Biodiversity of Herbivores Triggers Species Differentiation of Coprophilous Fungi: A Case Study of Snow Inkcap (Coprinopsis sect. Niveae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Morphological Characterization
2.2. DNA Extraction, PCR Amplification and DNA Sequencing
2.3. Alignment and Phylogenetic Analyses
| Taxon | Seq.-ID | Location | ITS | nrLSU | tef-1α | mtSSU | Reference |
|---|---|---|---|---|---|---|---|
| C. aesontiensis | LZ P-7614 (type) | Italy | KY554753 | KY554752 | [34] | ||
| C. afronivea | SFSU BAP 619 (type) | São Tomé | NR_148105 | [35] | |||
| C. afronivea | HMJAU46372 | China | MW822049 | OL376317 | [23] | ||
| C. afronivea | HMJAU67175 | China | OR921294 | This study | |||
| C. afronivea | HMJAU67192 | China | OR921295 | OR921344 | OR940179 | OR916193 | This study |
| C. afronivea | HMJAU67193 | China | OR921297 | This study | |||
| C. afronivea | HMJAU67194 | China | OR921298 | This study | |||
| C. afronivea | HMJAU67195 | China | OR921296 | This study | |||
| C. afronivea | HMJAU67196 | China | OR921282 | OR921333 | OR940170 | OR916192 | This study |
| C. atramentaria | SZMC-NL-4245 | Hungary | FN396123 | FN396347 | FN396225 | [36] | |
| C. caribaeonivea | PA-2023a ANGE1390 | Dominican Republic | OQ275140 | [37] | |||
| C. aesontiensis | LZ P-7614 (type) | Italy | KY554753 | KY554752 | [34] | ||
| C. cerkezii | CNF1/7253 (type) | Croatia | KX869912 | KX869913 | [38] | ||
| C. cinerea | SZMC-NL-2141 | Hungary | FN396149 | FN396190 | [36] | ||
| C. coniophorus | SZMC-NL-3414 | Hungary | FN396122 | FN396207 | [36] | ||
| C. cortinata | SZMC-NL-1621 | Hungary | FN396121 | FN396171 | FN396224 | [36] | |
| C. cothurnata | CBS 174.49 | Netherlands | MH856479 | MH868018 | [39] | ||
| C. furfuracea | HMJAU67156 (type) | China | OR921288 | OR921346 | OR940182 | OR916200 | This study |
| C. furfuracea | HMJAU67159 | China | OR921290 | This study | |||
| C. furfuracea | HMJAU67160 | China | OR921291 | This study | |||
| C. furfuracea | HMJAU67161 | China | OR921292 | This study | |||
| C. furfuracea | HMJAU67162 | China | OR921293 | OR921340 | OR940175 | OR916199 | This study |
| C. furfuracea | HMJAU67163 | China | OR921286 | This study | |||
| C. furfuracea | HMJAU67164 | China | OR921287 | This study | |||
| C. furfuracea | HMJAU67165 | China | OR921289 | This study | |||
| C. furfuracea | RAAA 2021 | Iraq | MZ265188 | [40] | |||
| C. furfuracea | Ghobad-Nejhad 4282 | Iran | MT535708 | MT554301 | [40] | ||
| C. igarashii | CBM-FB38829 (type) | Japan | AB854625 | [41] | |||
| C. igarashii | HMJAU67212 | China | OR921284 | OR921338 | OR916188 | This study | |
| C. igarashii | HMJAU67213 | Chin | OR921326 | This study | |||
| C. igarashii | HMJAU67214 | China | OR921324 | This study | |||
| C. iliensis | HMJAU67171 (type) | China | OR921305 | OR921331 | OR940169 | OR916195 | This study |
| C. iliensis | HMJAU67172 | China | OR921306 | OR921332 | This study | ||
| C. khorqinensis | HMJAU67147 (type) | China | OR921330 | OR921348 | OR940178 | OR940168 | This study |
| C. lagopus | SZMC-NL-0191 | Hungary | JN943127 | JQ045867 | [42] | ||
| C. marcescibilis | SZMC-NL-2140 | Hungary | FM878020 | FM876277 | FM897257 | [43] | |
| C. musae | JV06-179 | Sweden | NR_148070 | KC992965 | [44] | ||
| C. narcotica | SZMC-NL-2342 | Hungary | FM163180 | FM160729 | FN396290 | [45] | |
| C. nivea | 4585 | USA | JF907848 | [46] | |||
| C. nivea | SZMC-NL-0847 | Hungary | HQ847032 | HQ847117 | [36] | ||
| C. nivea | HMJAU58777 | China | MZ220450 | This study | |||
| C. nivea | HMJAU67201 | China | OR921317 | This study | |||
| C. nivea | HMJAU67145 | China | OR921309 | This study | |||
| C. nivea | HMJAU67202 | China | OR921315 | This study | |||
| C. nivea | HMJAU67203 | China | OR921307 | This study | |||
| C. nivea | HMJAU67204 | China | OR921313 | This study | |||
| C. nivea | HMJAU67205 | China | OR921314 | This study | |||
| C. nivea | HMJAU67206 | China | OR921312 | This study | |||
| C. nivea | HMJAU67207 | China | OR921308 | This study | |||
| C. nivea | HMJAU67208 | China | OR921281 | OR921339 | OR940174 | OR916189 | This study |
| C. nivea | HMJAU67209 | China | OR921310 | This study | |||
| C. nivea | HMJAU67210 | China | OR921311 | This study | |||
| C. nivea | HMJAU67211 | China | OR921316 | This study | |||
| C. psammophila | CNF 1/6401 | Libya | MK491274 | MK492278 | [47] | ||
| C. pseudomarcecibilis | AH:33711 | Spain | KY698008 | MF033345 | [48] | ||
| C. pseudonivea | SZMC:NL:2340 | Hungary | FM163181 | FM160728 | FN430698 | [41] | |
| C. pseudonivea | HMJAU46449 | China | MW822599 | OL376335 | [23] | ||
| C. pseudonivea | HFRG_EJ220922_1_FRDBI 28794927 | United Kingdom | OQ133583 | Unpublish-ed | |||
| C. pseudonivea | HMJAU67153 | China | OR921301 | OR921347 | OR940183 | OR916201 | This study |
| C. pseudonivea | HMJAU67198 | China | OR921328 | This study | |||
| C. pseudonivea | HMJAU67199 | China | OR921327 | This study | |||
| C. sericivia | HMJAU67200 (type) | China | OR921285 | OR921342 | OR940176 | OR916190 | This study |
| C. sericivia | HMJAU67201 | China | OR921317 | This study | |||
| C. sp. 1 | TPN-2017 CBM-FB42007 | Vietnam | LC259498 | [22] | |||
| C. sp. 2 | HMJAU67197 | China | OR921329 | OR921343 | OR940177 | OR916191 | This study |
| C. sp. 3 | HMJAU67173 | China | OR921300 | This study | |||
| C. sp. 3 | HMJAU67174 | China | OR921299 | OR921334 | OR940171 | OR916194 | This study |
| C. strossmayeri | SZMC-NL-0774 | Hungary | HQ847048 | HQ847129 | [34] | ||
| C. subigarashii | HMJAU67215 | China | OR921322 | This study | |||
| C. subigarashii | HMJAU67216 | China | OR921321 | This study | |||
| C. subigarashii | HMJAU67217 | China | OR921280 | OR921341 | OR916187 | This study | |
| C. subigarashii | HMJAU67218 | China | OR921279 | OR940180 | This study | ||
| C. subigarashii | HMJAU67219 | China | OR921283 | OR921345 | OR940181 | OR918461 | This study |
| C. subigarashii | HMJAU67220 | China | OR921318 | This study | |||
| C. subigarashii | HMJAU67221 | China | OR921319 | This study | |||
| C. subigarashii | HMJAU67222 | China | OR921320 | This study | |||
| C. subigarashii | HMJAU67223 (type) | China | OR921323 | This study | |||
| C. subigarashii | HMJAU67224 | China- | OR921325 | This study | |||
| C. tenuipes | HMJAU67168 | China | OR921304 | OR921337 | OR916198 | This study | |
| C. tenuipes | HMJAU67169 (type) | China | OR921303 | OR921335 | OR940172 | OR916197 | This study |
| C. tenuipes | HMJAU67170 | China | OR921302 | OR921336 | OR940173 | OR916196 | This study |
| C. udicola | AM1240 | Germany | KC992967 | KC992967 | KJ732831 | [44] | |
| C. utrifer | SZMC-NL-0591 | Hungary | FN396140 | FN396209 | [36] |
3. Results
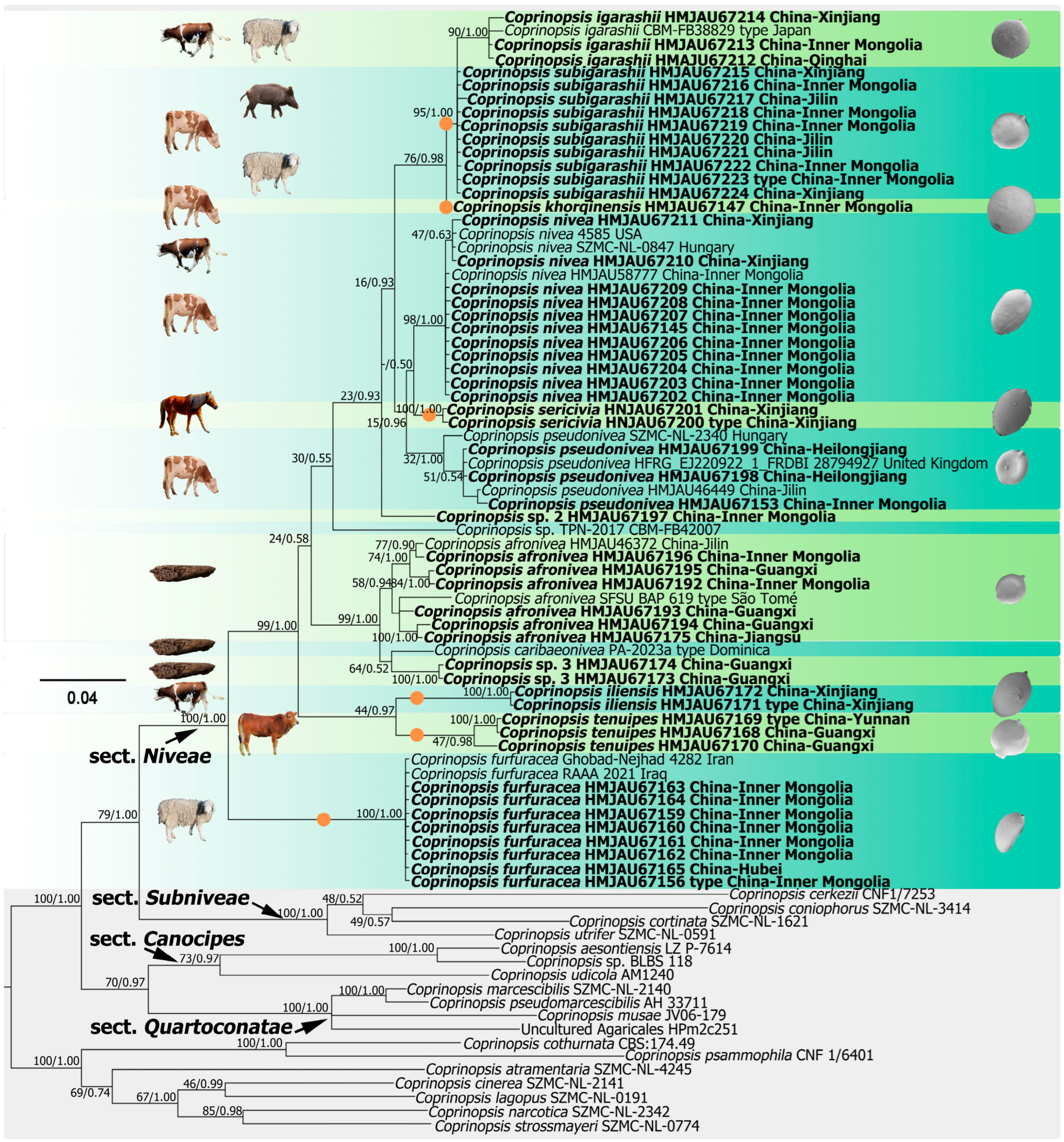
3.1. Molecular Phylogeny
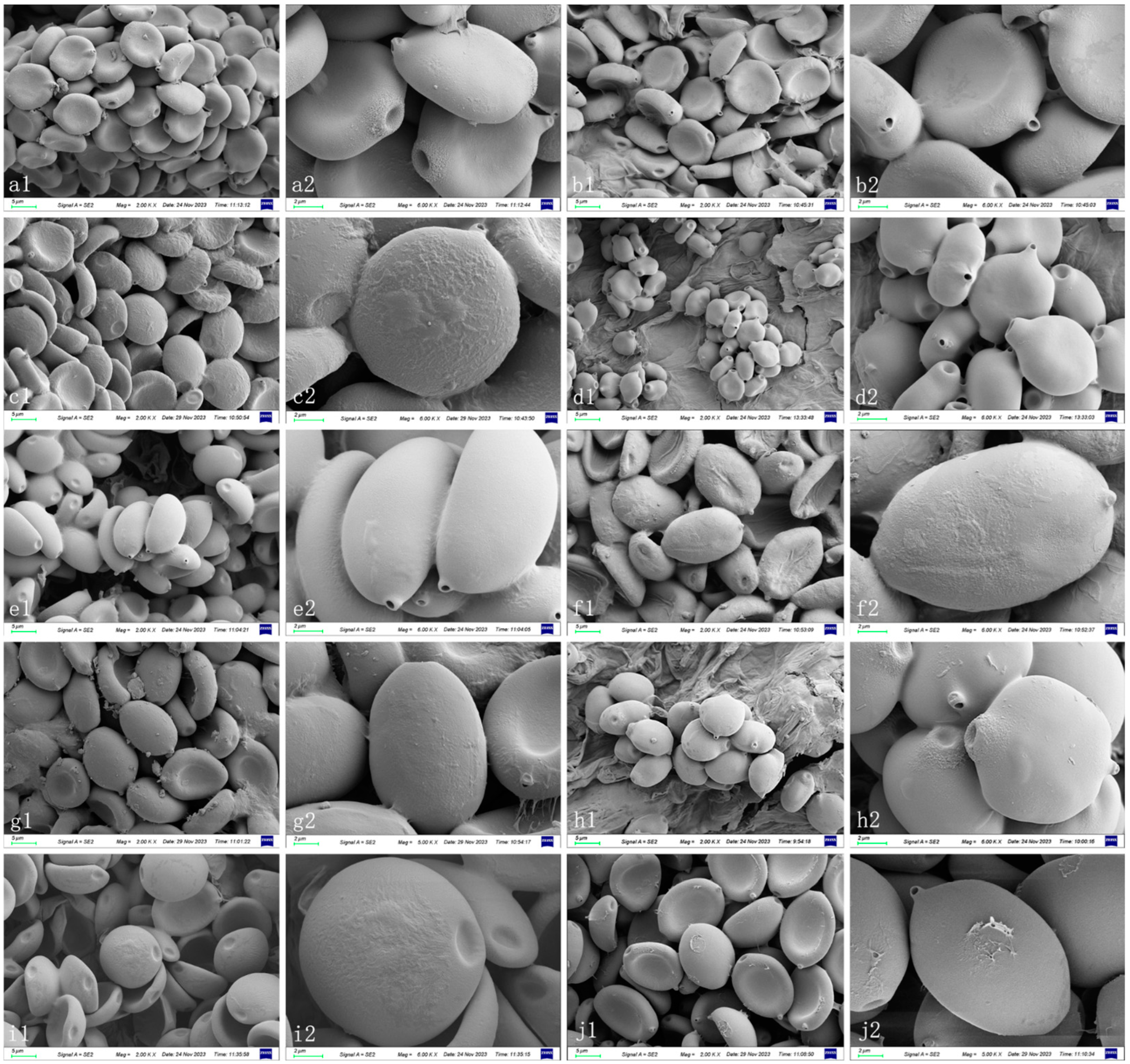
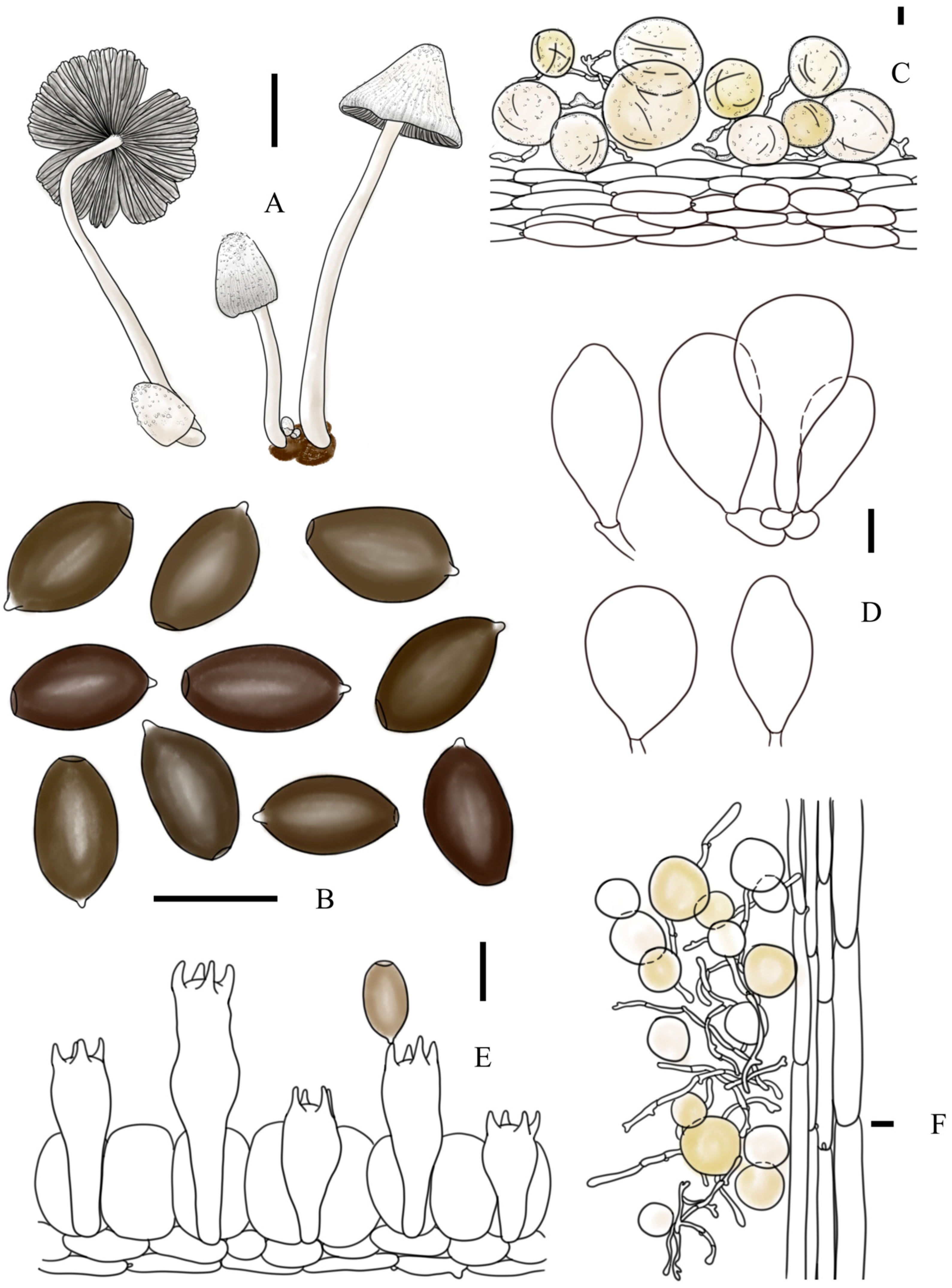
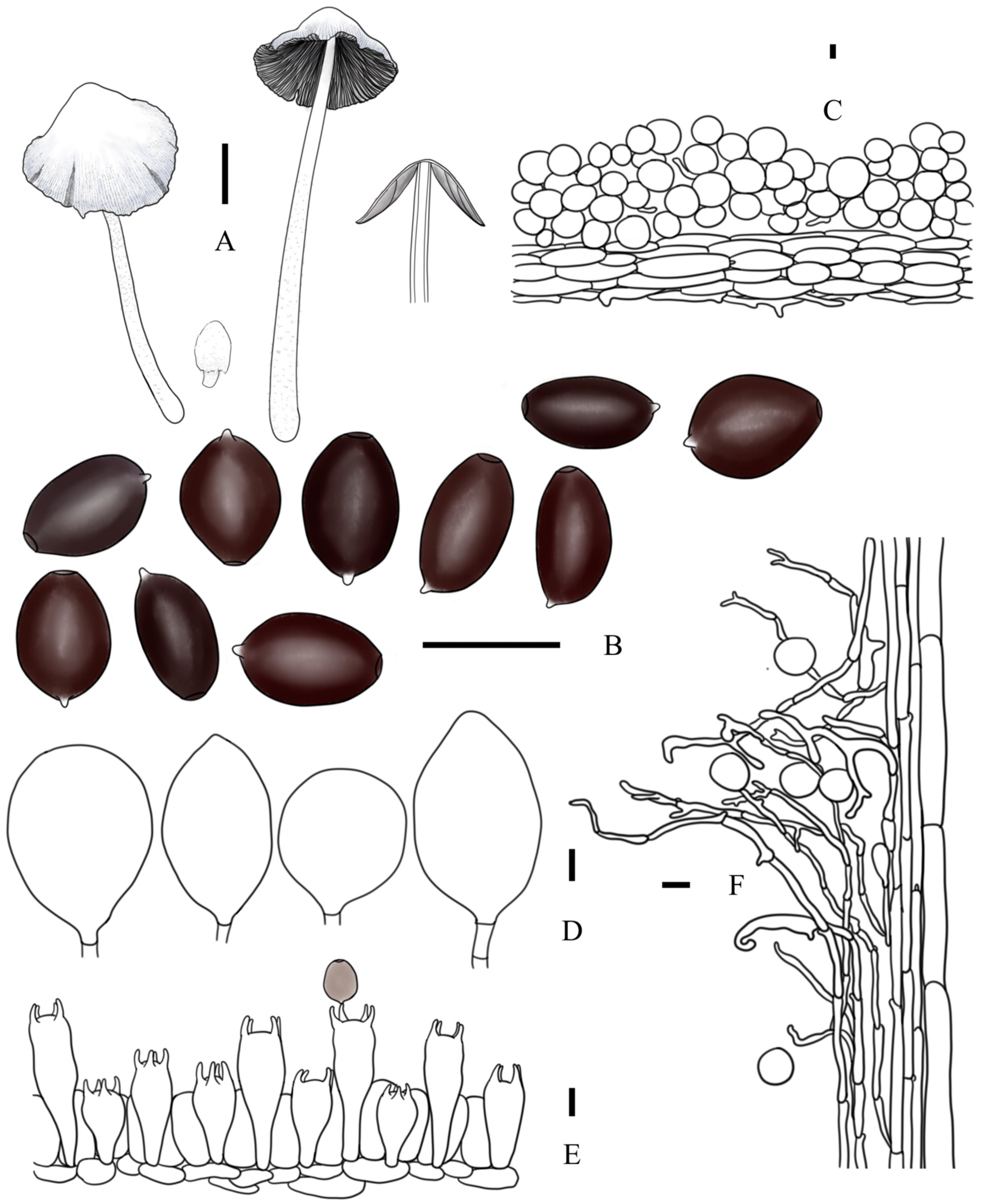
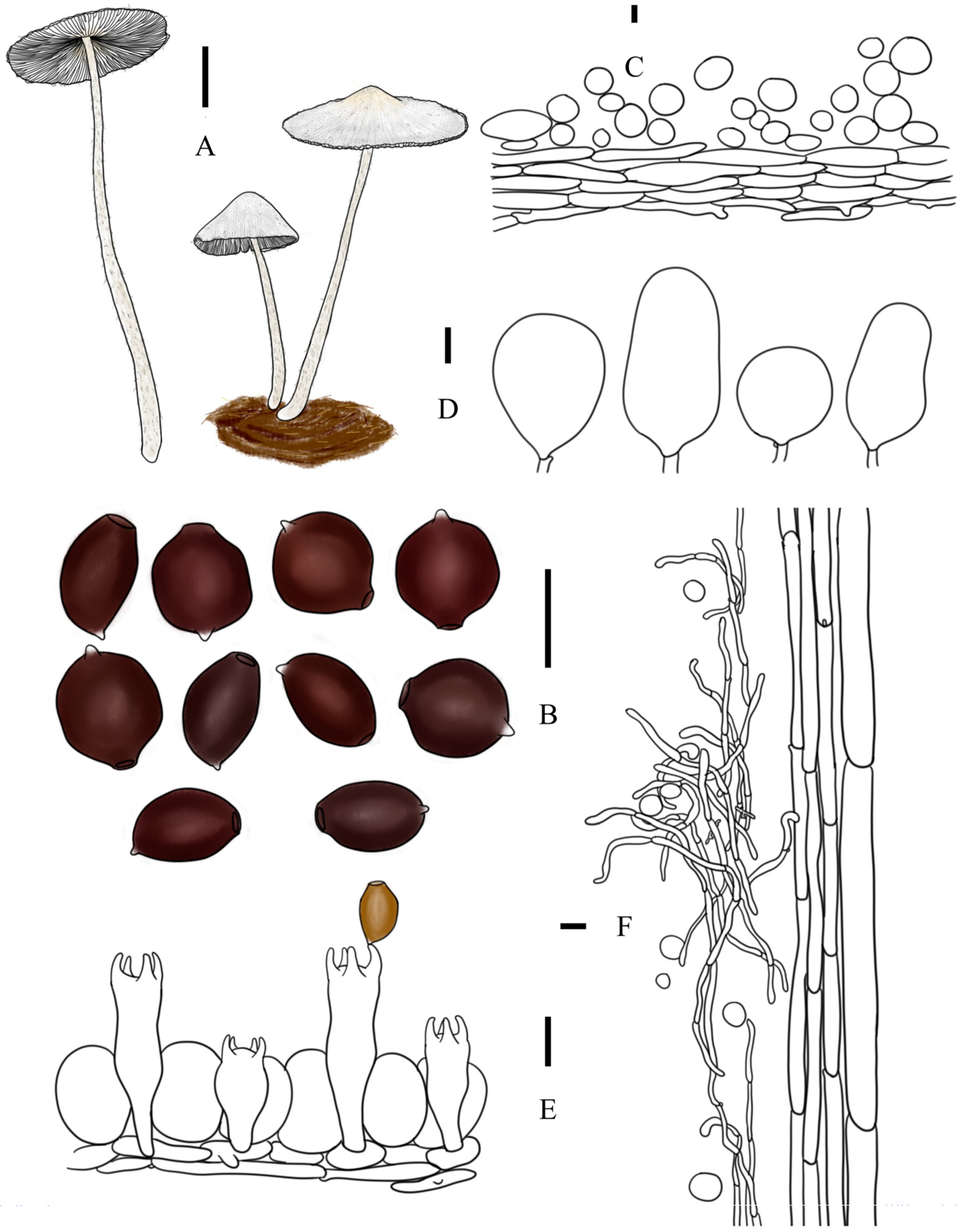
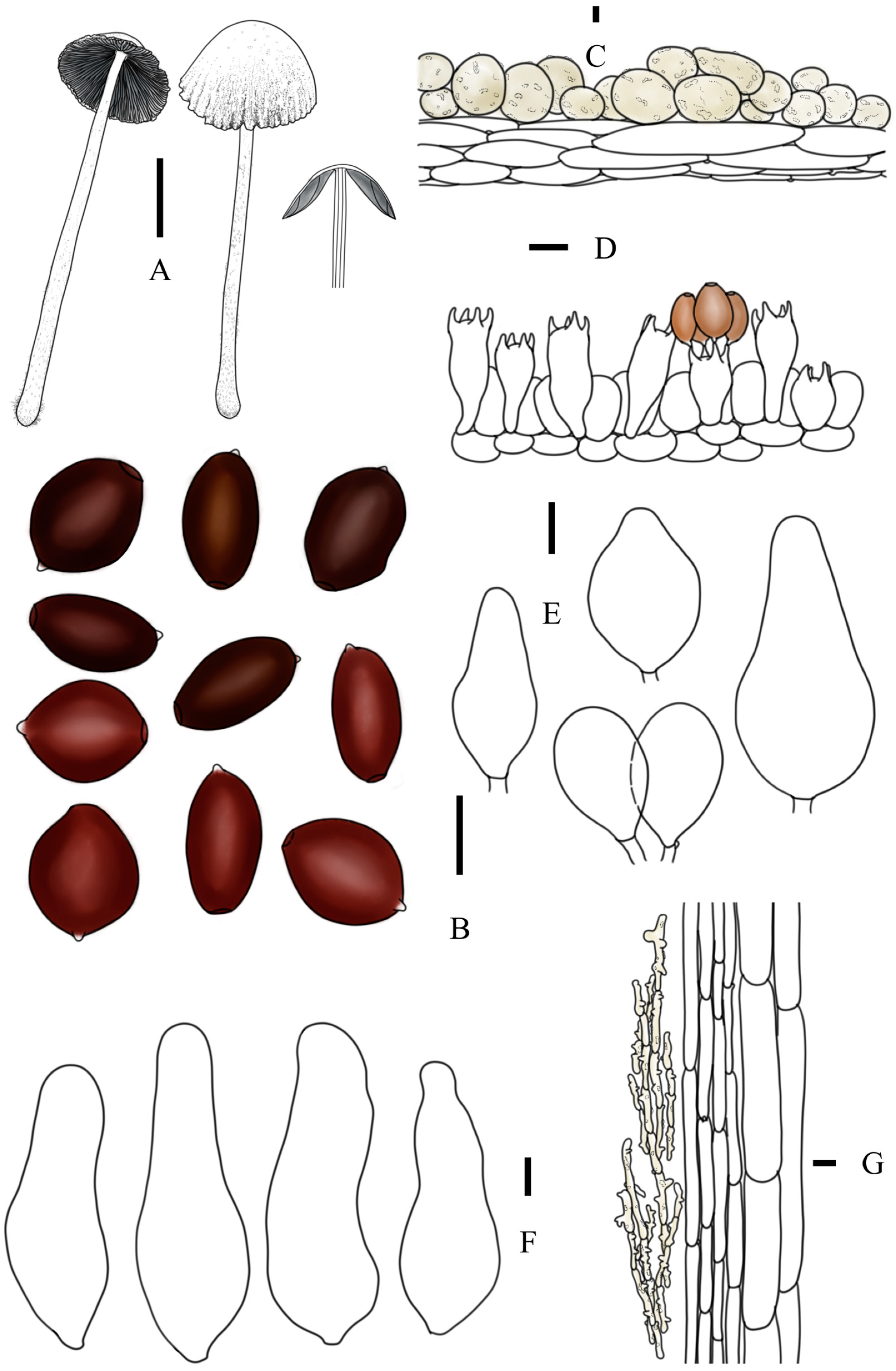
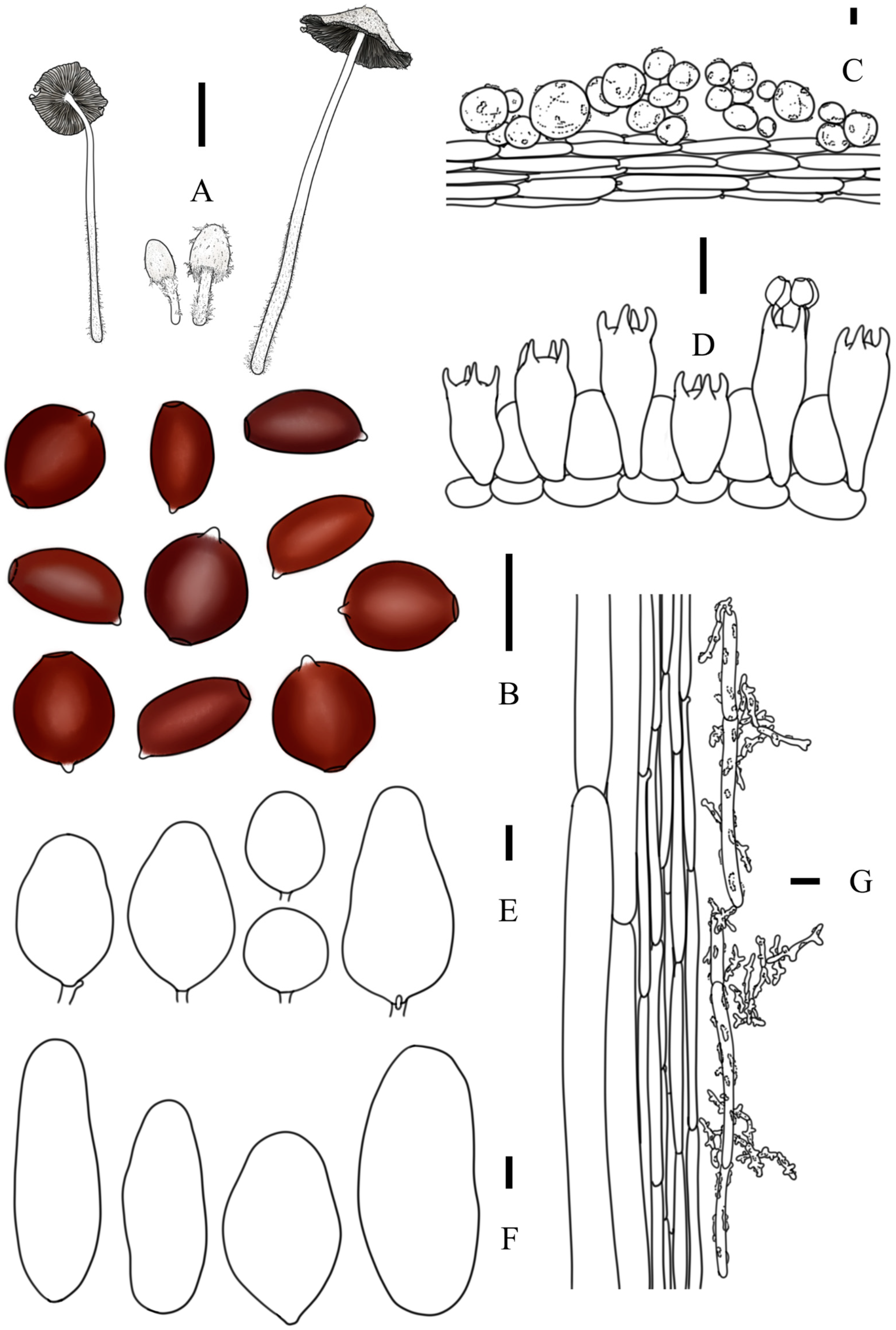
3.2. Taxonomy
3.3. Key to Species of Coprinopsis sect. Niveae
| 1 | Growing on little dead branches of deciduous trees or other plant residues ………………………………………… 2 |
| 1′ | Growing on herbivore dung or nutrient-rich soil …………………………………………………………………………3 |
| 2 | Diameter of pileus veil cells less than 40 μm ………………………………………………………………… C. afronivea |
| 2′ | Diameter of pileus veil cells more than 40 μm ……………………………………………………………C. caribaeonivea |
| 3 | Basidiomata small-sized, diameter of pileus usually less than 1.0 cm ………………………………………………… 4 |
| 3′ | Basidiomata middle- to large-sized, diameter of pileus usually larger than 1.5 cm. …………………………………6 |
| 4 | Central of pileus with light brown hue or pink-brown hue. ………………………………………………C. pseudonivea |
| 4′ | Central of pileus (almost) without light brown hue or pink-brown hue. ………………………………………………5 |
| 5 | Basidiopores five-angular or heart-shaped in frontal view, which with rough surface.………………… C. igarashii |
| 5′ | Basidiopores subglobose, rhomboid or limoniform in frontal view, which with smooth surface. ……C. subigarashii |
| 6 | Pileus with radiate plication. ……………………………………………………………………………………C. furfuracea |
| 6′ | Pileus without radiate plication. ……………………………………………………………………………………………7 |
| 7 | Pileus snowy white when mature. …………………………………………………………………………………………8 |
| 7′ | Pileus greyish white when mature. ………………………………………………………………………………………10 |
| 8 | Growing on horse dung. …………………………………………………………………………………………C. sericivia |
| 8′ | Growing on cow dung. ………………………………………………………………………………………………………9 |
| 9 | Pleurocystidia present. ………………………………………………………………………………………………C. nivea |
| 9′ | Pleurocystidia absent. ………………………………………………………………………………………………C. iliensis |
| 10 | Length of basidiospores less than 10 μm.……………………………………………………………………… C. tenuipes |
| 10′ | Length of basidiospores 11–14 μm. ……………………………………………………………………………C. khorqiensis |
4. Discussion
4.1. Species Delimitation of Coprinopsis sect. Niveae
4.2. Revision of Classification of Fecal Species in sect. Niveae Based on Ecological and Geographical Features
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Persoon, C.H. Tentamen Dispositionis Methodicae Fungorum in Classes, Ordines, Genera et Familiascum Supplemento Adjecto i-iv, 2nd ed.; Apud Petrum Philippum Wolf: Leipzig, Germany, 1797; pp. 1–76. [Google Scholar]
- Baker, A.G.; Bhagwat, S.A.; Willis, K.J. Do dung fungal spores make a good proxy for past distribution of large herbivores? Quat. Sci. Rev. 2013, 62, 21–31. [Google Scholar] [CrossRef]
- Cugny, C.; Mazier, F.; Galop, D. Modern and fossil non-pollen palynomorphs from the Basque mountains (western Pyrenees, France): The use of coprophilous fungi to reconstruct pastoral activity. Veg. Hist. Archaeobot. 2010, 19, 391–408. [Google Scholar] [CrossRef]
- Van Geel, B.; Buurman, J.; Brinkkemper, O.; Schelvis, J.; Aptroot, A.; Van Reenen, G.; Hakbijl, T. Environmental reconstruction of a Roman Period settlement site in Uitgeest (The Netherlands), with special refer ence to coprophilous fungi. J. Archaeol. Sci. 2003, 30, 873–883. [Google Scholar] [CrossRef]
- Persoon, C.H. Synopsis Methodica Fungirum; Apud Henricum Dieterich: Gottingae, Germany, 1801; pp. 1–400. [Google Scholar]
- Massee, G. A revision of the genus Coprinus. Ann. Bot. 1896, 10, 123–184. [Google Scholar] [CrossRef]
- Buller, A.H.R. The Function and Fate of the Cystidia of Coprinus atramentarius, together with some General Remarks on Coprinus Fruit-bodies. Ann. Bot. 1910, 24, 613–628. [Google Scholar] [CrossRef]
- Buller, A.H.R. Some critical remarks on the generic positions of Psathyra urticaecola Berk. et Broome, Coprinus plicatilis Fr., and Psathyrella disseminata Pers. Trans. Br. Mycol. Soc. 1914, 5, 482–489. [Google Scholar] [CrossRef]
- Fires, E.M. Systematis Mycologici seu Synopsis Hymenomycetum; Upsaliæ: Stockholm, Sweden, 1836–1838; pp. 1–612. [Google Scholar]
- Lange, J.E. Studies in the Agarics of Denmark. II. Dansk bot. Ark. 1915, 2, 32–53. [Google Scholar]
- Lange, J.E. Flora Agaricina Danica, Vol. 4; Recato: Copenhagen, Denmark, 1939; pp. 104–114. [Google Scholar]
- Kühner, R.; Romagnesi, H. Flore Analytique des Champignons Superieurs; Masson et Cie: Paris, France, 1953; pp. 1–385. [Google Scholar]
- Kits van Waveren, E. The ‘stercorarius group’ of the genus Coprinus. Persoonia 1968, 5, 131–176. [Google Scholar]
- Orton, P.D.; Watling, R. Agarics and Boleti. Br. Fung. Fl. 2/Coprinacae. Part 1: Coprinus; Royal Botanic Garden Edinburgh: Edinburgh, UK, 1979; pp. 1–149. [Google Scholar]
- Citerin, M. Clé analitique du Genre Coprinus. Docums. Mycol. 1992, 86, 1–28. [Google Scholar]
- Uljé, C.B.; Noordeloos, M.E. Studies in Coprinus III—Coprinus section Veliformes. Subdivision and revision of subsection Nivei emend. Persoonia 1993, 15, 257–301. [Google Scholar]
- Hopple, J.S., Jr.; Vilgalys, R. Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 1994, 86, 96–107. [Google Scholar] [CrossRef]
- Hopple, J.S., Jr.; Vilgalys, R. Phylogenetic relationships in the mushroom genus Coprinus and dark-spored allies based on sequence data from the nuclear gene coding for the large ribosomal subunit RNA: Divergent domains, outgroups, and monophyly. Mol. Phylogenet. Evol. 1999, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Redhead, S.A.; Vilgalys, R.; Moncalvo, J.M.; Johnson, J.; Hopple, J.S., Jr. Coprinus Pers. and the disposition of Coprinus species sensu lato. Taxon 2001, 50, 203–241. [Google Scholar] [CrossRef]
- Schafer, D.J. Keys to sections of Parasola, Coprinellus, Coprinopsis and Coprinus in Britain. Field Mycol. 2010, 11, 44–51. [Google Scholar] [CrossRef]
- Gierczyk, B.; Rodriguez-Flakus, P.; Pietras, M.; Gryc, M.; Czerniawski, W.; Piątek, M. Coprinopsis rugosomagnispora: A distinct new coprinoid species from Poland (Central Europe). Plant Syst. Evol. 2017, 303, 915–925. [Google Scholar] [CrossRef][Green Version]
- Wächter, D.; Melzer, A. Proposal for a subdivision of the family Psathyrellaceae based on a taxon-rich phylogenetic analysis with iterative multigene guide tree. Mycol. Prog. 2020, 19, 1151–1265. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Huang, M.; Bau, T. Taxonomy of coprinoid fungi in China. Mycosystema 2022, 41, 878–898. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, H. Methuen Handbook of Colour; Methuen & Co., Ltd.: London, UK, 1978; pp. 1–30. [Google Scholar]
- Bas, C. Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 1969, 5, 285–573. [Google Scholar]
- Zhu, L.Y.; Bau, T. Species clarification of fairy inkcap (“Coprinellus disseminatus”) in China. Mycology 2024, 15, 424–470. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Bau, T. Species diversity of Tulosesus (Psathyrellaceae, Agaricales) in China. Mycosystema 2024, 43, 230300. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Bau, T. Four new species of Cystolepiota (Agaricaceae, Agaricales) from northeastern China. Front. Microbiol. 2024, 15, 1358612. [Google Scholar] [CrossRef] [PubMed]
- Mou, G.F.; Bau, T. Asproinocybaceae fam. nov. (Agaricales, Agaricomycetes) for Accommodating the Genera Asproinocybe and Tricholosporum, and Description of Asproinocybe sinensis and Tricholosporum guangxiense sp. nov. J. Fungi 2021, 7, 1086. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bact. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Hughes, K.W.; Petersen, R.H.; Lodge, D.J.; Bergemann, D.J.; Baumgartner, K.; Tulloss, R.E.; Lickey, E.; Cifuentes, J. Evolutionary consequences of putative intra-and interspecific hybridization in agaric fungi. Mycologia 2013, 105, 1577–1594. [Google Scholar] [CrossRef]
- Melzer, A.; Ferisin, G.; Dovana, F. Coprinopsis aesontiensis, una nuova specie trovata in Friuli Venezia Giulia, Italia. Micol. Veget. Medit. 2017, 31, 123–132. [Google Scholar]
- Desjardin, D.E.; Perry, B.A. Dark-spored species of Agaricineae from Republic of São Tomé and Príncipe, West Africa. Mycosphere 2016, 7, 359–391. [Google Scholar] [CrossRef]
- Nagy, L.G.; Walther, G.; Hazi, J.; Vágvölgyi, C.; Papp, T. Understanding the evolutionary processes of fungal fruiting bodies: Correlated evolution and divergence times in the Psathyrellaceae. Syst. Biol. 2011, 60, 303–317. [Google Scholar] [CrossRef]
- Angelini, C.; Voto, P.; Alvarado, P. First report of coprinoid fungi (Psathyrellaceae, Agaricales) in the Dominican Republic. Mycol. Obs. 2023, 6, 54–76. [Google Scholar]
- Tibpromma, S.; Hyde, K.D.; Jeewon, R.; Maharachchikumbura, S.S.N.; Liu, J.K. Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 1–261. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; De Vries, M.; Gehrmann, T.; Stielow, B. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Al Anbagi, R.A.; Suliaman, S.Q.; Al-Khesraji, T.O. Morphological and Molecular Identification of New species of Coprinopsis iraqicus sp. nov. from Iraq. Indian J. Ecol. 2022, 49, 1424–1432. [Google Scholar] [CrossRef]
- Fukiharu, T.; Shimizu, K.; Nakajima, A.; Miyamoto, T.; Raut, J.K.; Kinjo, N. Coprinopsis igarashii sp. nov., a coprophilous agaric fungus from Hokkaido, northern Japan. Mycoscience 2015, 56, 413–418. [Google Scholar] [CrossRef]
- Nagy, L.G.; Desjardin, D.E.; Vágvölgyi, C.; Kemp, R.; Papp, T. Phylogenetic analyses of Coprinopsis sections Lanatuli and Atramentarii identify multiple species within morphologically defined taxa. Mycologia 2013, 105, 112–124. [Google Scholar] [CrossRef][Green Version]
- Nagy, L.G.; Urban, A.; Örstadius, L.; Papp, T.; Larsson, E.; Vágvölgyi, C. The evolution of autodigestion in the mushroom family Psathyrellaceae (Agaricales) inferred from Maximum Likelihood and Bayesian methods. Mol. Phylogenet. Evol. 2010, 57, 1037–1048. [Google Scholar] [CrossRef]
- Örstadius, L.; Ryberg, M.; Larsson, E. Molecular phylogenetics and taxonomy in Psathyrellaceae (Agaricales) with focus on psathyrelloid species: Introduction of three new genera and 18 new species. Mycol. Prog. 2015, 14, 1–42. [Google Scholar] [CrossRef]
- Nagy, L.G.; Kocsubé, S.; Papp, T.; Vágvölgyi, C. Phylogeny and character evolution of the coprinoid mushroom genus Parasola as inferred from LSU and ITS nrDNA sequence data. Persoonia 2009, 22, 28–37. [Google Scholar] [CrossRef]
- Osmundson, T.W.; Robert, V.A.; Schoch, C.L.; Baker, L.J.; Smith, A. Filling gaps in biodiversity knowledge for macrofungi: Contributions and assessment of an herbarium collection DNA barcode sequencing project. PLoS ONE 2013, 8, e62419. [Google Scholar] [CrossRef]
- Hyde, K.D.; Tennakoon, D.S.; Jeewon, R.; Bhat, D.J.; Maharachchikumbura, S.S.N. Fungal diversity notes 1036–1150: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2019, 96, 1–242. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.S.J.; Barber, P.A.; Alvarado, P.; Barnes, C.W.; Buchanan, P.K.; Heykoop, M.; Moreno, G.; et al. Fungal Planet description sheets: 558–624. Persoonia 2017, 38, 240–384. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 2016, 32, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Farris, J.S.; Kallersjo, M.; Kluge, A.G.; Bult, C. Testing significance of incongruence. Cladistics 1994, 10, 315–319. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods); Version 4.0b10; Sinauer Associate: Sunderland, MA, USA, 2002. [Google Scholar]
- Cunningham, C.W. Can three incongruence tests predict when data should be combined? Mol. Biol. Evol. 1997, 14, 733–740. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Hillis, D.M. Success of phylogenetic methods in the four-taxon case. Syst. Biol. 1993, 42, 247–264. [Google Scholar] [CrossRef]
- Leaché, A.D.; Reede, T.W. Molecularsystematics of the eastern fence lizard (Sceloporus undulatus): A comparison of parsimony, likelihood, and Bayesian approaches. Syst. Biol. 2002, 51, 44–68. [Google Scholar] [CrossRef]
- Uljé, C.B. Four new species of Coprinus from the Netherlands. Persoonia 1988, 13, 479–488. [Google Scholar]
- Redhead, S.A.; Norvell, L.L. Report of the Nomenclature Committee for Fungi 19: Official repositories for fungal names. Taxon 2013, 62, 173–174. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018; pp. 1–310. [Google Scholar]
- Aime, M.C.; Miller, A.N.; Aoki, T.; Bensch, T.; Cai, L. How to publish a new fungal species, or name, version 3.0. IMA Fungus 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Doveri, F.; Granito, V.M.; Lunghini, D. Nuovi ritrovamenti di Coprinus sl fimicoli in Italia. Riv. Micol. 2005, 48, 319–340. [Google Scholar]
- Prydiuk, M.P. New records of dung inhabiting Coprinus species in Ukraine II. Section Coprinus. Czech Mycol. 2011, 63, 13–32. [Google Scholar] [CrossRef]
- Van Vuure, T. History, morphology and ecology of the Aurochs (Bos taurus primigenius). Lutra 2002, 45, 3–17. [Google Scholar]
- Bonfiglio, S.; Ginja, C.; De Gaetano, A.; Achilli, A. Origin and spread of Bos taurus: New clues from mitochondrial genomes belonging to haplogroup T1. PLoS ONE 2012, 7, e38601. [Google Scholar] [CrossRef]
- Olivieri, A.; Gandini, F.; Achilli, A.; Fichera, A. Mitogenomes from Egyptian cattle breeds: New clues on the origin of haplogroup Q and the early spread of Bos tsautus from the Near East. PLoS ONE 2015, 10, e0141170. [Google Scholar] [CrossRef]
- Angel, S.K.; Wicklow, D.T. Relationships between coprophilous fungi and fecal substrares in Colorado grassland. Mycologia 1975, 67, 63–74. [Google Scholar] [CrossRef]
- Kruys, Å.; Ericson, L. Species richness of coprophilous ascomycetes in relation to variable food intake by herbivores. Fungal Divers. 2008, 30, 73–81. [Google Scholar]
- Lokare, P.; Fatima, S. Study of diversity if coprophilous fungi from selected dung sample. In Research Interventions and Advancements in Plant Science; Desai, N., Pawar, U., Aparadh, V., Patil, M., Eds.; Bhumi Publishing: Maharashtra, India, 2020; pp. 259–274. [Google Scholar]
- Halbwachs, H.; Bässler, C. Gone with the wind—A review on basidiospores of lamellate agarics. Mycosphere 2015, 6, 78–112. [Google Scholar] [CrossRef]
- Wheeler, W.; Noller, C. Gasteointestinal tract pH and starch in feces of ruminants. J. Anim. Sci. 1977, 44, 131–135. [Google Scholar] [CrossRef]
- Russell, J.B. Rumen Microbiology and Its Role in Ruminant Nutrition; Cornell University Press: Ithaca, NY, USA, 2002. [Google Scholar]
- Van Weyenberg, S.; Sales, J.; Janssens, G.P.J. Passage rate of digesta through the equine gastrointestinal tract: A review. Livest. Sci. 2006, 99, 3–12. [Google Scholar] [CrossRef]
- Ran, L.; Yang, Y.; Qiu, M.; Brunson, K. Direct dating of the earliest domesticated cattle and caprines in northwestern China reveals the history of pastoralism in the Gansu-Qinghai region. J. Archaeol. Sci. 2002, 44, 105627. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, N.; Zhu, S.; Chen, Q. Ancient DNA reveals evidence of abundant aurochs (Bos primigenius) in Neolithic Northeast China. J. Archeaol. Sci. 2018, 98, 72–80. [Google Scholar] [CrossRef]
- Frelius, M.; Koolmees, P.A.; Theunissen, B.; European Cattle Genetic Diversity Consortium. On the breeds of cattle—Historic and current classifications. Diversity 2011, 3, 660–692. [Google Scholar] [CrossRef]
- Lundqvist, N. Nordic Sordariaceae s. lat. Symb. Bot. Ups. 1972, 20, 1–374. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Bau, T. Biodiversity of Herbivores Triggers Species Differentiation of Coprophilous Fungi: A Case Study of Snow Inkcap (Coprinopsis sect. Niveae). J. Fungi 2024, 10, 835. https://doi.org/10.3390/jof10120835
Zhu L, Bau T. Biodiversity of Herbivores Triggers Species Differentiation of Coprophilous Fungi: A Case Study of Snow Inkcap (Coprinopsis sect. Niveae). Journal of Fungi. 2024; 10(12):835. https://doi.org/10.3390/jof10120835
Chicago/Turabian StyleZhu, Liyang, and Tolgor Bau. 2024. "Biodiversity of Herbivores Triggers Species Differentiation of Coprophilous Fungi: A Case Study of Snow Inkcap (Coprinopsis sect. Niveae)" Journal of Fungi 10, no. 12: 835. https://doi.org/10.3390/jof10120835
APA StyleZhu, L., & Bau, T. (2024). Biodiversity of Herbivores Triggers Species Differentiation of Coprophilous Fungi: A Case Study of Snow Inkcap (Coprinopsis sect. Niveae). Journal of Fungi, 10(12), 835. https://doi.org/10.3390/jof10120835






