The Stress of Fungicides Changes the Expression of Clock Protein CmFRQ and the Morphology of Fruiting Bodies of Cordyceps militaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. Militaris Strains
2.2. Strain Activation
2.3. Culture of Mycelium with the Treatment of Fungicides
2.4. Transfer Culture of C. Militaris Mycelium after the Treatment of Fungicides
2.5. Culture of Fruiting Bodies for S1
2.6. Sample Treatment and Western Blot Analysis
3. Results
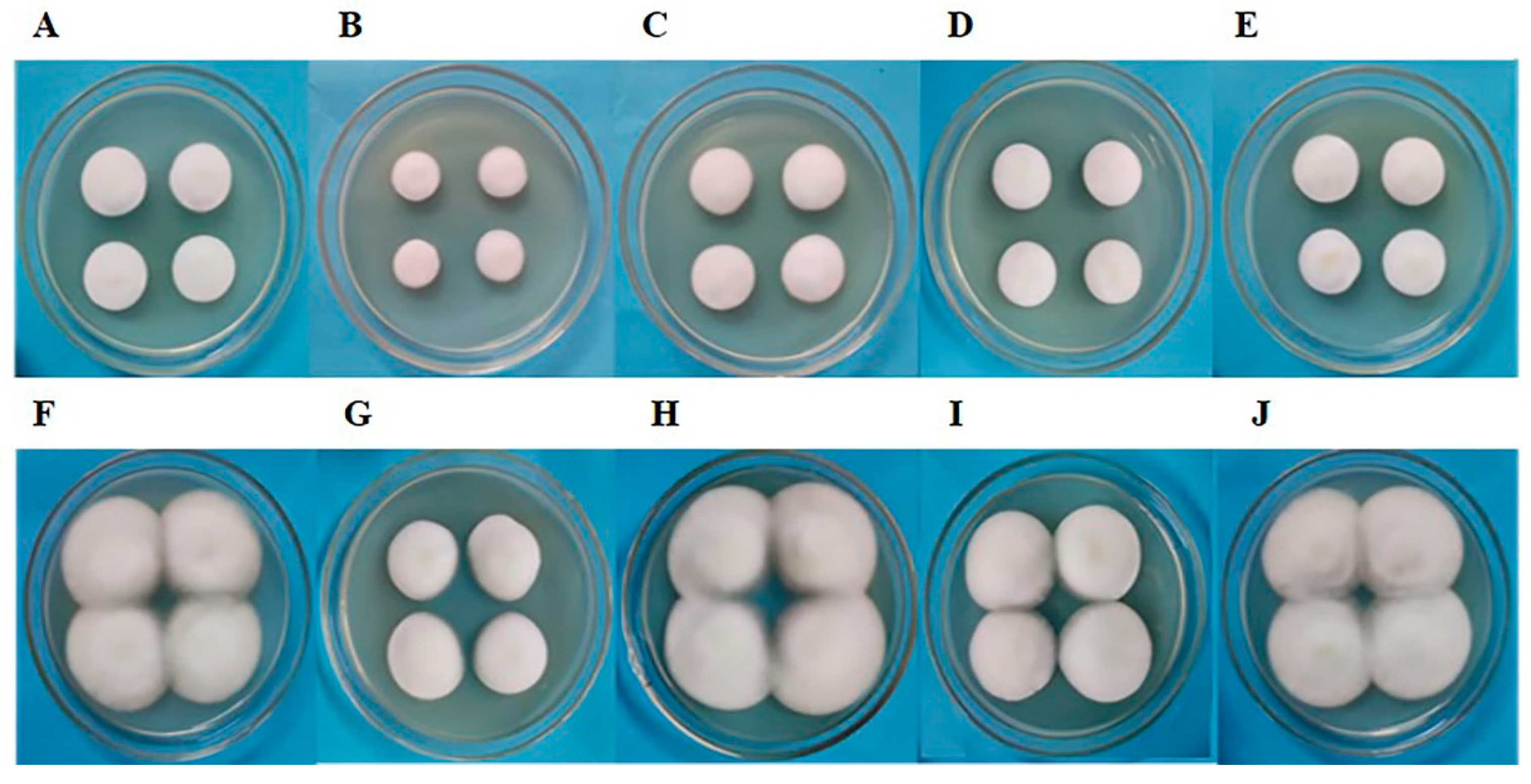
3.1. Morphology of C. militaris Strain TN Colony Treated with Fungicides
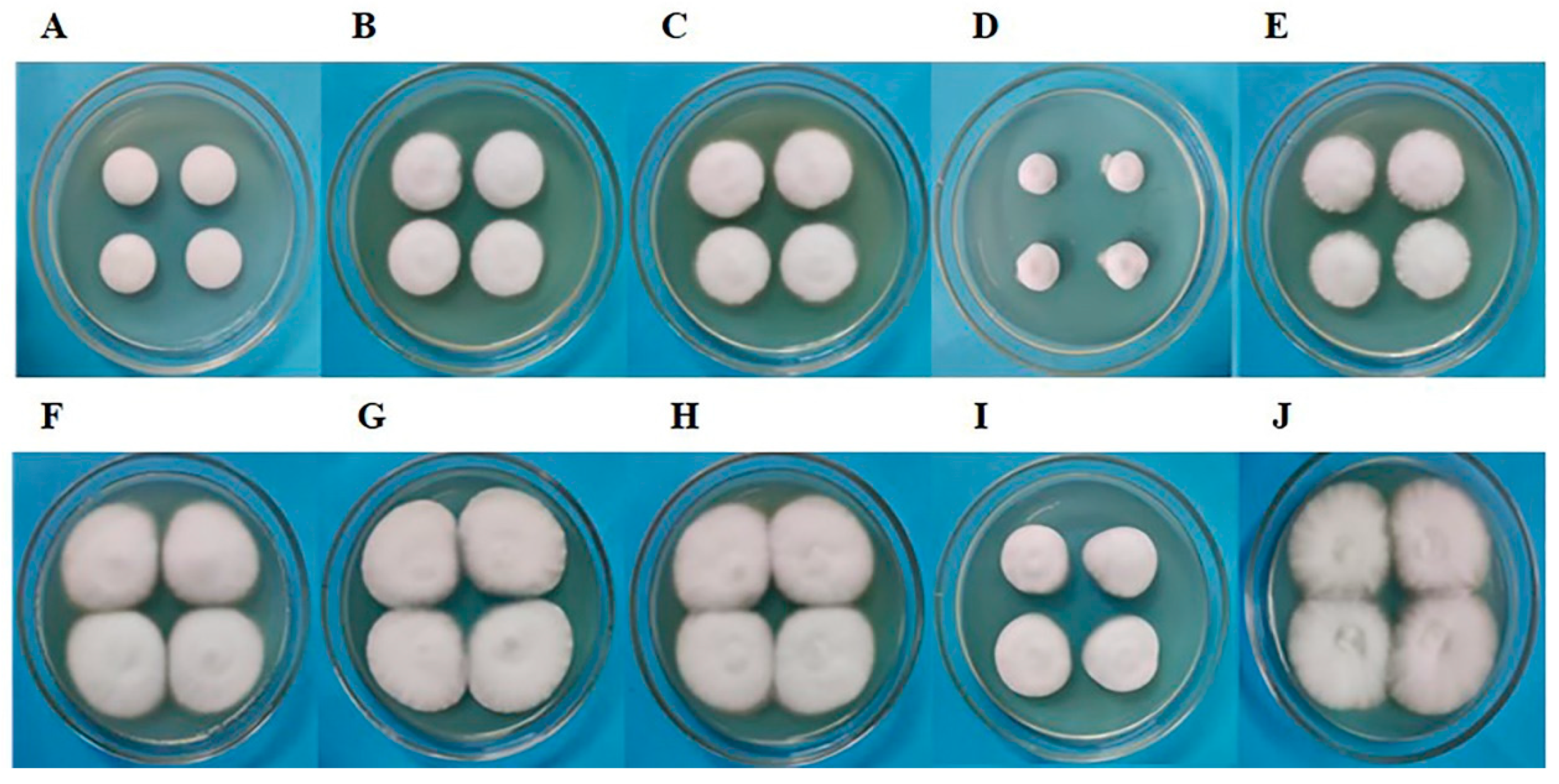
3.2. Morphology of C. militaris Strain CmFRQ454 Colony Treated with Fungicides
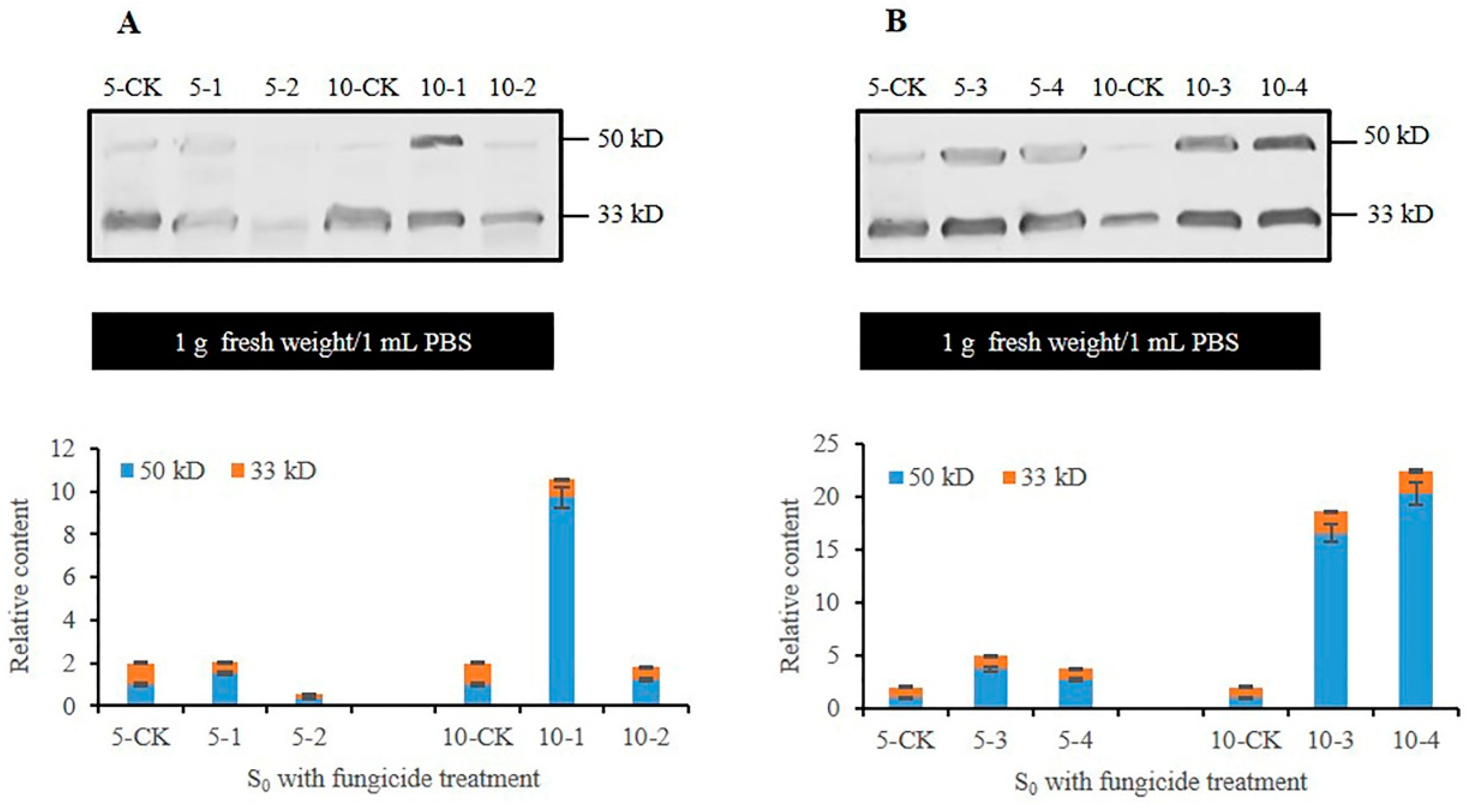
3.3. Analysis of CmFRQ in the Subculture S0 Mycelium of C. militaris Strain TN Treated with Fungicides
3.4. Analysis of CmFRQ in Subculture S0 Mycelium of C. militaris Strain CmFRQ454 Treated with Fungicides
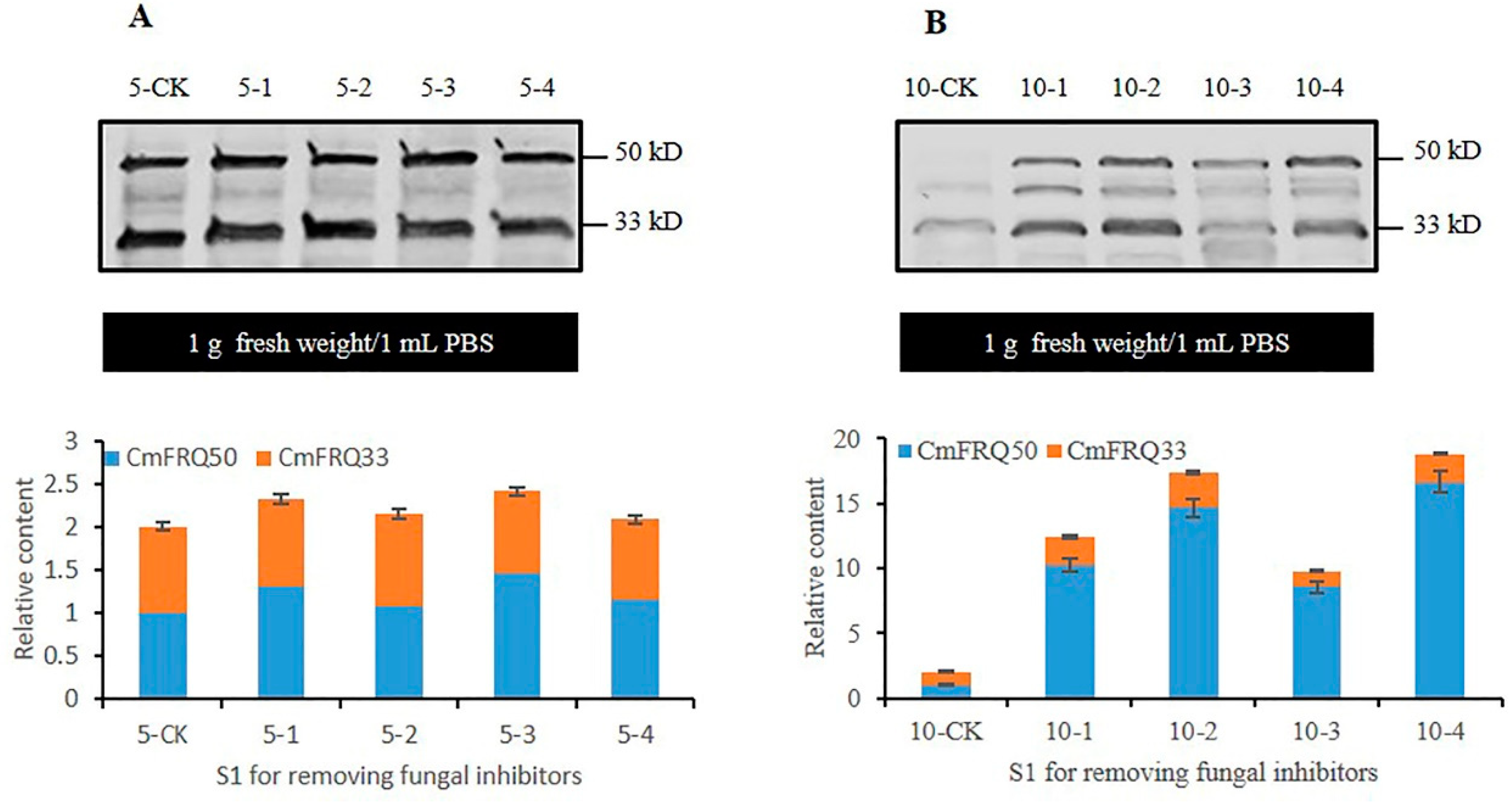
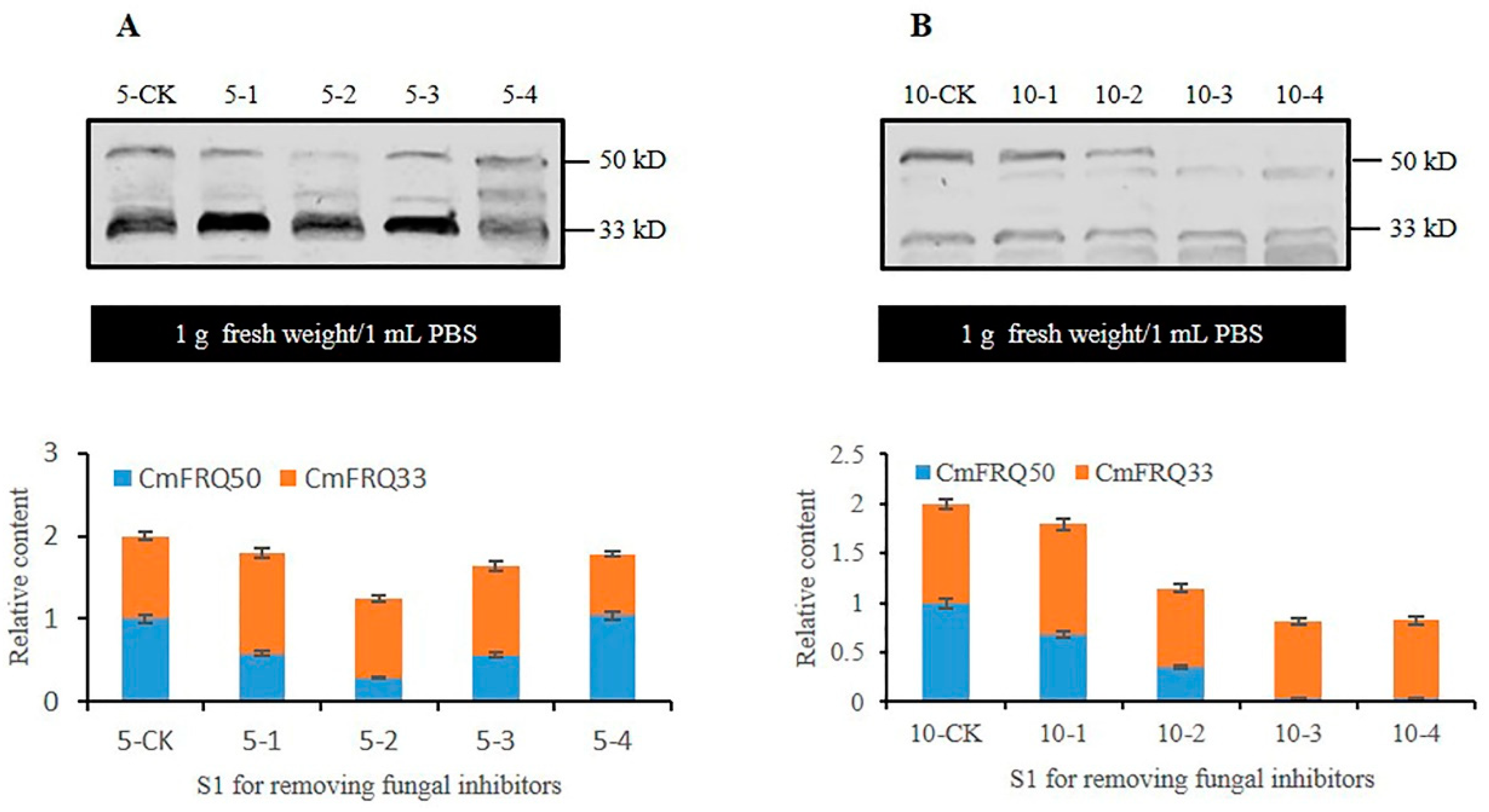
3.5. Analysis of CmFRQ in Subculture S1 Mycelium of C. militaris Strain TN after Removal of Fungicide
3.6. Analysis of CmFRQ in Subculture S1 Mycelium of C. militaris Strain CmFRQ454 after Removal of Fungicide
3.7. Morphological Analysis of Subculture S1 Fruiting Bodies of C. militaris Strain TN after Removal of Fungicides
3.8. Morphological Analysis of Subculture S1 Fruiting Bodies of C. militaris Strain CmFRQ454 after Removal of Fungicide
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scanlan, J.L.; Mitchell, A.C.; Marcroft, S.J.; Forsyth, L.M.; Idnurm, A.; Van de Wouw, A.P. Deep amplicon sequencing reveals extensive allelic diversity in the erg11/CYP51 promoter and allows multi-population DMI fungicide resistance monitoring in the canola pathogen Leptosphaeria maculans. Fungal. Genet. Biol. 2023, 168, 103814. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Everhart, S.E.; Bryson, P.K.; Luo, C.; Song, X.; Liu, X.; Schnabel, G. Fungicide-induced transposon movement in Monilinia fructicola. Fungal. Genet. Biol. 2015, 85, 38–44. [Google Scholar] [CrossRef]
- Gambhir, N.; Kamvar, Z.N.; Higgins, R.; Amaradasa, B.S.; Everhart, S.E. Spontaneous and Fungicide-Induced Genomic Variation in Sclerotinia sclerotiorum. Phytopathology 2021, 111, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.T.; Froelich, M.H.; Boatwrigh, H.; Wang, H.; Schnabel, G.; Kerrigan, J. Genetic diversity and fungicide sensitivity of Cytospora plurivora on peach. Plant Dis. 2023, 107, 2112–2118. [Google Scholar] [CrossRef]
- Brito, F.S.D.; Santos, J.R.P.; Azevedo, V.C.R.; Peixouto, Y.S.; de Oliveira, S.A.; Ferreira, C.F.; Haddad, F.; Amorim, E.P.; Fraaije, B.; Miller, R.N.G. Genetic diversity and azole fungicide sensitivity in Pseudocercospora musae field populations in Brazil. Front. Microbiol. 2020, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.K.; Cochran, A.; Mengistu, A.; Lamour, K.; Castro-Rocha, A.; Young-Kelly, H. Genetic diversity, QoI fungicide resistance, and mating type distribution of Cercospora sojina-Implications for the disease dynamics of frogeye leaf spot on soybean. PLoS ONE 2017, 12, e0177220. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.S.; Ravigne, V.; Rieux, A.; Ali, S.; Carpentier, F.; Fournier, E. Fungal adaptation to contemporary fungicide applications: The case of Botrytis cinerea populations from Champagne vineyards (France). Mol. Ecol. 2017, 26, 1919–1935. [Google Scholar] [CrossRef]
- Barber, A.E.; Riedel, J.; Sae-Ong, T.; Kang, K.; Brabetz, W.; Panagiotou, G.; Deising, H.B.; Kurzai, O. Effects of agricultural fungicide use on Aspergillus fumigatus abundance, antifungal susceptibility, and population structure. mBio 2020, 11, e02213-20. [Google Scholar] [CrossRef]
- Mahmoud, D.E.; Faraag, A.H.I.; Abu El-Wafa, W.M. In vitro study on the potential fungicidal effects of atorvastatin in combination with some azole drugs against multidrug resistant Candida albicans. World J. Microbiol. Biotechnol. 2021, 37, 191. [Google Scholar] [CrossRef]
- Woods, M.; McAlister, J.A.; Geddes-McAlister, J. A One Health approach to overcoming fungal disease and antifungal resistance. WIREs Mech. Dis. 2023, 15, e1610. [Google Scholar] [CrossRef]
- Dunlap, J.C. Molecular bases for circadian clocks. Cell 1999, 96, 271–290. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 2001, 63, 647–676. [Google Scholar] [CrossRef] [PubMed]
- Bell-Pedersen, D. Understanding circadian rhythmicity in Neurospora crassa: From behavior to genes and back again. Fungal. Genet. Biol. 2000, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pregueiro, A.M.; Liu, Q.; Baker, C.L.; Dunlap, J.C.; Loros, J.J. The Neurospora checkpoint kinase 2: A regulatory link between the circadian and cell cycles. Science 2006, 313, 644–649. [Google Scholar] [CrossRef]
- Liu, Y.; Merrow, M.; Loros, J.J.; Dunlap, J.C. How temperature changes reset a circadian oscillator. Science 1998, 281, 825–829. [Google Scholar] [CrossRef]
- Devlin, P.F. Signs of the time: Environmental input to the circadian clock. J. Exp. Bot. 2002, 53, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.L.; Loros, J.J.; Dunlap, J.C. The circadian clock of Neurospora crassa. FEMS Microbiol. Rev. 2012, 36, 95–110. [Google Scholar] [CrossRef]
- Chen, C.H.; Dunlap, J.C.; Loros, J.J. Neurospora illuminates fungal photoreception. Fungal. Genet. Biol. 2010, 47, 922–929. [Google Scholar] [CrossRef]
- Heintzen, C.; Liu, Y. The Neurospora crassa circadian clock. Adv. Genet. 2007, 58, 25–66. [Google Scholar]
- Crosthwaite, S.K.; Dunlap, J.C.; Loros, J.J. Neurospora wc-1 and wc-2: Transcription, photoresponses, and the origins of circadian rhythmicity. Science 1997, 76, 763–769. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Y.; Gardner, K.H.; Liu, Y. PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady-state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora. Mol. Cell Biol. 2002, 22, 517–524. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Y.; Wang, L.; He, Q.; Liu, Y. WHITE COLLAR-1, a multifunctional Neurospora protein involved in the circadian feedback loops, light sensing, and transcription repression of wc-2. J. Biol. Chem. 2003, 278, 3801–3808. [Google Scholar] [CrossRef]
- Froehlich, A.C.; Loros, J.J.; Dunlap, J.C. Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc. Natl. Acad. Sci. USA 2003, 100, 5914–5919. [Google Scholar] [CrossRef]
- Belden, W.J.; Loros, J.J.; Dunlap, J.C. Execution of the circadian negative feedback loop in Neurospora requires the ATPdependent chromatin-remodeling enzyme CLOCKSWITCH. Mol. Cell 2007, 25, 587–600. [Google Scholar] [CrossRef]
- Aronson, B.D.; Johnson, K.A.; Loros, J.J.; Dunlap, J.C. Negative feedback defining a circadian clock: Autoregulation of the clock gene frequency. Science 1994, 263, 1578–1584. [Google Scholar] [CrossRef]
- Cheng, P.; He, Q.L.; Wang, L.; Liu, Y. Regulation of the Neurospora circadian clock by an RNA helicase. Genes Dev. 2005, 19, 234–241. [Google Scholar] [CrossRef]
- Dunlap, J.C.; Loros, J.J. The Neurospora circadian system. J. Biol. Rhythm. 2004, 19, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Loros, J.; Dunlap, J.C. Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc. Natl. Acad. Sci. USA 2000, 97, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Görl, M.; Merrow, M.; Huttner, B.; Johnson, J.; Roenneberg, T.; Brunner, M. A PEST-like element in FREQUENCY determines the length of the circadian period in Neurospora crassa. EMBO J. 2001, 20, 7074–7084. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Cheng, P.; Yang, Y.; He, Q.; Yu, H.; Liu, Y. FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J. 2003, 22, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Cha, J.; Lee, H.C.; Yang, Y.; Liu, Y. CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 2006, 20, 2552–2565. [Google Scholar] [CrossRef]
- He, Q.; Cheng, P.; He, Q.; Liu, Y. The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev. 2005, 19, 1518–1531. [Google Scholar] [CrossRef]
- He, Q.; Liu, Y. Degradation of the Neurospora circadian clock protein FREQUENCY through the ubiquitin-proteasome pathway. Biochem. Soc. Trans. 2005, 33, 953–956. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, P.; Zhi, G.; Liu, Y. Identification of a calcium/calmodulin-dependent protein kinase that phosphorylates the Neurospora circadian clock protein FREQUENCY. J. Biol. Chem. 2001, 276, 41064–41072. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, P.; He, Q.; Wang, L.; Liu, Y. Phosphorylation of FREQUENCY protein by casein kinase II is necessary for the function of the Neurospora circadian clock. Mol. Cell. Biol. 2003, 23, 6221–6228. [Google Scholar] [CrossRef]
- Schafmeier, T.; Haase, A.; Káldi, K.; Scholz, J.; Fuchs, M.; Brunner, M. Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell 2005, 122, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kettenbach, A.N.; Zhou, X.; Loros, J.J.; Dunlap, J.C. The Phospho-Code Determining Circadian Feedback Loop Closure and Output in Neurospora. Mol. Cell 2019, 74, 771–784.e3. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Marr, K.A.; Rex, J.H.; Cohen, S.H. Amphotericin B: Time for a new “gold standard”. Clin. Infect. Dis. 2003, 37, 415–425. [Google Scholar] [PubMed]
- Zhao, P. Effect of amino acids on growth and development of Auricularia auricula. J. Gansu Agric. Univ. 1997, 32, 159–192. (In Chinese) [Google Scholar]
- Pan, K.; Wu, F. Effects of amino acids on growth and development of Fusarium oxysporum f. sp. Cucumerinum. North. Hortic. 2008, 2, 228–231. (In Chinese) [Google Scholar]
- Liu, A.; Chen, J.; Zhang, J.; Fang, Q.; Wang, M.; Zha, Q. Effect of organic acids and amino acids on the mycelial growth of Agaricus bisporus 2796. Chin. Agric. Sci. Bull. 2010, 26, 46–51. (In Chinese) [Google Scholar]
- Kang, H.; Qiao, Y.; Liu, C.; Zhang, Y.; Sun, D. Inhibitory effect of L-cysteine against Alternaria alternata rot of grapes. Food Sci. 2020, 41, 228–235. (In Chinese) [Google Scholar]
- Zhang, L.; Zhao, X.; Guo, Y.; Hu, D.; Liang, W. Inhibitory effect of L-cysteine on the mycelial growth of Villosiclava virens and its transcriptome analysis. Acta Phytopathol. Sin. 2022, 52, 352–363. (In Chinese) [Google Scholar]
- Hou, S.; Deng, L.; Cai, J.; Sun, R. Agricultural antifungal activity of terbinafine and terbinafine hydrochloride. J. Trop. Biol. 2021, 12, 448–455. (In Chinese) [Google Scholar]
- Waldorf, A.R.; Polak, A. Mechanisms of action of 5-fluorocytosine. Antimicrob. Agents Chemother. 1983, 23, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Polak, A. Mode of action of 5-fluorocytosine and 5-fluorouracil in dematiaceous fungi. Sabouraudia 1983, 21, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Polak, A.; Wain, W.H. The influence of 5-fluorocytosine on nucleic acid synthesis in Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus. Chemotherapy 1977, 23, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Wain, W.H.; Polak, A. The effect of 5-fluorocytosine on the synthesis of 80S ribosomes by pathogenic fungi. Sabouraudia 1981, 19, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Whelan, W.L. The genetic basis of resistance to 5-fluorocytosine in Candida species and Cryptococcus neoformans. Crit. Rev. Microbiol. 1987, 15, 45–56. [Google Scholar] [CrossRef]
- Lou, H.; Lin, J.; Guo, L.; Wang, X.; Tian, S.; Liu, C.; Zhao, Y.; Zhao, R. Advances in research on Cordyceps militaris degeneration. Appl. Microbiol. Biotechnol. 2019, 103, 7835–7841. [Google Scholar] [CrossRef]
- Cha, J.; Zhou, M.; Liu, Y. Mechanism of the Neurospora Circadian Clock, a FREQUENCY-centric View. Biochemistry 2015, 54, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, A.C.; Basaiah, T.; Adams, M.L.; Quesada-Ocampo, L.M. Genetic diversity, fungicide sensitivity, and host resistance to Ceratocystis fimbriata infecting sweetpotato in north carolina. Plant Dis. 2017, 101, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C.; Zeng, X.G.; Guo, C.; Zhang, Q.H.; Chen, F.Y.; Ren, L.; Chen, W.D.; Qin, L. Reproduction response of Colletotrichum fungi under the fungicide stress reveals new aspects of chemical control of fungal diseases. Microb. Biotechnol. 2022, 15, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Scheckhuber, C.Q.; Mitterbauer, R.; Osiewacz, H.D. Molecular basis of and interference into degenerative processes in fungi: Potential relevance for improving biotechnological performance of microorganisms. Appl. Microbiol. Biotechnol. 2009, 85, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Silar, P.; Lalucque, H.; Vierny, C. Cell degeneration in the model system Podospora anserina. Biogerontology 2001, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, M.; Gredilla, R.; Müller-Ohldach, M.; Werner, A.; Bohr, V.A.; Osiewacz, H.D.; Stevnsner, T. A potential impact of DNA repair on ageing and lifespan in the ageing model organism Podospora anserina: Decrease in mitochondrial DNA repair activity during ageing. Mech. Ageing Dev. 2009, 130, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Dufour, E.; Boulay, J.; Rincheval, V.; Sainsard-Chanet, A. A causal link between respiration and senescence in Podospora anserina. Proc. Natl. Acad. Sci. USA 2000, 97, 4138–4143. [Google Scholar] [CrossRef]
- Gyöngyösi, N.; Nagy, D.; Makara, K.; Ella, K.; Káldi, K. Reactive oxygen species can modulate circadian phase and period in Neurospora crassa. Free Radic. Biol. Med. 2013, 58, 134–143. [Google Scholar] [CrossRef]
- Gyöngyösi, N.; Káldi, K. Interconnections of reactive oxygen species homeostasis and circadian rhythm in Neurospora crassa. Antioxid. Redox Signal. 2014, 20, 3007–3023. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, Z.; Wang, S.; Guan, D.; Liu, F. Modeling the crosstalk between the circadian clock and ROS in Neurospora crassa. J. Theor. Biol. 2018, 458, 125–132. [Google Scholar] [CrossRef]
- Wang, C.S.; Butt, T.M.; St Leger, R.J. Colony sectorization of Metarhizium anisopliae is a sign of ageing. Microbiology 2005, 151 Pt 10, 3223–3236. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pischetsrieder, M.; St Leger, R.J.; Wang, C.S. Associated links among mtDNA glycation, oxidative stress and colony sectorization in Metarhizium anisopliae. Fungal Genet. Biol. 2008, 45, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Q.; Qiu, X.H.; Zheng, Z.L.; Xie, C.H.; Xu, Z.F.; Han, R.H. Characteristics of the degenerate strains of Cordyceps militaris. Mycosystema 2010, 29, 670–677. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.-M.; Zhang, D.-D.; Huang, Z.-Y.; Fu, M.-J. The Stress of Fungicides Changes the Expression of Clock Protein CmFRQ and the Morphology of Fruiting Bodies of Cordyceps militaris. J. Fungi 2024, 10, 150. https://doi.org/10.3390/jof10020150
Peng J-M, Zhang D-D, Huang Z-Y, Fu M-J. The Stress of Fungicides Changes the Expression of Clock Protein CmFRQ and the Morphology of Fruiting Bodies of Cordyceps militaris. Journal of Fungi. 2024; 10(2):150. https://doi.org/10.3390/jof10020150
Chicago/Turabian StylePeng, Jing-Mei, Dan-Dan Zhang, Zi-Yan Huang, and Ming-Jia Fu. 2024. "The Stress of Fungicides Changes the Expression of Clock Protein CmFRQ and the Morphology of Fruiting Bodies of Cordyceps militaris" Journal of Fungi 10, no. 2: 150. https://doi.org/10.3390/jof10020150




