Biotransformation of the Fluoroquinolone Antibiotic, Levofloxacin, by the Free and Immobilized Secretome of Coriolopsis gallica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fungal Strain and Culture Conditions
2.3. Production of Coriolopsis gallica Secretome
2.4. Purification of Laccase
2.5. Determination of Laccase Activity
2.6. Immobilization of Coriolopsis gallica Proteins
2.7. Determination of the Percentage of Levofloxacin Biotransformation
2.8. Determination of the Effect of Physical–Chemical Factors on Levofloxacin Biotransformation by the Coriolopsis gallica Secretome Proteins
2.9. UHPLC–UV and HR-MS Analyses of Levofloxacin in the Presence of Coriolopsis gallica Secretome or Laccase for the Dereplication of Levofloxacin Degradation Products
3. Results
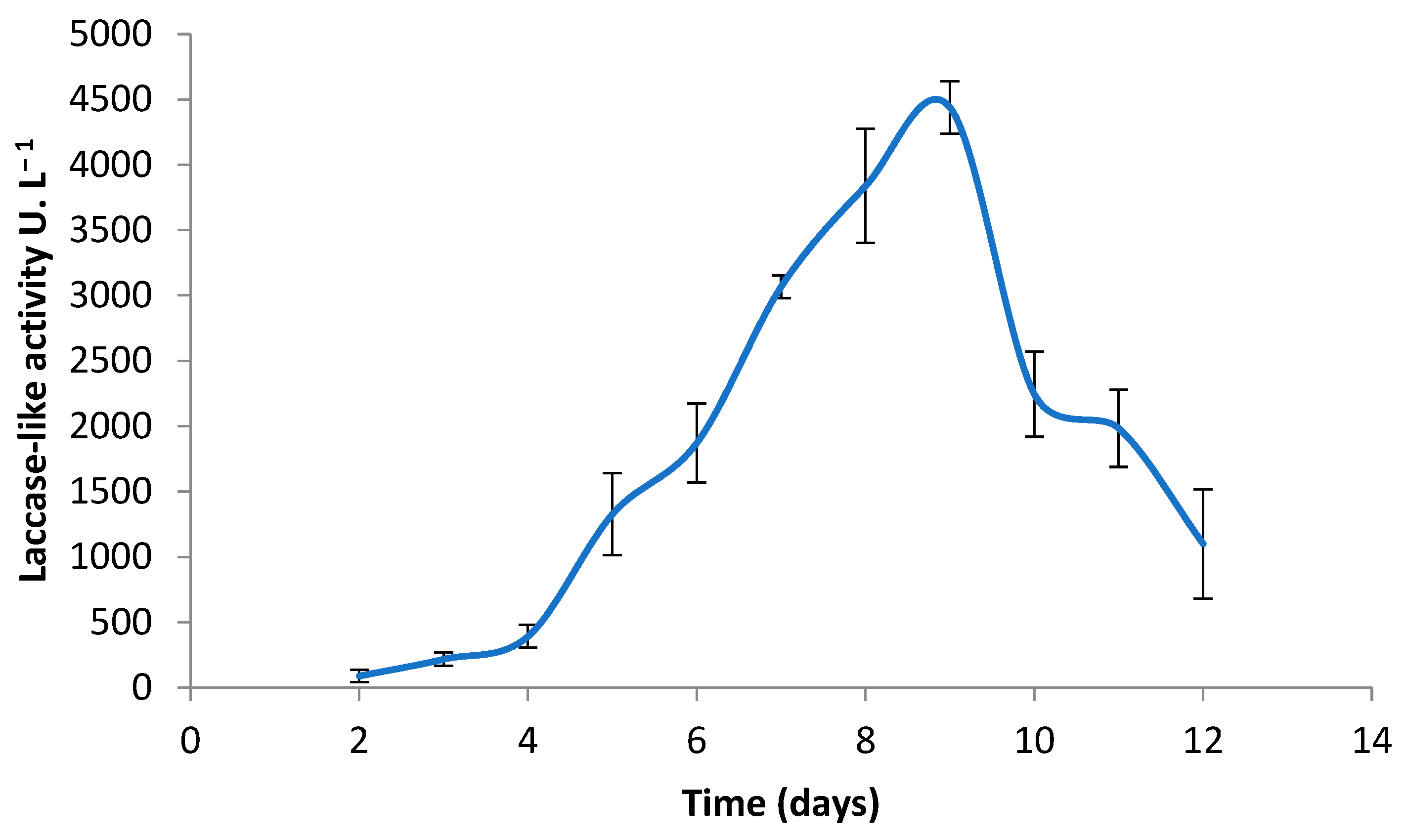
3.1. Production of Coriolopsis gallica Secretome
3.2. Purification of Laccase
3.3. Laccase Immobilization into Alginate Beads
3.4. Effect of Physical–Chemical Factors on Levofloxacin Biotransformation by Free and Immobilized C. gallica Secretome
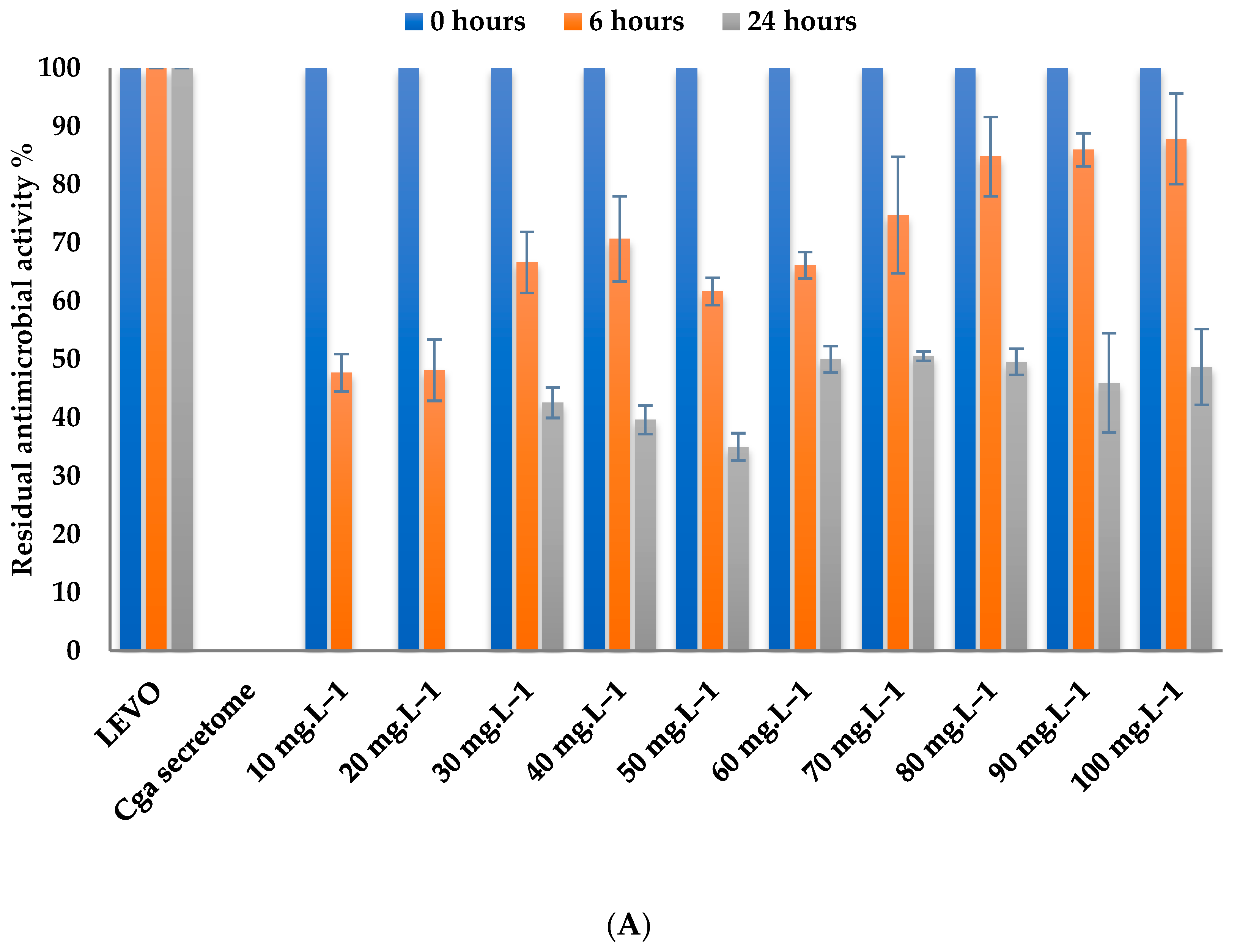
3.4.1. Effect of Levofloxacin Concentration
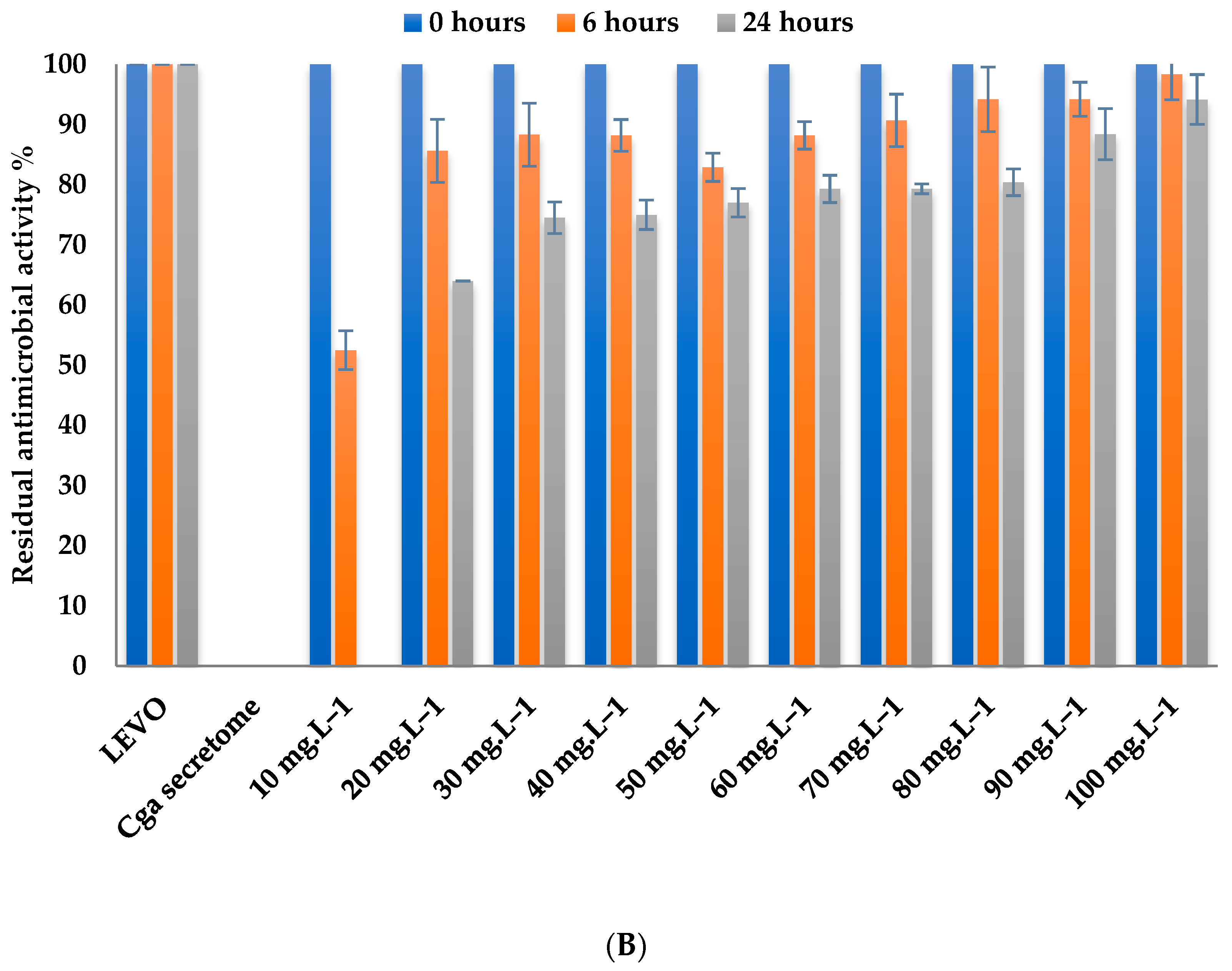
3.4.2. Effect of Mediator Concentration
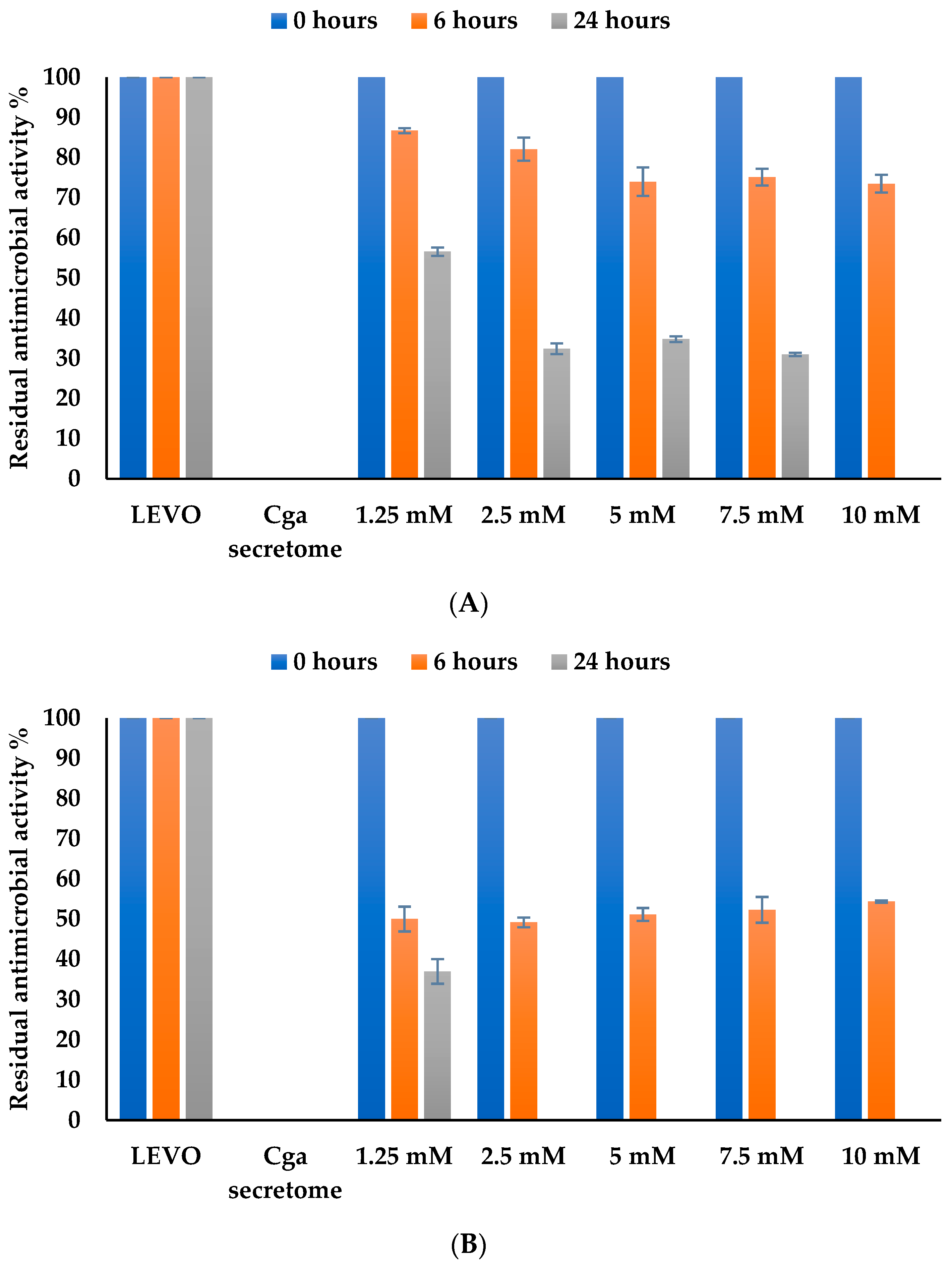
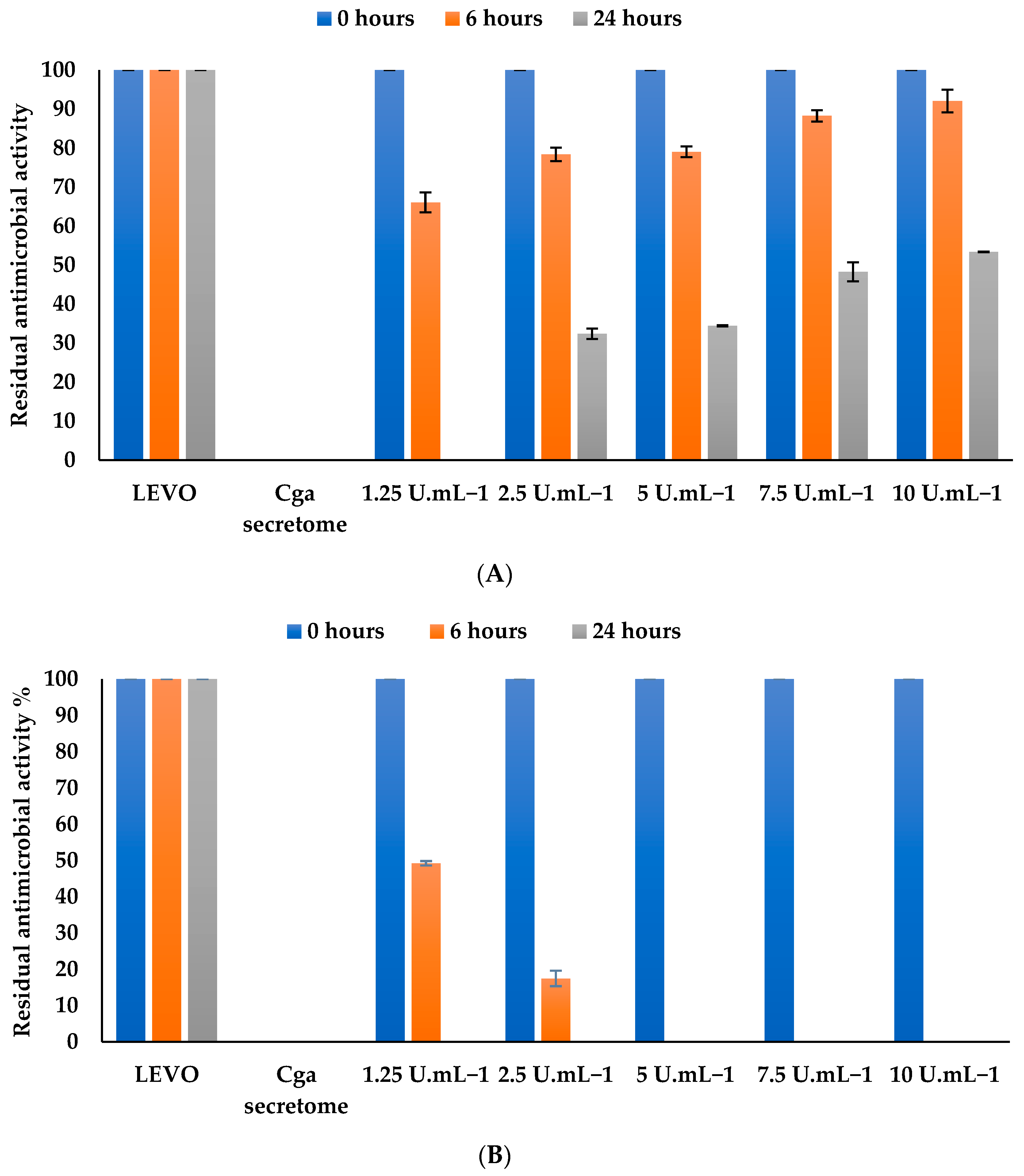
3.4.3. Effect of Enzyme Concentration on Levofloxacin
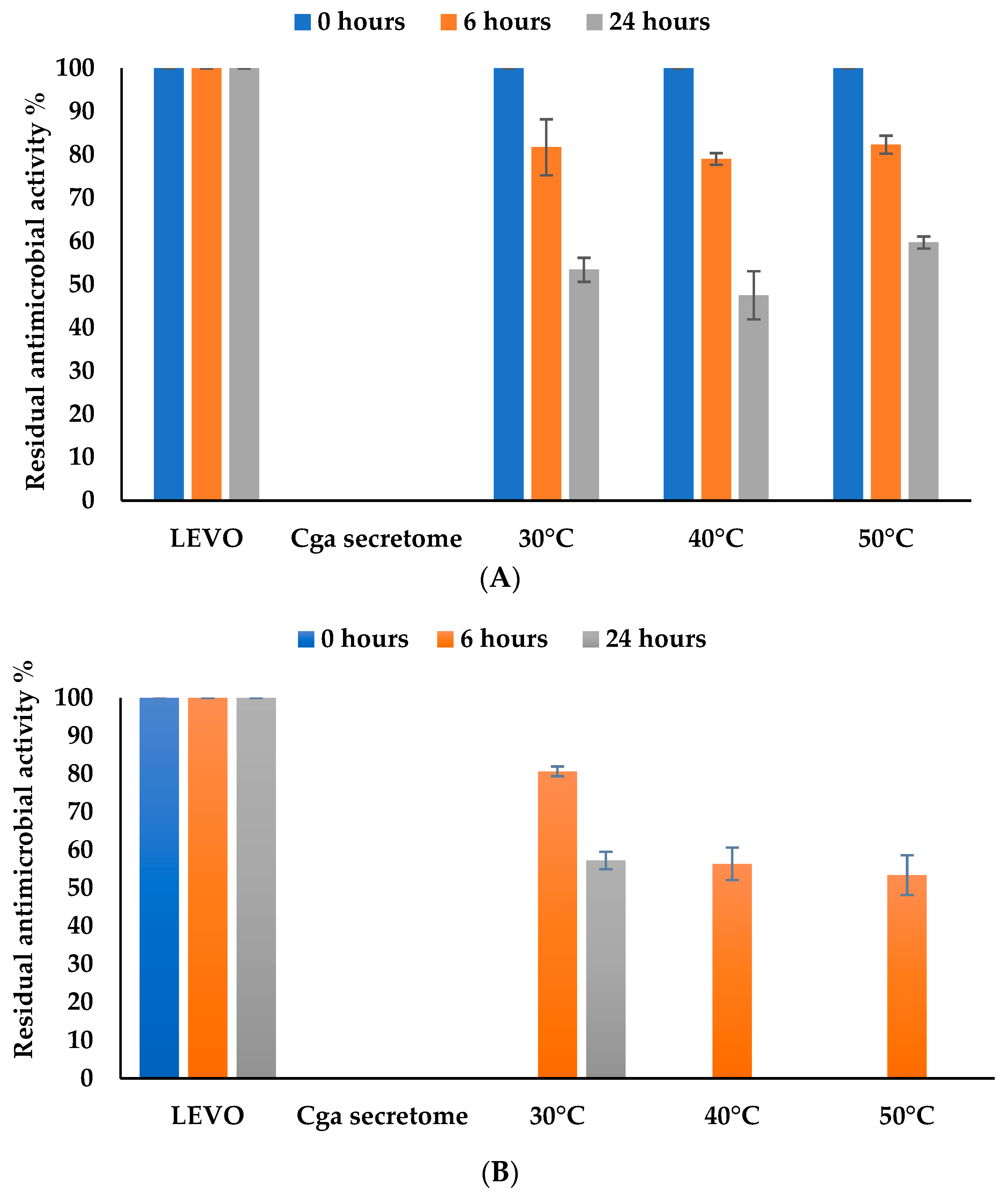
3.4.4. Effect of Temperature
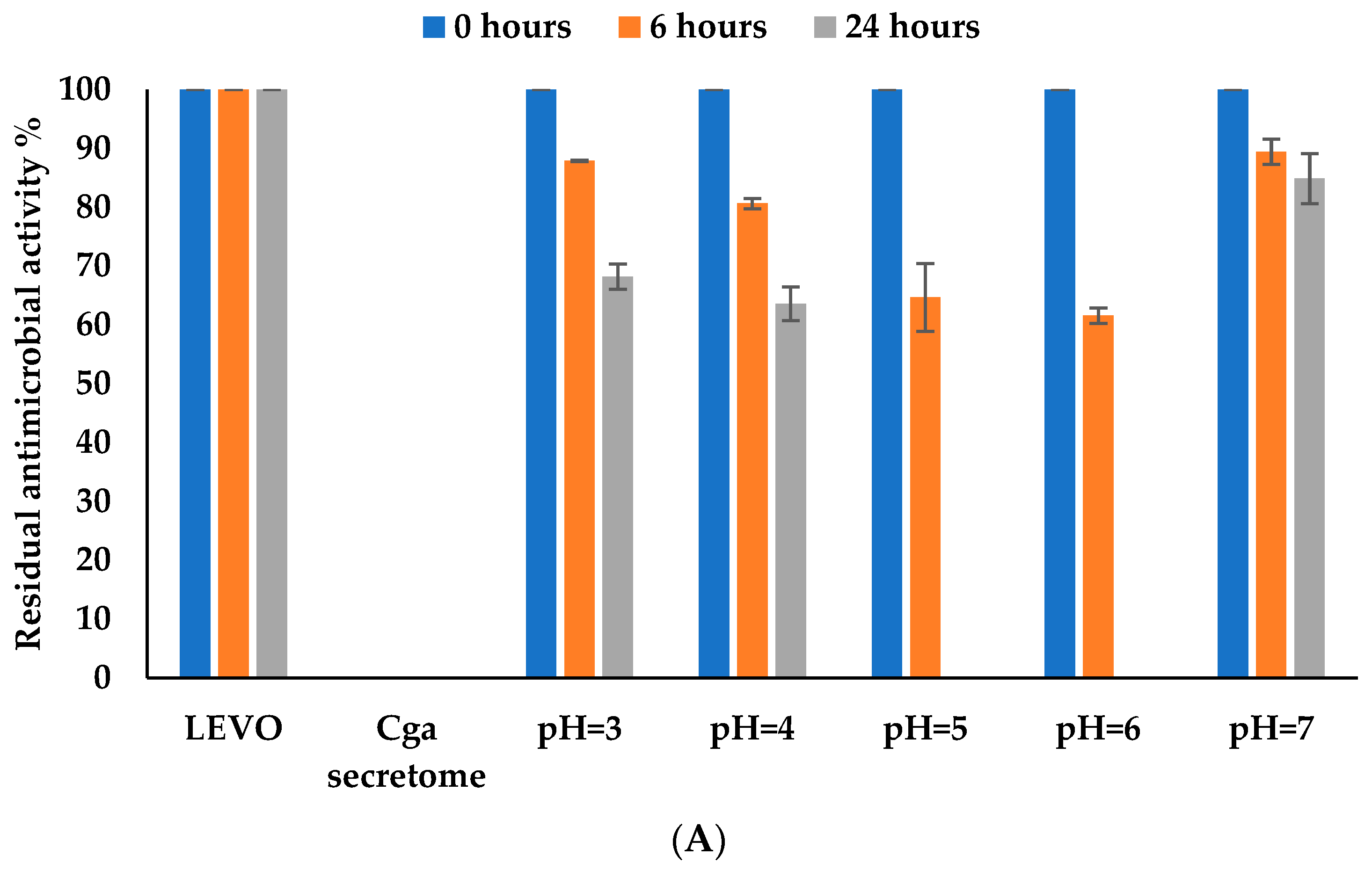
3.4.5. Effect of pH
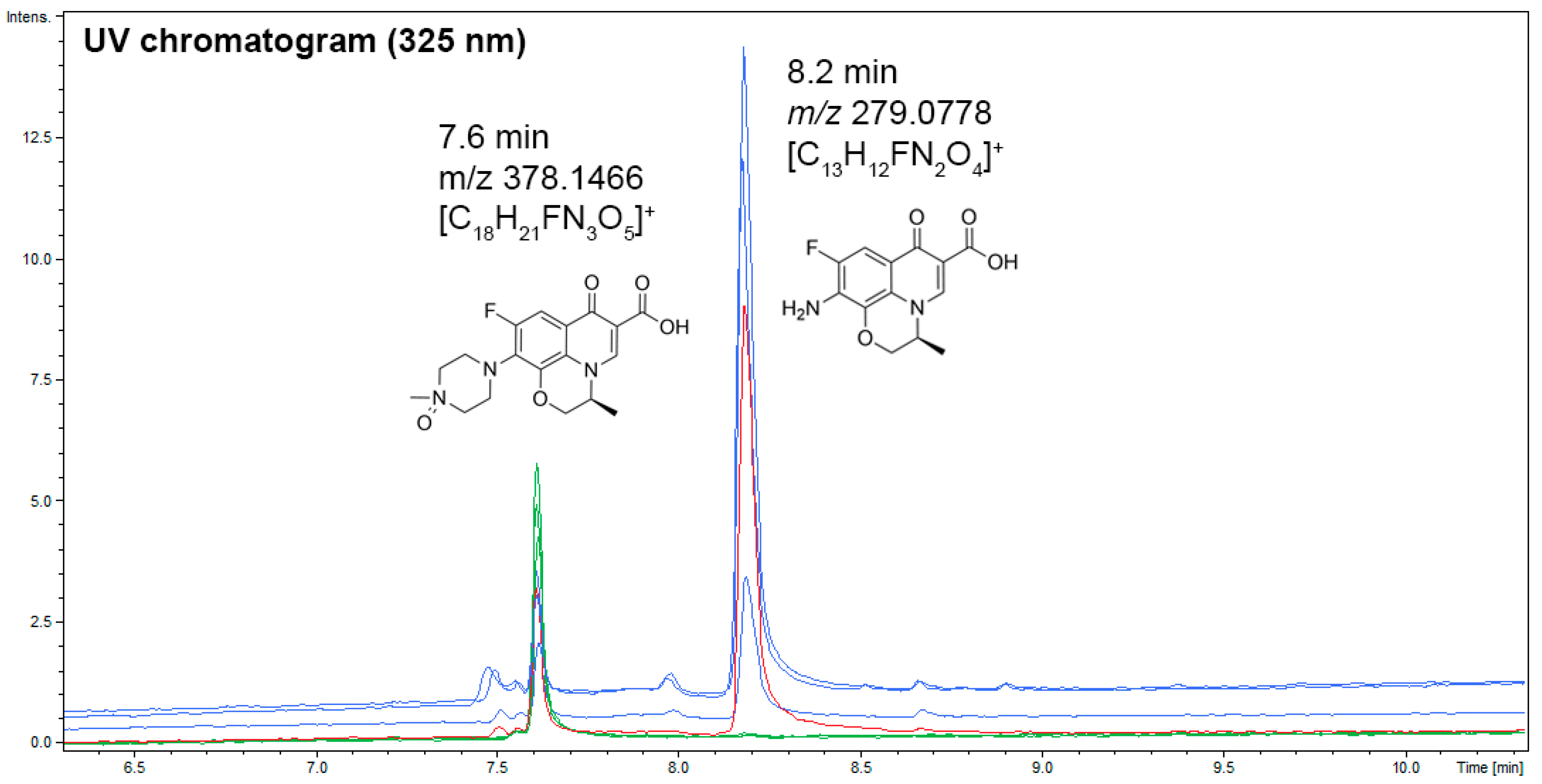
3.5. UHPLC–UV–MS Analyses of Coriolopsis gallica Secretome Extracts, or Laccase, in the Presence of Levofloxacin for the Dereplication of Antibiotic Degradation Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sommet, A.; Benevent, J.; Rousseau, V.; Chebane, L.; Douros, A.; Montastruc, J.L.; Montastruc, F. What fluoroquinolones have the highest risk of aortic aneurysm? A case/non-case Study in VigiBase (R). J. Gen. Intern. Med. 2019, 34, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Wierzbiński, P.; Hubska, J.; Henzler, M.; Kucharski, B.; Bieś, R.; Krzystanek, M. Depressive and other adverse CNS effects of fluoroquinolones. Pharmaceuticals 2023, 16, 1105. [Google Scholar] [CrossRef]
- Ding, G.; Chen, G.; Liu, Y.; Li, M.; Liu, X. Occurrence and risk assessment of fluoroquinolone antibiotics in reclaimed water and receiving groundwater with different replenishment pathways. Sci. Tot. Environ. 2020, 738, 139802. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Qu, S.; Li, R.; Huo, Z.Y.; Gao, Y.; Luo, Y. Biotransformation of antibiotics by electrochemical advanced oxidation processes (EAOPs): Performance, mechanisms, and perspectives. Sci. Total. Environ. 2023, 856, 159092. [Google Scholar] [CrossRef]
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.H.; Oeurng, C.; et al. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total. Environ. 2021, 764, 142865. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pinacho, D.G.; Pascual, N.; Granados, M.; Companyó, R.; Marco, M.P. Development and validation of an enzyme linked immunosorbent assay for fluoroquinolones in animal feeds. Food Control 2015, 57, 195–201. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Occurrence of antibiotics and antibiotic resistance genes in soils from wastewater irrigation areas in the Pearl River Delta region, southern China. Sci. Tot. Environ. 2018, 624, 145–152. [Google Scholar] [CrossRef]
- Masud, M.A.A.; Shin, W.S.; Septian, A.; Samaraweera, H.; Khan, I.J.; Mohamed, M.M.; Billah, M.M.; López-Maldonado, E.A.; Rahman, M.M.; Islam, A.R.M.T.; et al. Exploring the environmental pathways and challenges of fluoroquinolone antibiotics: A state-of-the-art review. Sci. Total. Environ. 2024, 926, 171944. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water. Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Q.; Wang, Z.X.; Zhang, Y.; Zhang, Y.; Ma, J.; Shao, L. Bio-inspired loose nanofiltration membranes with optimized separation performance for antibiotics removals. J. Memb. Sci. 2018, 554, 385–394. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, X.; Xu, Z.; Hu, W.; Zhou, Y.; Wan, Z.; Yang, Y.; Wei, Y.; Yang, J.; Tsang, D.C. Fabrication of sustainable manganese ferrite modified biochar from vinasse for enhanced adsorption of fluoroquinolone antibiotics: Effects and mechanisms. Sci. Tot. Environ. 2019, 709, 136079. [Google Scholar] [CrossRef] [PubMed]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The impact of tetracycline pollution on the aquatic environment and removal strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Erust, C.; Ozdemiroglu, S.; Fonti, V.; Beolchini, F. A review of approaches and techniques used in aquatic contaminated sediments: Metal removal and stabilization by chemical and biotechnological processes. J. Clean. Prod. 2015, 86, 24–36. [Google Scholar] [CrossRef]
- Cai, Z.; Dwivedi, A.D.; Lee, W.N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano. 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Cornelissen, E.; Harmsen, D.; Blankert, B.; Wessels, L.; Van Der Meer, W. Effect of minimal pre-treatment on reverse osmosis using surface water as a source. Desalination 2021, 509, 115056. [Google Scholar] [CrossRef]
- Zad, T.J.; Astuti, M.P.; Padhye, L.P. Fate of environmental pollutants. Water. Environ. Res. 2018, 90, 1104–1170. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Giri, B.S.; Shukla, P.; Gupta, P. Recent advancement in remediation of synthetic organic antibiotics from environmental matrices: Challenges and perspective. Bioresour. Technol. 2021, 319, 124161. [Google Scholar] [CrossRef]
- Khmaissa, M.; Zouari-Mechichi, H.; Sciara, G.; Record, E.; Mechichi, T. Pollution from livestock farming antibiotics an emerging environmental and human health concern: A Review. J. Hazard. Mater. Adv. 2024, 13, 100410. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob. Change Biol. 2022, 26, 4506–4520. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.B.; Madhavan, A.; Tarafdar, A.; Sirohi, R.; Anoopkumar, A.N.; Kuriakose, L.L.; Kumar Awasthi, M.; Binod, P.; Varjani, S.; Sindhu, R. Filamentous fungi for pharmaceutical compounds degradation in the environment: A sustainable approach. Env. Technolo. Innov. 2023, 31, 103182. [Google Scholar]
- Kathiravan, A.; Gnanadoss, J.J. White-rot fungi-mediated bioremediation as a sustainable method for xenobiotic degradation. Environ. Exp. Biol. 2021, 19, 103–119. [Google Scholar] [CrossRef]
- Akrout, I.; Staita, K.; Zouari-Mechichi, H.; Ghariani, B.; Khmaissa, M.; Navarro, D.; Doan, A.; Albert, Q.; Faulds, C.; Sciara, G.; et al. Valorizing fungal diversity for the degradation of fluoroquinolones. Heliyon 2024, 10, e30611. [Google Scholar] [CrossRef]
- Ben Ayed, A.B.; Akrout, I.; Staita, K.; Albert, Q.; Greff, S.; Simmler, C.; Ahrendt, S.; LaButti, K.; Lipzen, A.; He, G.; et al. Genome sequencing of Porostereum spadiceum to study the degradation of levofloxacin. Ecotoxicol. Environ. Safe. 2024, 270, 115808. [Google Scholar] [CrossRef]
- Ghariani, B.; Alessa, A.H.; Ben Atitallah, I.; Louati, I.; Alsaigh, A.A.; Mechichi, T.; Zouari-Mechichi, H. Fungal Bioremediation of the β-Lactam Antibiotic Ampicillin under Laccase-Induced Conditions. Antibiotics 2024, 13, 407. [Google Scholar] [CrossRef]
- Benali, J.; Ben Atitallah, I.; Ghariani, B.; Mechichi, T.; Hadrich, B.; Zouari-Mechichi, H. Optimized decolorization of two poly azo dyes Sirius Red and Sirius Blue using laccase-mediator system. 3 Biotech. 2024, 14, 93. [Google Scholar] [CrossRef]
- Zouari-Mechichi, H.; Benali, J.; Alessa, A.H.; Hadrich, B.; Mechichi, T. Efficient Decolorization of the Poly-Azo Dye Sirius Grey by Coriolopsis gallica Laccase-Mediator System: Process Optimization and Toxicity Assessment. Molecules 2024, 29, 477. [Google Scholar] [CrossRef]
- Park, H.; Choi, I.G. Genomic and transcriptomic perspectives on mycoremediation of polycyclic aromatic hydrocarbons. Appl. Microbiolo. Biotechnolo. 2020, 104, 6919–6928. [Google Scholar] [CrossRef]
- Wang, L.; Qiang, Z.; Li, Y.; Ben, W. An insight into the removal of fluoroquinolones in activated sludge process: Sorption and biodegradation characteristics. J. Environ. Sci. 2017, 263–271. [Google Scholar] [CrossRef]
- Čvančarová, M.M.; Moeder, M.; Filipová, A.; Cajthaml, T. Biotransformation of fluoroquinolone antibiotics by ligninolytic fungi—metabolites, enzymes and residual antibacterial activity. Chemosphere 2015, 136, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ben Ayed, A.; Akrout, I.; Albert, Q.; Greff, S.; Simmler, C.; Armengaud, J.; Kielbasa, M.; Turbé-Doan, A.; Chaduli, D.; Navarro, D.; et al. Biotransformation of the fluoroquinolone, levofloxacin, by the white-rot fungus Coriolopsis gallica. J. Fungi. 2022, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Lueangjaroenkit, P.; Teerapatsakul, C.; Sakka, K.; Sakka, M.; Kimura, T.; Kunitake, E.; Chitradon, L. Two manganese peroxidases and a laccase of Trametes polyzona KU-RNW027 with novel properties for dye and pharmaceutical product degradation in redox mediator-free system. Mycobiology 2019, 47, 217–229. [Google Scholar] [CrossRef]
- Ashe, B.; Nguyen, L.N.; Hai, F.I.; Lee, D.; Van De Merwe, J.P.; Leusch, F.D.L.; Price, W.E.; Nghiem, L.D. Impacts of redox-mediator type on trace organic contaminants degradation by laccase: Degradation efficiency, laccase stability and effluent toxicity. Int. Biodeterior. Biodegrad. 2016, 113, 169–176. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, expression regulation, and applications in pharmaceutical biodegradation. Front. Microbiol. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Choudhury, B. Lignolytic Enzymes and Their Role in the Bioremediation of Environmental Pollutants: Prospects and Challenges. In Microbial Remediation of Azo Dyes with Prokaryotes; CRC Press: Boca Raton, FL, USA, 2022; pp. 117–128. [Google Scholar]
- Gu, Y.; Yuan, L.; Jia, L.; Xue, P.; Yao, H. Recent developments of a co-immobilized laccase–mediator system: A review. RSC Adv. 2021, 11, 29498–29506. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Biodegradation of a simulated textile effluent by immobilised-coated laccase in laboratory-scale reactors. Appl. Catal. A: General. 2010, 373, 147–153. [Google Scholar] [CrossRef]
- Bilal, M.; Ashraf, S.S.; Barceló, D.; Iqbal, H.M. Biocatalytic degradation/redefning “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Tot. Env. 2019, 691, 1190–1211. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Bachosz, K.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Kaczorek, E.; Jesionowski, T. A promising laccase immobilization using electrospun materials for biocatalytic degradation of tetracycline: Effect of process conditions and catalytic pathways. Catal. Today 2020, 348, 127–136. [Google Scholar] [CrossRef]
- Daâssi, D.; Rodríguez-Couto, S.; Nasri, M.; Mechichi, T. Biodegradation of textile dyes by immobilized laccase from Coriolopsis gallica into Ca-alginate beads. Int. Biodeterior. Biodegradation. 2014, 90, 71–78. [Google Scholar] [CrossRef]
- Damayanti, A.C.K.A.; Kumoro, A.C.; Bahlawan, Z.A.S. Review calcium alginate beads as immobilizing matrix of functional cells: Extrusion dripping method, characteristics, and application. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012017. [Google Scholar] [CrossRef]
- Contreras-Cortés, A.G.; Almendariz-Tapia, F.J.; Gómez-Álvarez, A.; Burgos-Hernández, A.; Luque-Alcaraz, A.G.; Rodríguez-Félix, F.; Quevedo-López, M.Á.; Plascencia-Jatomea, M. Toxicological Assessment of Cross-Linked Beads of Chitosan-Alginate and Aspergillus australensis Biomass, with Efficiency as Biosorbent for Copper Removal. Polymers. 2019, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Maqbool, A.; Sun, K.; Si, Y. Immobilization of Trametes Versicolor laccase on Cu-alginate beads for biocatalytic degradation of bisphenol A in water: Optimized immobilization, degradation and toxicity assessment. J. Env. Chem. Eng. 2022, 10, 107089. [Google Scholar] [CrossRef]
- Becker, D.; Varela-Della-Giustina, S.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase–degradation of compounds does not always eliminate toxicity. Bioresour. Technolo. 2016, 219, 500–509. [Google Scholar] [CrossRef]
- Zouari-Mechichi, H.; Mechichi, T.; Dhouib, A.; Sayadi, S.; Martínez, A.T.; Martinez, M.J. Laccase purification and characterization from Trametes trogii isolated in Tunisia: Decolorization of textile dyes by the purified enzyme. Enzyme. Microb. Technol. 2006, 39, 141–148. [Google Scholar] [CrossRef]
- Songulashvili, G.; Flahaut, S.; Demarez, M.; Tricot, C.; Bauvois, C.; Debaste, F.; Penninckx, M.J. High yield production in seven days of Coriolopsis gallica 1184 laccase at 50 L scale; enzyme purification and molecular characterization. Fungal. Biol. 2016, 120, 481–488. [Google Scholar] [CrossRef]
- Olajuyigbe, F.M.; Fatokun, C.O. Biochemical characterization of an extremely stable pH-versatile laccase from Sporothrix carnis CPF-05. Int. J. Biol. Macromol. 2017, 94, 535–543. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, M.A.; Elshafei, A.M.; Hassan, M.M. Purification and biochemical characterization of two isolated laccase isoforms from Agaricus bisporus CU13 and their potency in dye decolorization. Int. J. Biol. Macromol. 2018, 113, 1142–1148. [Google Scholar] [CrossRef]
- Qamar, S.A.; Asgher, M.; Bilal, M. Immobilization of alkaline protease from Bacillus brevis using Ca-alginate entrapment strategy for improved catalytic stability, silver recovery, and dehairing potentialities. Catal. Letters. 2020, 150, 3572–3583. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, J.; Xing, L. Preparation of active corn peptides from zein through double enzymes immobilized with calcium alginate–chitosan beads. Process. Biochem. 2014, 49, 1682–1690. [Google Scholar] [CrossRef]
- Devi, M.L.; Chandrasekhar, K.B. A validated stability-indicating RP-HPLC method for levofloxacin in the presence of degradation products, its process related impurities and identification of oxidative degradant. J. Pharm. Biomed. Anal. 2009, 50, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Mtibaà, R.; Barriuso, J.; de Eugenio, L.; Aranda, E.; Belbahri, L.; Nasri, M.; Martínez, M.J.; Mechichi, T. Purification and characterization of a fungal laccase from the ascomycete Thielavia sp. and its role in the decolorization of a recalcitrant dye. Int. J. Biol. Macromol. 2018, 120, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Cen, Q.; Wu, X.; Cao, L.; Lu, Y.; Lu, X.; Chen, J.; Fu, G.; Liu, Y.; Ruan, R. Green production of a yellow laccase by Coriolopsis gallica for phenolic pollutants removal. AMB Express. 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Geethanjali, S.; Subash, A. Optimization and immobilization of purified Labeo rohita visceral protease by entrapment method. Enzyme. Res. 2013, 2013, 874050. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancing catalytic functionality of Trametes versicolor IBL-04 laccase by immobilization on chitosan microspheres. Chem. Eng. Res. Design. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Kumar, D.; Muthukumar, M.; Garg, N. Kinetics of fungal extracellular [alpha]-amylase from Fusarium solani immobilized in calcium alginate beads. J. Environ. Biol. 2012, 33, 1021. [Google Scholar]
- Norouzian, D.; Akbarzadeh, A.; Inanlou, D.N.; Farahmand, B.; Saleh, M.; Sheikh-ul-Eslam, F.; Vaez, J. Biotransformation of alcohols to aldehydes by immobilized cells of Saccharomyces cerevisiae PTCC5080. Enzyme. Microb. Technol. 2003, 33, 150–153. [Google Scholar] [CrossRef]
- Forootanfar, H.; Rezaei, S.; Zeinvand-Lorestani, H.; Tahmasbi, H.; Mogharabi, M.; Ameri, A.; Faramarzi, M.A. Studies on the laccase-mediated decolorization, kinetic, and microtoxicity of some synthetic azo dyes. J. Environ. Health Sci. Eng. 2016, 14, 7. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Nasseri, S.; Alimohammadi, M.; Mahvi, A.H.; Faramarzi, M.A. Application of free and immobilized laccase for removal and detoxification of fluoroquinolones from aqueous solution. Global. Nest. J. 2020, 22, 240–249. [Google Scholar]
- Munk, L.; Andersen, M.L.; Meyer, A.S. Influence of mediators on laccase catalyzed radical formation in lignin. Enzyme. Microb. Technol. 2018, 116, 48–56. [Google Scholar] [CrossRef]
- Khlifi, R.; Sayadi, S.; Belbahri, L.; Woodward, S.; Mechichi, T. Effect of HBT on the stability of laccase during the decolourization of textile wastewaters. J. Chem. Technol. Biotechnol. 2009, 84, 1828–1833. [Google Scholar] [CrossRef]
- Mechichi, T.; Mhiri, N.; Sayadi, S. Remazol Brilliant Blue R decolourization by the laccase from Trametes trogii. Chemosphere 2006, 64, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Hooda, S.; Beniwal, S.; Sindhu, A. Partial purification and immobilization of laccase isolated from medicinal mushroom (Ganoderma lucidum). J. Chem. Technol. 2016, 23, 313–317. [Google Scholar]
- Kurniawati, S.; Nicell, J.A. Characterization of Trametes versicolor laccase for the transformation of aqueous phenol. Bioresour. Technol. 2008, 99, 7825–7834. [Google Scholar] [CrossRef]
- Champagne, P.P.; Ramsay, J.A. Dye decolorization and detoxification by laccase immobilized on porous glass beads. Bioresour. Technol. 2010, 101, 2230–2235. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Sanromán, M.Á.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases–occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Lomascolo, A.; Record, E.; Herpoël-Gimbert, I.; Delattre, M.; Robert, J.L.; Georis, J.; Dauvrin Sigoillot, J.C.; Asther, M. Overproduction of laccase by a monokaryotic strain of Pycnoporus cinnabarinus using ethanol as inducer. J. Appl. Microbiol. 2003, 94, 618–624. [Google Scholar] [CrossRef]
- Record, E.; Punt, P.J.; Chamkha, M.; Labat, M.; van Den Hondel, C.A.; Asther, M. Expression of the Pycnoporus cinnabarinus laccase gene in Aspergillus niger and characterization of the recombinant enzyme. Eur. J. Biochem. 2002, 269, 602–609. [Google Scholar] [CrossRef]
- Czyrski, A.; Anusiak, K.; Teżyk, A. The degradation of levofloxacin in infusions exposed to daylight with an identification of a degradation product with HPLC-MS. Sci. Rep. 2019, 9, 3621. [Google Scholar] [CrossRef]
- Foti, L.; Coviello, D.; Zuorro, A.; Lelario, F.; Bufo, S.A.; Scrano, L.; Sauvetre, A.; Chiron, S.; Brienza, M. Comparison of sunlight-AOPs for levofloxacin removal: Kinetics, transformation products, and toxicity assay on Escherichia coli and Micrococcus flavus. Environ. Sci. Pollut. Res. 2022, 29, 58201–58211. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Patil, D.V.; Jang, M.; Paeng, K.J.; Jeon, B.H. Biodegradation and metabolic fate of levofloxacin via a freshwater green alga, Scenedesmus obliquus in synthetic saline wastewater. Algal. Res. 2017, 25, 54–61. [Google Scholar] [CrossRef]
| Step | Total Activity (U) | Protein Quantity (mg) | Specific Activity (U/mg) | Purification Factor | Activity Recovery (%) |
|---|---|---|---|---|---|
| Culture medium | 1471 | 24.9 | 59 | 1 | 100 |
| Concentration with PEG | 1375 | 13.3 | 103 | 1.74 | 93.4 |
| Ammonium sulfate precipitation | 403 | 2.2 | 184 | 3.12 | 27.4 |
| Q-Sepharose | 270 | 0.9 | 300 | 5.1 | 18.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staita, K.; Khmaissa, M.; Akrout, I.; Greff, S.; Ghariani, B.; Turbé-Doan, A.; Lambert, J.; Lomascolo, A.; Albert, Q.; Faulds, C.B.; et al. Biotransformation of the Fluoroquinolone Antibiotic, Levofloxacin, by the Free and Immobilized Secretome of Coriolopsis gallica. J. Fungi 2024, 10, 861. https://doi.org/10.3390/jof10120861
Staita K, Khmaissa M, Akrout I, Greff S, Ghariani B, Turbé-Doan A, Lambert J, Lomascolo A, Albert Q, Faulds CB, et al. Biotransformation of the Fluoroquinolone Antibiotic, Levofloxacin, by the Free and Immobilized Secretome of Coriolopsis gallica. Journal of Fungi. 2024; 10(12):861. https://doi.org/10.3390/jof10120861
Chicago/Turabian StyleStaita, Karima, Marwa Khmaissa, Imen Akrout, Stéphane Greff, Bouthaina Ghariani, Annick Turbé-Doan, Julien Lambert, Anne Lomascolo, Quentin Albert, Craig B. Faulds, and et al. 2024. "Biotransformation of the Fluoroquinolone Antibiotic, Levofloxacin, by the Free and Immobilized Secretome of Coriolopsis gallica" Journal of Fungi 10, no. 12: 861. https://doi.org/10.3390/jof10120861
APA StyleStaita, K., Khmaissa, M., Akrout, I., Greff, S., Ghariani, B., Turbé-Doan, A., Lambert, J., Lomascolo, A., Albert, Q., Faulds, C. B., Sciara, G., Zouari-Mechichi, H., Record, E., & Mechichi, T. (2024). Biotransformation of the Fluoroquinolone Antibiotic, Levofloxacin, by the Free and Immobilized Secretome of Coriolopsis gallica. Journal of Fungi, 10(12), 861. https://doi.org/10.3390/jof10120861







