practical work, J.-J.H., Z.-X.Q., D.-H.J. and X.-F.L.; statistical analyses, J.-J.H.; validation, J.-J.H. and B.Z.; writing—original draft preparation, J.-J.H.; writing—review and editing, B.Z.; supervision, Y.L. and B.Z.; project administration, J.-J.H., B.Z. and Y.L.; funding acquisition, J.-J.H., B.Z. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the National Natural Science Foundation of China (32400011); Youth Doctoral Program of Zhejiang Normal University for the study on species diversity of macrofungi in Baishanzu National Park (2023QB043), Zhejiang Normal University Doctoral Initiation Fund (YS304024921), Key R&D Projects in Jilin Province “Diversity and Conservation of Specialized Fungus Germplasm Resources in Different Forest Vegetation Types of Changbaishan Region” (20230202119NC), and Key R&D Projects in Jiangxi Province “Research on the Creation of Excellent Edible Mushroom Resources and High-Quality, Efficient Ecological Cultivation Technology” (20212BBF61002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would express the grateful to Jin-Bao Pu (Center for Medicinal Resources Research, Zhejiang Academy of Traditional Chinese Medicine, China) for specimen collection.
Conflicts of Interest
The authors state no conflicts of interest.
References
- Mata, J.L.; Hughes, K.W.; Petersen, R.H. An investigation of/Omphalotaceae (Fungi: Euagarics) with emphasis on the genus Gymnopus. Sydowia 2006, 58, 191–289. [Google Scholar]
- Wu, F.; Zhou, L.W.; Yang, Z.L.; Bau, T.; Li, T.H.; Dai, Y.C. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Huang, Y.; Qu, Y.; Zhang, G.; Wang, D. Analgesic effects of Marasmius androsaceus mycelia ethanol extract and possible mechanisms in mice. Braz. J. Med. Biol. Res. 2018, 51, e7124. [Google Scholar] [CrossRef] [PubMed]
- Persoon, C.H. Synopsis Methodica Fungorum 1; Apud Henricum Dieterich: Göttingen, Germany, 1801. [Google Scholar]
- Fries, E. Systema mycologicum: Sistens Fungorum Ordines, Genera et Species, Huc usque Cognitas; Ex Officina Berlingiana: Berling, Germany, 1821; Volume 1. [Google Scholar]
- Staude, F. Die Schwämme Mitteldeutschlands, Insbesondere des Herzogtums Coburg; Dietz: Coburg, Germany, 1857; Volume 1. [Google Scholar]
- Singer, R. The Agaricales in modern taxonomy. Lilloa 1949, 22, 1–832. [Google Scholar]
- Singer, R. The Agaricales in Modern Taxonomy, 2nd ed.; J. Cramer: Weinheim, Germany, 1962. [Google Scholar]
- Singer, R. The Agaricales in Modern Taxonomy, 3rd ed.; J. Cramer: Weinheim, Germany, 1975. [Google Scholar]
- Singer, R. The Agaricales in Modern taxonomy, 4th ed.; Koeltz Botanical Books: Koenigstein, Germany, 1986. [Google Scholar]
- Kühner, R.; Romagnesi, H. Flore Analytique des Champignons Supérieurs (Agarics, Bolets, Chanterelles): Comprenant les Espèces de l’Europe Occidentale et Centrale Ainsi que la Plupart de Celles de l’Algérie et du Maroc; Masson: Paris, Franch, 1953. [Google Scholar]
- Lennox, J. Collybioid genera in the Pacific Northwest. Mycotaxon 1979, 9, 117–231. [Google Scholar]
- Halling, R.E. The genus Collybia (Agaricales) in the northeastern United States and adjacent Canada. Mycologia 1983, 8, 1–148. [Google Scholar]
- Antonín, V.; Noordeloos, M.E. A monograph of Marasmius, Collybia and related genera in Europe. Part 1: Marasmius, Setulipes, and Marasmiellus. Libri. Botanici. 1993, 8, 1–229. [Google Scholar]
- Antonín, V.; Noordeloos, M.E. A monograph of Marasmius, Collybia and related genera in Europe. Part 2: Collybia, Gymnopus, Rhodocollybia, Crinipellis, Chaetocalathus, and additions to Marasmiellus. Libri. Botanici. 1997, 17, 1–256. [Google Scholar]
- Antonín, V.; Halling, R.; Noordeloos, M. Generic concepts within the groups of Marasmius and Collybia sensu lato. Mycotaxon 1997, 63, 359–368. [Google Scholar]
- Moncalvo, J.M.; Vilgalys, R.; Redhead, S.A.; Johnson, J.E.; James, T.Y.; Aime, M.C.; Hofstetter, V.; Verduin, S.J.; Larsson, E.; Baroni, T.J. One hundred and seventeen clades of euagarics. Mol. Phylogenet. Evol. 2002, 23, 357–400. [Google Scholar] [CrossRef]
- Mata, J.L.; Hughes, K.W.; Petersen, R.H. Phylogenetic placement of Marasmiellus juniperinus. Mycoscience 2004, 45, 214–221. [Google Scholar] [CrossRef]
- Wilson, A.; Desjardin, D. Phylogenetic relationships in the gymnopoid and marasmioid fungi (Basidiomycetes, euagarics clade). Mycologia 2005, 97, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Noordeloos, M.E.; Antonín, V. Contribution to a monograph of marasmioid and collybioid fungi in Europe. Czech Mycol. 2008, 60, 21–27. [Google Scholar] [CrossRef]
- Oliveira, J.J.; Vargas-Isla, R.; Cabral, T.S.; Rodrigues, D.P.; Ishikawa, N.K. Progress on the phylogeny of the Omphalotaceae: Gymnopus s. str., Marasmiellus s. str., Paragymnopus gen. nov. and Pusillomyces gen. nov. Mycol. Prog. 2019, 18, 713–739. [Google Scholar] [CrossRef]
- Petersen, R.H.; Hughes, K.W. Collybiopsis and its type species, Co. ramealis. Mycotaxon 2021, 136, 263–349. [Google Scholar] [CrossRef]
- Hu, J.J.; Zhao, G.P.; Tuo, Y.L.; Rao, G.; Zhang, Z.H.; Qi, Z.X.; Yue, L.; Liu, Y.J.; Zhang, T.; Li, Y.; et al. Morphological and Molecular Evidence Reveal Eight New Species of Gymnopus from Northeast China. J. Fungi 2022, 8, 349. [Google Scholar] [CrossRef]
- Antonín, V.; Noordeloos, M.E. A Monograph of Marasmioid and Collybioid Fungi in Europe; IHW Verl: Eching, Germany, 2010. [Google Scholar]
- Teng, S.Q. Fungi of China; Science Press, Academic Sinica: Beijing, China, 1963; pp. 599–601. [Google Scholar]
- Liu, B.; Rong, F.X.; Jing, H.S.; Cao, J.Z. Three New Species of Holobasidiomycetes from China. J. Shanxi Univ. 1984, 4, 48–52. [Google Scholar]
- Mešić, A.; Tkalčec, Z.; Deng, C.Y.; Li, T.H.; Pleše, B.; Ćetković, H. Gymnopus fuscotramus (Agaricales), a new species from southern China. Phytotaxa 2011, 117, 321–330. [Google Scholar] [CrossRef]
- Deng, S.F.; Li, T.H.; Jiang, Z.D.; Song, B. Gymnopus ramulicola sp. nov., a pinkish species from southern China. Mycotaxon 2016, 131, 663–670. [Google Scholar] [CrossRef]
- Li, J.P.; Antonín, V.; Gates, G.; Jiang, L.; Li, T.H.; Li, Y.; Song, B.; Deng, C.Y. Emending Gymnopus sect. Gymnopus (Agaricales, Omphalotaceae) by including two new species from southern China. MycoKeys 2022, 87, 183–204. [Google Scholar] [CrossRef]
- Li, J.P.; Li, Y.; Li, T.H.; Antonin, V.; Hosen, M.I.; Song, B.; Xie, M.L.; Feng, Z. A preliminary report of Gymnopus sect. Impudicae (Omphalotaceae) from China. Phytotaxa 2021, 497, 263–276. [Google Scholar] [CrossRef]
- Li, J.P.; Pan, M.C.; Li, Y.; Deng, C.Y.; Wang, X.M.; Zhang, B.X.; Li, C.T.; Li, Y. Morpho-Molecular Evidence Reveals Four Novel Species of Gymnopus (Agaricales, Omphalotaceae) from China. J. Fungi 2022, 8, 398. [Google Scholar] [CrossRef]
- Li, J.P.; Song, B.; Feng, Z.; Wang, J.; Deng, C.Y.; Yang, Y.H. A new species of Gymnopus sect. Androsacei (Omphalotaceae, Agaricales) from China. Phytotaxa 2021, 521, 1–14. [Google Scholar] [CrossRef]
- Hu, J.J.; Song, L.R.; Tuo, Y.L.; Zhao, G.P.; Lei, Y.; Zhang, B.; Li, Y. Multiple evidences reveal new species and a new record of smelly Gymnopus (Agaricales, Omphalotaceae) from China. Front. Microbiol. 2022, 13, 968617. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Zhao, G.P.; Tuo, Y.L.; Dai, D.; Guo, D.Z.; Rao, G.; Qi, Z.X.; Zhang, Z.H.; Li, Y.; Zhang, B. Morphology and molecular study of three new Cordycipitoid fungi and its related species collected from Jilin Province, northeast China. MycoKeys 2021, 83, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Royal Botanic Garden, E. Flora of British Fungi: Colour Identification Chart; HM Stationery Office: Edinburgh, UK, 1969. [Google Scholar]
- César, E.; Bandala, V.M.; Montoya, L.; Ramos, A. A new Gymnopus species with rhizomorphs and its record as nesting material by birds (Tyrannideae) in the subtropical cloud forest from eastern Mexico. MycoKeys 2018, 42, 21–34. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Cubeta, M.; Echandi, E.; Abernethy, T.; Vilgalys, R. Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 1991, 81, 1395–1400. [Google Scholar] [CrossRef]
- Morehouse, E.A.; James, T.Y.; Ganley, A.R.; Vilgalys, R.; Berger, L.; Murphy, P.J.; Longcore, J.E. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol. Ecol. 2003, 12, 395–403. [Google Scholar] [CrossRef]
- Coimbra, V.R.; Pinheiro, F.G.; Wartchow, F.; Gibertoni, T.B. Studies on Gymnopus sect. Impudicae (Omphalotaceae, Agaricales) from Northern Brazil: Two new species and notes on G. montagnei. Mycol. Prog. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Ryoo, R.; Antonín, V.; KA, K.H.; Tomšovský, M. Marasmioid and gymnopoid fungi of the Republic of Korea. 8. Gymnopus section Impudicae. Phytotaxa 2016, 286, 75–88. [Google Scholar] [CrossRef]
- Xu, J.; Yu, X.; Zhang, C.; Li, Y. Morphological characteristics and phylogenetic analyses revealed a new Calocybe (Lyophyllaceae, Basidiomycota) species from northeast China. Phytotaxa 2021, 490, 203–210. [Google Scholar] [CrossRef]
- Xu, J.; Yu, X.; Suwannarach, N.; Jiang, Y.; Zhao, W.; Li, Y. Additions to Lyophyllaceae sl from China. J. Fungi 2021, 7, 1101. [Google Scholar] [CrossRef]
- Petersen, R.H.; Hughes, K.W. Micromphale sect. Perforantia (Agaricales, Basidiomycetes); expansion and phylogenetic placement. MycoKeys 2016, 18, 1–122. [Google Scholar] [CrossRef]
- Thonpson, J. The CLUSTAL X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 24, 4876–4882. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Farris, J.S.; Kallersjo, M.; Kluge, A.G.; Bult, C. Testing significance of incongruence. Cladistics 1994, 10, 315–319. [Google Scholar]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods) 4.0 b10; Sinauer Associates: Sunderland, UK, 2002. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. RaxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Wilson, A.; Desjardin, D.; Horak, E. Agaricales of Indonesia: 5. The genus Gymnopus from Java and Bali. Sydowia 2004, 56, 137–210. [Google Scholar]
- Antonín, V.; Sedlák, P.; Tomšovský, M. Taxonomy and phylogeny of European Gymnopus subsection levipedes (Basidiomycota, Omphalotaceae). Persoonia 2013, 31, 179–187. [Google Scholar] [CrossRef]
- Hu, J.J. Taxonomic, Molecular Phylogenetic, and Biogeographic Evolution of Collybia s.l. in China. Ph.D. Thesis, Northeast Normal University, Changchun, China, 2024. [Google Scholar]
- Vilgalys, R.; Miller, O., Jr. Mating relationships within the Collybia dryophila group in Europe. Trans. Br. Mycol. Soc. 1987, 89, 295–300. [Google Scholar] [CrossRef]
- Vilgalys, R.; Miller, O., Jr. Morphological studies on the Collybia dryophila group in Europe. Trans. Br. Mycol. Soc. 1987, 88, 461–472. [Google Scholar] [CrossRef]
- Halling, R.E. Notes on Collybia V. Gymnopus section Levipedes in tropical South America, with comments on Collybia. Brittonia 1996, 48, 487–494. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).