Aphids May Facilitate the Spread of Sclerotinia Stem Rot in Oilseed Rape by Carrying and Depositing Ascospores
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Aphids
2.3. Determination of Host Preference by Y-Tube Olfactometer
2.4. Fungi
2.5. Identification of S. sclerotiorum
2.6. Disease Assessment
2.7. Relationship between Aphid Incidence and Incidence of S. sclerotiorum in the Field
2.8. Determining If Aphids Could Transfer SSR from Symptomatic Oilseed Rape Seedlings to Healthy Oilseed Rape Seedlings in a Laboratory
2.8.1. Influence of Aphid Feeding on the Incidence of S. sclerotiorum
2.8.2. Ability of Aphids to Carry Ascospores of S. sclerotiorum
2.9. EPG Experiments
2.10. Statistical Analyses
3. Results
3.1. Host Selection Studies
3.2. Sclerotinia Disease Assessment
3.3. Relationship between Aphid Populations and the Incidence of S. sclerotiorum in the Field
3.4. Influence of Aphid Feeding on the Development of S. sclerotiorum
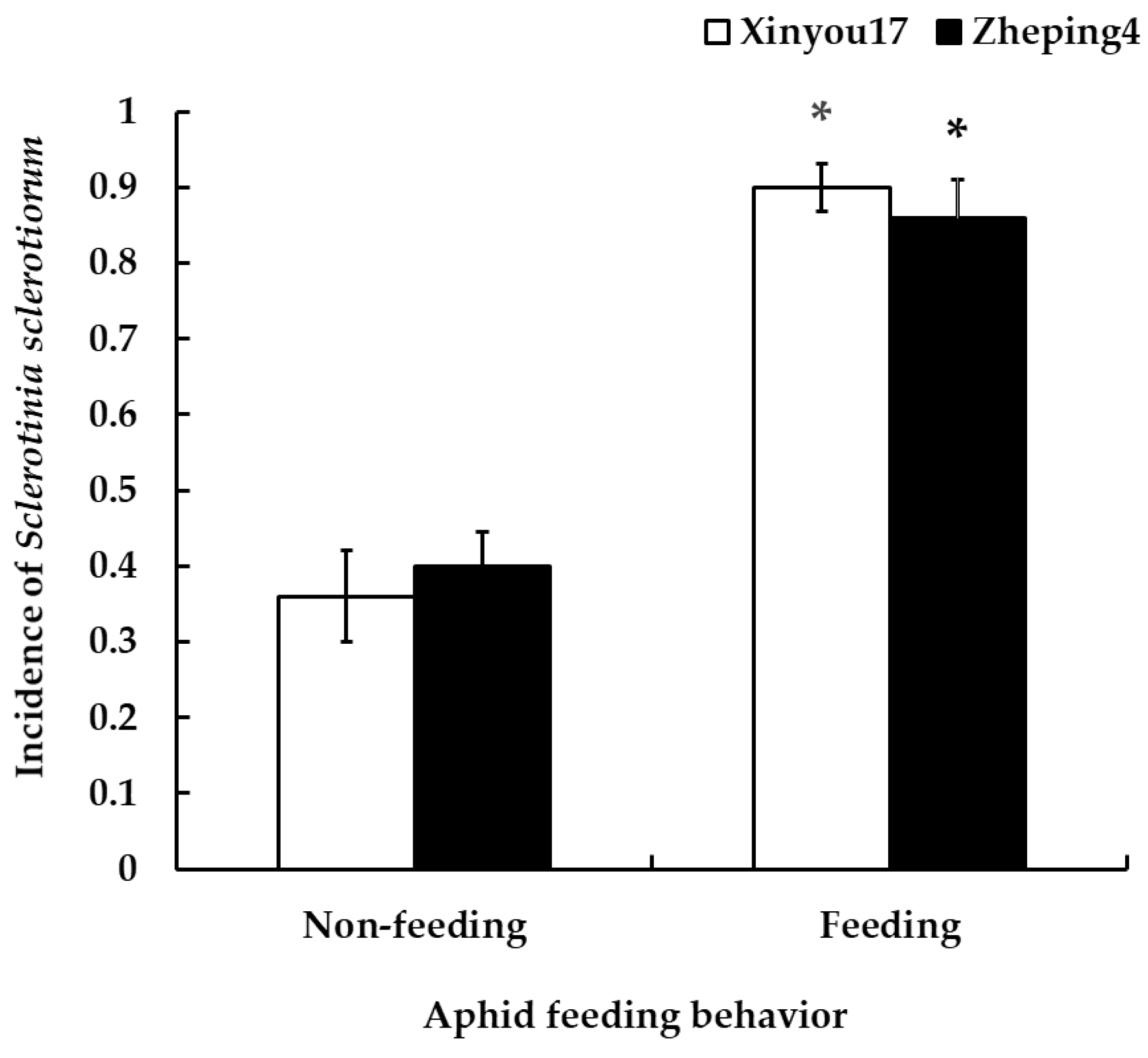
3.4.1. Influence of Aphid Feeding and Non-Feeding on the Incidence of S. sclerotiorum
3.4.2. Ability of Aphids to Transmit Ascospores
3.5. Determination of Aphid Feeding Behavior by the EPG Technique
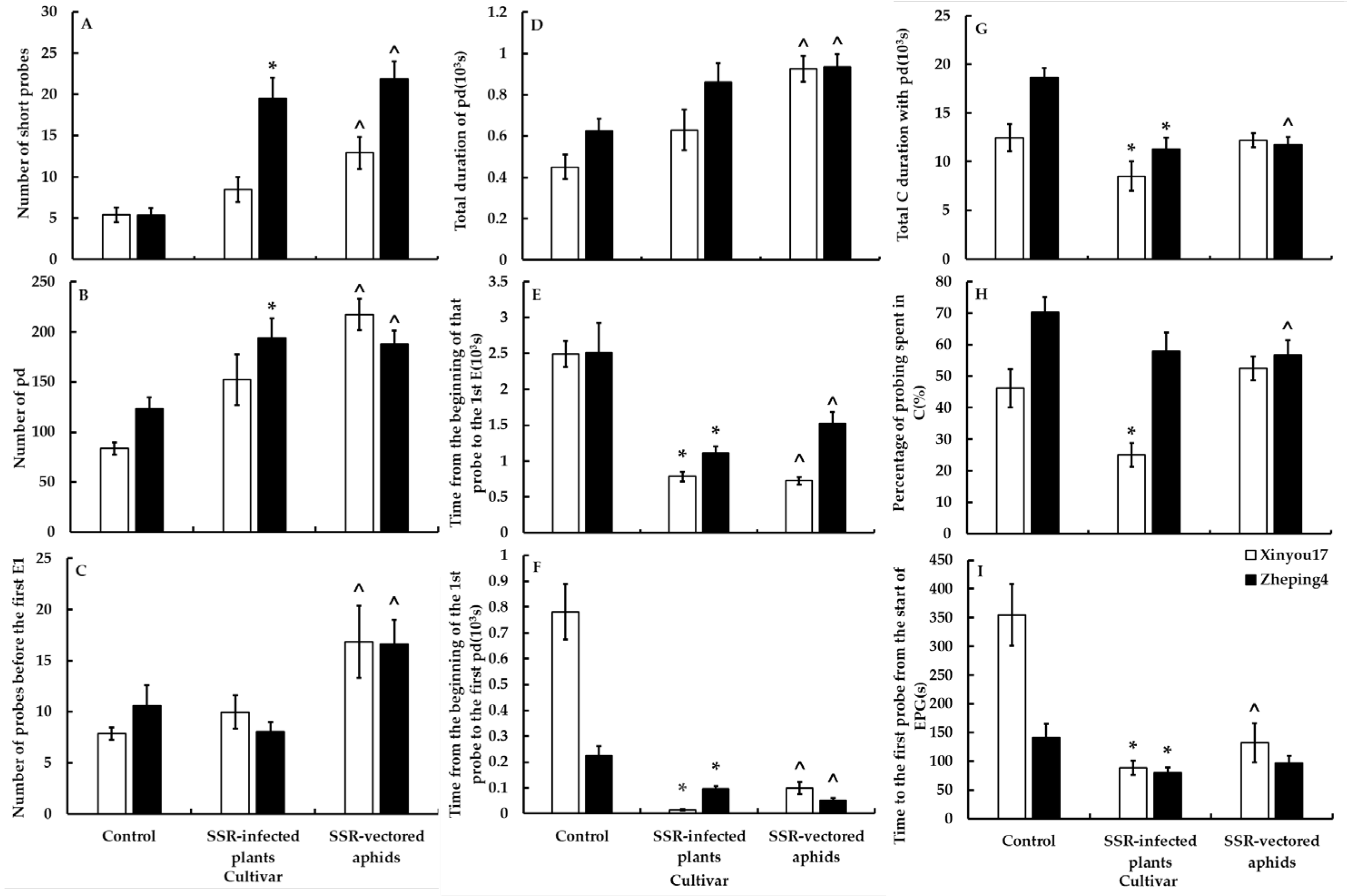
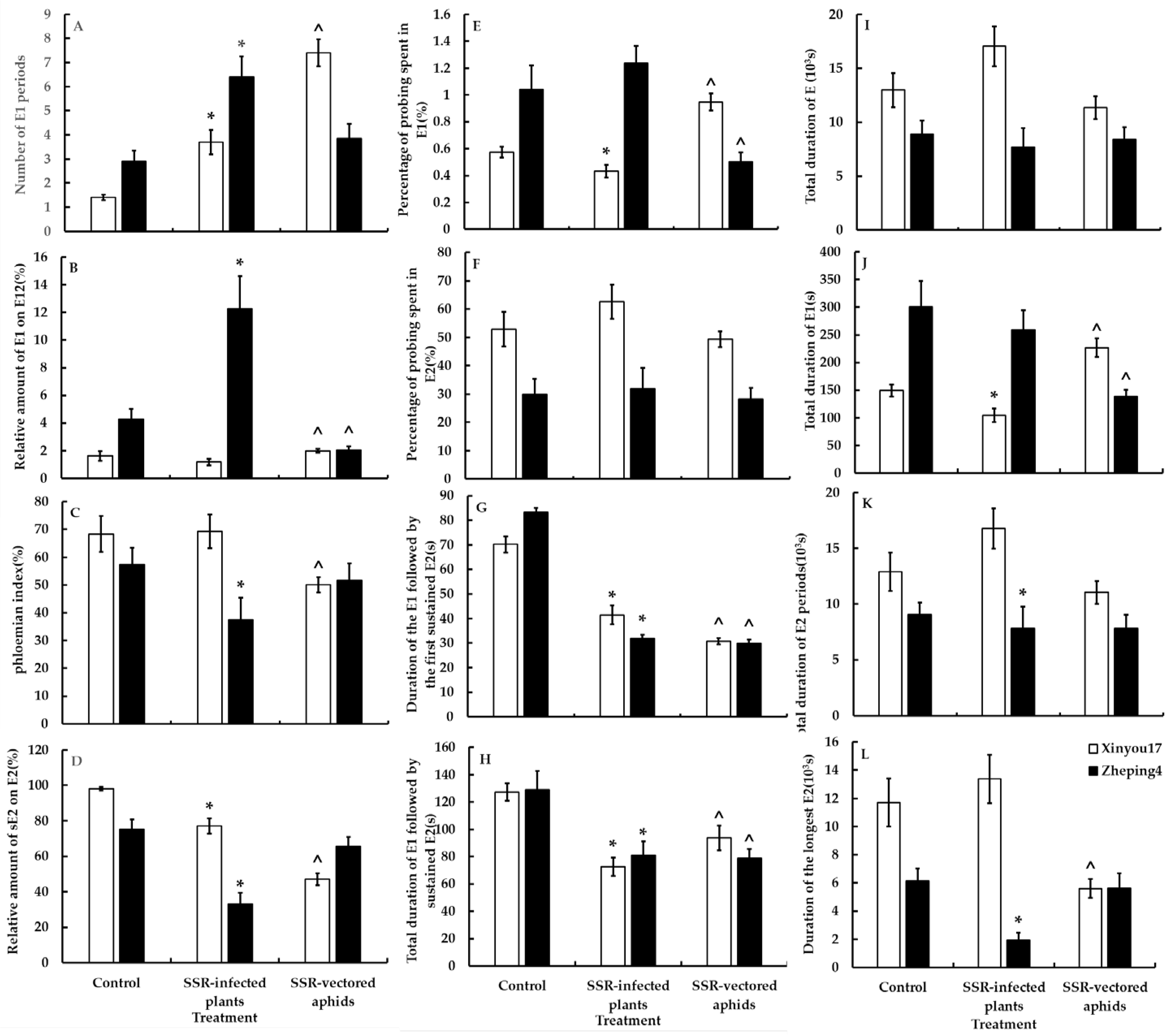
3.5.1. Treatment I—Changes in the Feeding Behavior of Aphids in Plants Infected with S. sclerotiorum
3.5.2. Treatment II—The Effect of Ascospores on the Feeding Behavior of Aphids
3.5.3. Analysis of Cultivar and S. sclerotiorum Infection Main Effects
4. Discussion
4.1. Differences in Resistance between the Two Cultivars
4.2. The Relationship between Aphids and the Occurrence of S. sclerotiorum
4.3. Effect of S. sclerotiorum-Infected Plants on the Feeding Behavior of Aphids
4.4. Changes in the Feeding Behavior of Aphids Carrying Ascospores
4.5. Relationship between Aphids and S. sclerotiorum and the Effect of Oilseed Rape Cultivars
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, X.; Koopmann, B.; Ulber, B.; von Tiedemann, A. A global survey on diseases and pests in oilseed rape-current challenges and innovative strategies of control. Front. Agron. 2020, 2, 590908. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, W.; Huang, C. Statistics and analysis of oilseed rape losses caused by main diseases and insect pests in recent 10 years. Plant Prot. 2018, 44, 24–30. [Google Scholar]
- Fening, K.O.; Forchibe, E.E.; Wamonje, F.O.; Adama, I.; Afreh-Nuamah, K.; Carr, J.P. First report and distribution of the indian mustard aphid, Lipaphis erysimi pseudobrassicae (Hemiptera: Aphididae) on cabbage (Brassica oleracea var capitata) in Ghana. J. Econ. Entomol. 2020, 113, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, M.C.; Denton-Giles, M. The control of Sclerotinia stem rot on oilseed rape (Brassica napus): Current practices and future opportunities. Plant Pathol. 2016, 65, 859–877. [Google Scholar] [CrossRef]
- Khan, M.A.; Cowling, W.; Banga, S.S.; You, M.P.; Tyagi, V.; Bharti, B.; Barbetti, M.J. Patterns of inheritance for cotyledon resistance against Sclerotinia sclerotiorum in Brassica napus. Euphytica 2020, 216, 79. [Google Scholar] [CrossRef]
- Sharma, P.; Meena, P.; Verma, P.; Saharan, G.; Mehta, N.; Singh, D.; Kumar, A. Sclerotinia sclerotiorum (lib) de Bary causing sclerotinia rot in oilseed Brassicas: A review. J. Oilseed Brassica 2016, 1, 1–44. [Google Scholar]
- Duarte, P.; Menze, L.; Abdelrasoul, G.N.; Yosinski, S.; Kobos, Z.; Stuermer, R.; Reed, M.; Yang, J.; Li, S.; Chen, J. Single ascospore detection for the forecasting of Sclerotinia stem rot of canola. Lab Chip 2020, 20, 3644–3652. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.P.; Staveley, J.; Phelps, K.; Young, C.S.; Whipps, J.M. Ascospore release and survival in Sclerotinia sclerotiorum. Mycol. Res. 2003, 107, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Mclean, D.M. Role of dead flower parts in infection of certain crucifers by Sclerotinia sclerotiorum (Lib.) de Bary. Plant Dis. Rep. 1958, 42, 663. [Google Scholar]
- Inglis, G.D.; Boland, G.J. The microflora of bean and rapeseed petals and the influence of the microflora of bean petals on white mold. Can. J. Plant Pathol. 1990, 12, 129–134. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Guo, S.; Yang, G.; Dan, B. Advances in research on rape Sclerotia and resistance breeding. Asian Agric. Res. 2020, 12, 55–60. [Google Scholar] [CrossRef]
- Jamaux, I.; Gelie, B.; Lamarque, C. Early stages of infection of rapeseed petals and leaves by Sclerotinia sclerotiorum revealed by scanning electron microscopy. Plant Pathol. 1995, 44, 22–30. [Google Scholar] [CrossRef]
- Luo, Z.W.; Lv, J. Studies on the control of Sclerotinia sclerotiorum at seedling stage under different treatment conditions of oilseed rape seeds before sowing. Heilongjiang Sci. Technol. Inf. 2011, 22, 261. [Google Scholar]
- Dillard, H.R.; Cobb, A.C. Relationship between leaf injury and colonisation of cabbage by Sclerotinia sclerotiorum. Crop Prot. 1995, 14, 677–682. [Google Scholar] [CrossRef]
- Yamoah, E. A Model System Using Insects to Vector Fusarium tumidum for Biological Control of Gorse (Ulex europaeus). Ph.D. Thesis, Lincoln University, Canterbury, New Zealand, 2007. [Google Scholar]
- Feldman, T.S.; O’Brien, H.E.; Arnold, A.E. Moths that vector a plant pathogen also transport Endophytic fungi and Mycoparasitic antagonists. Microb. Ecol. 2008, 56, 742–750. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Hu, H. Prelimiary study on insects carrying Sclerotinia Sclerotiorum. J. Anhui Agric. Univ. 1996, 23, 493–495. [Google Scholar]
- Syazwan, S.A.; Mohd-Farid, A.; Wan-Muhd-Azrul, W.-A.; Syahmi, H.M.; Zaki, A.M.; Ong, S.P.; Mohamed, R. Survey, identification, and pathogenicity of Ceratocystis fimbriata complex associated with wilt disease on Acacia mangium in Malaysia. Forests 2021, 12, 1782. [Google Scholar] [CrossRef]
- de Nooij, M.P. The role of weevils in the infection process of the fungus Phomopsis subordinaria in Plantago lanceolata. Oikos 1988, 52, 51–58. [Google Scholar] [CrossRef]
- Fermaud, M.; LeMenn, R. Association of Botrytis cinerea with grape berry moth larvae. Phytopathology 1989, 79, 651–656. [Google Scholar] [CrossRef]
- Fermaud, M.; Menn, R.I.; Le Menn, R. Transmission of Botrytis cinerea to grapes by grape berry moth larvae. Phytopathology 1992, 82, 1393–1398. [Google Scholar] [CrossRef]
- Isaacs, R.; Mason, K.S.; Maxwell, E. Stage-specific control of grape berry moth, Endopiza viteana (Clemens) (Lepidoptera: Tortricidae), by selective and broad spectrum insecticides. J. Econ. Entomol. 2005, 98, 415–422. [Google Scholar] [CrossRef]
- Klein, T.A.; Auld, B.A. Wounding can improve efficacy of Colletotrichum orbiculare as a mycoherbicide for Bathurst burr. Aust. J. Exp. Agric. 1996, 36, 185–187. [Google Scholar] [CrossRef]
- Hatcher, P.E. Three-way interactions between plant pathogenic fungi, herbivorous insects and their host plants. Biol. Rev. 1995, 70, 639–694. [Google Scholar] [CrossRef]
- Murray, D.C.; Walters, D.R. Increased photosynthesis and resistance to rust infection in upper, uninfected leaves of rusted broad bean (Vicia faba). New Phytol. 1992, 120, 235–242. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006, 57, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Tjallingii, W.F.; Hogen Esch, T. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 1993, 18, 317–328. [Google Scholar] [CrossRef]
- Will, T.; Tjallingii, W.F.; Thonnessen, A.; van Bel, A.J.E. Molecular sabotage of plant defence by aphid saliva. Proc. Natl. Acad. Sci. USA 2007, 104, 10536–10541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.X.; Zhong, T.S. New species of Chinese Aphidinae (Homoptera: Aphididae). Entomotaxonomia 1983, 5, 37–42. [Google Scholar]
- Liu, H.Q.; Xie, M. Field management of oilseed rape cultivation and control measures of Sclerotinia sclerotiorum. Farmers Consult. 2021, 22, 21–22. [Google Scholar]
- Hao, Z.P.; Sheng, L.; Feng, Z.B.; Fei, W.X.; Hou, S.M. Turnip mosaic virus infection differentially modifies cabbage aphid probing behavior in spring and winter oilseed rape (Brassica napus). Insects 2022, 13, 791. [Google Scholar] [CrossRef]
- Boszoradova, E.; Libantova, J.; Matusikova, I.; Poloniova, Z.; Jopcik, M.; Berenyi, M.; Moravcikova, J. Agrobacterium-mediated genetic transformation of economically important oilseed rape cultivars. Plant Cell Tissue Organ Cult. 2011, 107, 317–323. [Google Scholar] [CrossRef]
- Seifbarghi, S.; Borhan, M.H.; Wei, Y.; Coutu, C.; Robinson, S.J.; Hegedus, D.D. Changes in the Sclerotinia sclerotiorum transcriptome during infection of Brassica napus. BMC Genom. 2017, 18, 266. [Google Scholar] [CrossRef]
- Hao, Z.P.; Hou, S.M.; Hu, B.C.; Huang, F.; Dang, X.L. Assessment of probing behavior of the cabbage aphid, Brevicoryne brassicae (Hemiptera: Aphididae), on three Brassica napus cultivars at three developmental stages using Electropenetrography (EPG). J. Kans. Entomol. Soc. 2017, 90, 11–23. [Google Scholar] [CrossRef]
- Hao, Z.P.; Zhan, H.X.; Wang, Y.L.; Hou, S.M. How cabbage aphids Brevicoryne brassicae (L.) make a choice to feed on Brassica napus cultivars. Insects 2019, 10, 75. [Google Scholar] [CrossRef]
- George, J.; Jenkins, N.E.; Blanford, S.; Thomas, M.B.; Baker, T.C. Malaria mosquitoes attracted by fatal fungus. PLoS ONE 2013, 8, e62632. [Google Scholar] [CrossRef] [PubMed]
- Qandah, I.S. Epidemiological Studies on Sclerotinia Stem Rot Caused by Sclerotinia sclerotiorum (Lib.) de Bary on Canola. Ph.D. Thesis, North Dakota State University, Fargo, ND, USA, 2008. [Google Scholar]
- Ziesman, B.R.; Turkington, T.K.; Basu, U.; Strelkov, S.E. A quantitative PCR system for measuring Sclerotinia sclerotiorum in canola (Brassica napus). Plant Dis. 2016, 100, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Qian, L.; Disi, J.O.; Yang, X.; Li, Q.; Li, J.; Frauen, M.; Cai, D.; Qian, W. Identification of resistant sources against Sclerotinia sclerotiorum in Brassica species with emphasis on B. oleracea. Euphytica 2011, 177, 393–399. [Google Scholar] [CrossRef]
- Mei, J.; Wei, D.; Disi, J.O.; Ding, Y.; Liu, Y.; Qian, W. Screening resistance against Sclerotinia sclerotiorum in Brassica crops with use of detached stem assay under controlled environment. Eur. J. Plant Pathol. 2012, 134, 599–604. [Google Scholar] [CrossRef]
- Zhu, Z.H. Identification and evaluation of Sclerotinia sclerotiorum resistant varieties for oilseed rape. Anhui Agric. Sci. Bull. 2018, 24, 50–52, 57. [Google Scholar]
- Uloth, M.B.; You, M.P.; Barbetti, M.J. Host resistance to Sclerotinia stem rot in historic and current Brassica napus and B. juncea varieties: Critical management implications. Crop Pasture Sci. 2015, 66, 841–848. [Google Scholar] [CrossRef]
- Zhao, J.; Buchwaldt, L.; Rimmer, S.R.; Sharpe, A.; Mcgregor, L.; Bekkaoui, D.; Hegedus, D. Patterns of differential gene expression in Brassica napus cultivars infected with Sclerotinia sclerotiorum. Mol. Plant Pathol. 2009, 10, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, S.R.; Kutcher, H.R.; Morrall, R.A.A. Diseases of canola and mustard. In Diseases of Field Crops in Canada, 3rd ed.; Bailey, K.L., Gossen, B.D., Gugel, R.K., Morrall, R.A.A., Eds.; University Extension Press: Saskatoon, SK, Canada, 2003; pp. 129–146. [Google Scholar]
- Qin, Y.L.; Cao, Y.H.; Zheng, P.B.; Zhou, W. Comparison of Sclerotinia sclerotiorum resistance in different oilseed rape varieties. Hubei Plant Prot. 2020, 178, 31–32, 35. [Google Scholar]
- Al-Shindah, R.S.D.; Hassan, A.A.; Mansour, M.S. Isolation and identification of entomopathogenic fungi from of green peach sphid Myzus persicae and evaluation of their activity for insect control. IOP Conf. Ser. Earth Environ. Sci. 2022, 1060, 012093. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical recording of stylet penetration activities. In Aphids, Their Biology, Natural Enemies and Control, 1st ed.; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 95–108. [Google Scholar]
- Zamani-Noor, N. Baseline sensitivity and control efficacy of various group of fungicides against Sclerotinia sclerotiorum in oilseed rape cultivation. Agronomy 2021, 11, 1758. [Google Scholar] [CrossRef]
- Hao, Z.-P.; Feng, Z.-B.; Sheng, L.; Fei, W.-X.; Hou, S.-M. Aphids on aphid-susceptible cultivars have easy access to turnip mosaic virus, and effective inoculation on aphid-resistant cultivars of oilseed rape (Brassica napus). Plants 2023, 12, 1972. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 3rd ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Awuni, G.A. Rice Injury and Ecology of the Rice Stink Bug, Oebalus pugnax (F.) in the Delta Region of Mississippi. Ph.D. Thesis, Mississippi State University, Starkville, MS, USA, 2013. [Google Scholar]
- Douglas, W.A.; Tullis, E.L. Insects and fungi as causes of pecky rice. USDA Tech. Bull. 1950, 1015, 1–20. [Google Scholar]
- Palmer, L.T.; Kommedahl, T. Root-infecting Fusarium species in relation to rootworm infestations in corn. Phytopathology 1969, 59, 1613–1617. [Google Scholar]
- Warren, H.L.; Kommedahl, T. Prevalence and pathogenicity to corn of Fusarium species from corn roots, rhizosphere, residues, and soil. Phytopathology 1973, 63, 1288–1290. [Google Scholar] [CrossRef]
- Miller, N.L. Responses and Relationships among Fusarium Species, Sweet Corn, and Western Spotted Cucumber Beetles. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2007. [Google Scholar]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as transport devices for plant viruses. C. R. Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects- diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Moran, N.A. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol. Ecol. 2013, 23, 14731496. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.W. Pathogens of eriophyoid mites. In Eriophyoid Mites—Their Biology, Natural Enemies and Control, 1st ed.; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 481–490. [Google Scholar]
- Shamshad, A.; Clift, A.D.; Mansfield, S. The effect of tibia morphology on vector competency of mushroom sciarid flies. J. Appl. Entomol. 2009, 133, 484–490. [Google Scholar] [CrossRef]
- Cloonan, K.R.; Andreadis, S.S.; Chen, H.B.; Jenkins, N.E.; Baker, T.C. Attraction, oviposition and larval survival of the fungus gnat, Lycoriella ingenua, on fungal species isolated from adults, larvae, and mushroom compost. PLoS ONE 2016, 11, e0167074. [Google Scholar] [CrossRef] [PubMed]
- Mazin, M.; Harvey, R.; Andreadis, S.; Pecchia, J.; Cloonan, K.; Rajotte, E.G. Mushroom sciarid fly, Lycoriella ingenua (Diptera: Sciaridae) adults and larvae vector mushroom green mold (Trichoderma aggressivum ft. aggressivum) spores. Appl. Entomol. Zool. 2019, 54, 369–376. [Google Scholar] [CrossRef]
- Braun, S.E.; Sanderson, J.P.; Daughtrey, M.L.; Wraight, S.P. Attraction and oviposition responses of the fungus gnat Bradysia impatiens to microbes and microbe-inoculated seedlings in laboratory bioassays. Entomol. Exp. Appl. 2012, 145, 89–101. [Google Scholar] [CrossRef]
- McLeod, G.; Gries, R.; von Reuss, S.H.; Rahe, J.E.; McIntosh, R.; König, W.A.; Gries, G. The pathogen causing Dutch elm disease makes host trees attract insect vectors. Proc. R. Soc. B Biol. Sci. 2005, 272, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Martini, X.; Willett, D.S.; Kuhns, E.H.; Stelinski, L.L. Disruption of vector host preference with plant volatiles may reduce spread of insect-transmitted plant pathogens. J. Chem. Ecol. 2016, 42, 357–367. [Google Scholar] [CrossRef]
- Smith, R.M.; Alonso-Chavez, V.; Helps, J.; Shaw, M.W.; van den Bosch, F. Modelling lifestyle changes in insect endosymbionts, from insect mutualist to plant pathogen. Evol. Ecol. 2020, 34, 867–891. [Google Scholar] [CrossRef]
- Harrington, T.C. The genus Ceratocystis. Where does the oak wilt fungus fit? In Proceedings of the 2nd National Oak Wilt Symposium Texas Forest Service Publication 166; Billings, R.F., Appel, D.N., Eds.; USDA Forest Service, Forest Health Protection: Austin, TX, USA, 2009; pp. 21–35. [Google Scholar]
- Hulcr, J.; Mann, R.; Stelinski, L. The scent of a partner: Ambrosia beetles are attracted to volatiles from their fungal symbionts. J. Chem. Ecol. 2011, 37, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, R.J.; Wicklow, D.T. Volatiles from Fusarium verticillioides (Sacc.) Nirenb. and their attractiveness to Nitidulid beetles. J. Agric. Food Chem. 1999, 47, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Leath, K.T.; Byers, R.A. Attractiveness of diseased red clover roots to the clover root borer. Phytopathology 1973, 63, 428–431. [Google Scholar] [CrossRef]
- Martini, X.; Hughes, M.A.; Killiny, N.; George, J.; Lapointe, S.L.; Smith, J.A.; Stelinski, L.L. The fungus Raffaelea lauricola modifies behavior of its symbiont and vector, the redbay Ambrosia beetle (Xyleborus glabratus), by altering host plant volatile production. J. Chem. Ecol. 2017, 43, 519–531. [Google Scholar] [CrossRef]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant-pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Huang, L.; Buchenauer, H.; Han, Q.; Zhang, X.; Kang, Z. Ultrastructural and cytochemical studies on the infection process of Sclerotinia sclerotiorum in oilseed rape. J. Plant Dis. Prot. 2008, 115, 9–16. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Q.; Yang, Q.; Liu, H.; Li, Q.; Yi, X.; Cheng, Y.; Guo, L.; Fan, C.; Zhou, Y. Comparative transcriptomic analysis uncovers the complex genetic network for resistance to Sclerotinia sclerotiorum in Brassica napus. Sci. Rep. 2016, 6, 19007. [Google Scholar] [CrossRef]
- Zhao, J.; Peltier, A.J.; Meng, J.; Osborn, T.C.; Grau, C.R. Evaluation of Sclerotinia stem rot resistance in oilseed Brassica napus using a petiole inoculation technique under greenhouse conditions. Plant Dis. 2004, 88, 1033–1039. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simoes, L.C.; Simoes, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef]
- Kroymann, J.; Donnerhacke, S.; Schnabelrauch, D.; Mitchell-Olds, T. Evolutionary dynamics of an Arabidopsis insect resistance quantitative trait locus. Proc. Natl. Acad. Sci. USA 2003, 100, 14587–14592. [Google Scholar] [CrossRef]
- Dubuis, P.-H.; Marazzi, C.; Städler, E.; Mauch, F. Sulphur deficiency causes a reduction in antimicrobial potential and leads to increased disease susceptibility of oilseed rape. J. Phytopathol. 2005, 153, 27–36. [Google Scholar] [CrossRef]
- Kuhlmann, F.; Muller, C. Independent responses to ultraviolet radiation and herbivore attack in Broccoli. J. Exp. Bot. 2009, 60, 3467–3475. [Google Scholar] [CrossRef]
- Barah, P.; Winge, P.; Kusnierczyk, A.; Tran, D.H.; Bones, A.M. Molecular signatures in Arabidopsis thaliana in response to insect attack and bacterial infection. PLoS ONE 2013, 8, e58987. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.H.K.; Laila, R.; Abuyusuf, M.; Park, J.I.; Nou, I.S. Leptosphaeria maculans alters glucosinolate accumulation and expression of aliphatic and indolic glucosinolate biosynthesis genes in blackleg disease-resistant and -susceptible cabbage lines at the seedling stage. Front. Plant Sci. 2020, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, E.; Liu, Y.; Xu, Z.; Hui, M.; Zhang, X.; Cai, M. Transcriptome analysis of two lines of Brassica oleracea in response to early infection with Xanthomonas campestris pv. Campestris. Can. J. Plant Pathol. 2020, 43, 127–139. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates–A review. LWT Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Kurt, Ş.; Güneş, U.; Soylu, E.M. In vitro and in vivo antifungal activity of synthetic pure isothiocyanates against Sclerotinia sclerotiorum. Pest Manag. Sci. 2011, 67, 869–875. [Google Scholar] [CrossRef]
- Warmington, R.; Clarkson, J.P. Volatiles from biofumigant plants have a direct effect on carpogenic germination of sclerotia and mycelial growth of Sclerotinia sclerotiorum. Plant Soil 2016, 401, 213–229. [Google Scholar] [CrossRef]
- Rahmanpour, S.; Backhouse, D.; Nonhebel, H.M. Induced tolerance of Sclerotinia sclerotiorum to isothiocyanates and toxic volatiles from Brassica species. Plant Pathol. 2009, 58, 479–486. [Google Scholar] [CrossRef]
- Li, R.; Rimmer, R.; Buchwaldt, L.; Sharpe, A.G.; Séguin-Swartz, G.; Hegedus, D.D. Interaction of Sclerotinia sclerotiorum with Brassica napus: Cloning and characterization of endo- and exo-polygalacturonases expressed during saprophytic and parasitic modes. Fungal Genet. Biol. 2004, 41, 754–765. [Google Scholar] [CrossRef]
- Kars, I.; van Kan, J.A.L. Extracellular enzymes and metabolites involved in pathogenesis of Botrytis. In Botrytis: Biology, Pathology and Control, 1st ed.; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2004; pp. 99–118. [Google Scholar]
- Krokene, P.; Christiansen, E.; Solheim, H.; Franceschi, V.R.; Berryman, A.A. Induced resistance to pathogenic fungi in Norway spruce. Plant Physiol. 1999, 121, 565–569. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Krekling, T.; Christiansen, E. Phloem parenchyma cells are involved in local and distant defense responses to fungal inoculation or bark-beetle attack in Norway spruce (Pinaceae). Am. J. Bot. 2000, 87, 314–326. [Google Scholar] [CrossRef]
- Lieutier, F.; Yart, A.; Salle, A. Stimulation of tree defenses by ophiostomatoid fungi can explain attack success of bark beetles on conifers. Ann. For. Sci. 2009, 66, 801. [Google Scholar] [CrossRef]
- Kirisits, T. Fungi isolated from Picea abies infested by the bark beetle Ips typographus in the Białowieza forest in north-eastern Poland. For. Pathol. 2010, 40, 100–110. [Google Scholar] [CrossRef]
- Krokene, P. Conifer defense and resistance to bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 177–207. [Google Scholar]
- Netherer, S.; Hammerbacher, A. 4—The Eurasian spruce bark beetle in a warming climate: Phenology, behavior, and biotic interactions. In Bark Beetle Management, Ecology, and Climate Change; Gandhi, K.J.K., Hofstetter, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 89–131. [Google Scholar]
- Zhao, T.; Krokene, P.; Hu, J.; Christiansen, E.; Björklund, N.; Långström, B.; Solheim, H.; Borg-Karlson, A.-K. Induced terpene accumulation in Norway spruce inhibits bark beetle colonization in a dose-dependent manner. PLoS ONE 2011, 6, e26649. [Google Scholar] [CrossRef] [PubMed]
- Lahr, E.C.; Krokene, P. Conifer stored resources and resistance to a fungus associated with the spruce bark beetle Ips typographus. PLoS ONE 2013, 8, e72405. [Google Scholar] [CrossRef]
- Lu, Q.; Decock, C.; Zhang, X.Y.; Maraite, H. Leptographium sinoprocerum sp. nov., an undescribed species associated with Pinus tabuliformis-Dendroctonus valens in northern China. Mycologia 2008, 100, 275–290. [Google Scholar] [CrossRef]
- Lu, Q.; Decock, C.; Zhang, X.; Maraite, H. Ophiostomatoid fungi (Ascomycota) associated with Pinus tabuliformis infested by Dendroctonus valens (Coleoptera) in northern China and an assessment of their pathogenicity on mature trees. Antonie Leeuwenhoek 2009, 96, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Kloth, K.J.; Abreu, I.N.; Delhomme, N.; Petrík, I.; Villard, C.; Ström, C.; Amini, F.; Novák, O.; Moritz, T.; Albrectsen, B.R. PECTIN AceTyLeSTerase9 affects the transcriptome and metabolome and delays aphid feeding. Plant Physiol. 2019, 181, 1704–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhuo, C.; Wang, Z.; Liu, F.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T.; Tu, J. BnaA03.MKK5-BnaA06.MPK3/BnaC03.MPK3 module positively contributes to Sclerotinia sclerotiorum resistance in Brassica napus. Plants 2022, 11, 609. [Google Scholar] [CrossRef]
- Gandhi, A.; Kariyat, R.R.; Chappa, C.; Tayal, M.; Sahoo, N. Tobacco hornworm (Manduca sexta) oral secretion elicits reactive oxygen species in isolated tomato protoplasts. Int. J. Mol. Sci. 2020, 21, 8297. [Google Scholar] [CrossRef]
- Reymond, P. Receptor kinases in plant responses to herbivory. Curr. Opin. Biotechnol. 2021, 70, 143–150. [Google Scholar] [CrossRef]
- Si, H.; Liu, H.; Sun, Y.; Xu, Z.; Liang, S.; Li, B.; Ding, X.; Li, J.; Wang, Q.; Sun, L.; et al. Transcriptome and metabolome analysis reveal that oral secretions from Helicoverpa armigera and Spodoptera litura influence wound-induced host response in cotton. Crop J. 2020, 8, 929–942. [Google Scholar] [CrossRef]
- Hao, Z.-P.; Zhan, H.-X.; Gao, L.-L.; Huang, F.; Zhu, L.-N.; Hou, S.-M. Possible effects of leaf tissue characteristics of oilseed rape Brassica napus on probing and feeding behaviors of cabbage aphids Brevicoryne brassicae. Arthropod-Plant Interact. 2020, 14, 733–744. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Schultz, J.C. Rapid changes in tree leaf chemistry induced by damage: Evidence for communication between plants. Science 1983, 221, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Zeringue, H.J. Changes in cotton leaf chemistry induced by volatile elicitors. Phytochemistry 1987, 26, 1357–1360. [Google Scholar] [CrossRef]
- Choi, D.; Bostock, R.M.; Avdiushko, S.; Hildebrand, D.F. Lipid-derived signals that discriminate wound- and pathogen-responsive isoprenoid pathways in plants: Methyl jasmonate and the fungal elicitor arachidonic acid induce different 3-hydroxy-3-methylglutaryl-coenzyme a reductase genes and antimicrobial isopre. Proc. Natl. Acad. Sci. USA 1994, 91, 2329–2333. [Google Scholar] [CrossRef] [PubMed]
- Zebelo, S.A.; Maffei, M.E. Role of early signalling events in plant-insect interactions. J. Exp. Bot. 2015, 66, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Belete, T. Defense mechanisms of plants to insect pests: From morphological to biochemical approach. Trends Tech. Sci. Res. 2018, 2, 555584. [Google Scholar] [CrossRef]
- Gantner, M.; Najda, A.; Piesik, D. Effect of phenolic acid content on acceptance of hazel cultivars by filbert aphid. Plant Prot. Sci. 2019, 55, 116–122. [Google Scholar] [CrossRef]
- Chapela, I.H.; Rehner, S.A.; Schultz, T.R.; Mueller, U.G. Evolutionary history of the symbiosis between fungus growing ants and their fungi. Science 1994, 266, 1691–1694. [Google Scholar] [CrossRef]
- Farrell, B.D.; Sequeira, A.S.; O’Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [PubMed]
- Aanen, D.K.; Eggleton, P.; Rouland-Lefevre, C.; GuldbergFroslev, T.; Rosendahl, S.; Boomsma, J.J. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc. Natl. Acad. Sci. USA 2002, 99, 14887–14892. [Google Scholar] [CrossRef]
- Madelin, M.F. Fungal parasites of insects. Annu. Rev. Entomol. 1966, 11, 423–448. [Google Scholar] [CrossRef]
- Hofstetter, R.; Cronin, J.; Klepzig, K.; Moser, J.; Ayres, M. Antagonisms, mutualisms and commensalisms affect outbreak dynamics of the southern pine beetle. Oecologia 2006, 147, 679–691. [Google Scholar] [CrossRef]
- Wang, B.; Salcedo, C.; Lu, M.; Sun, J. Mutual interactions between an invasive bark beetle and its associated fungi. Bull. Entomol. Res. 2012, 102, 71–77. [Google Scholar] [CrossRef]
- Ollerton, J. “Biological barter”: Patterns of specialization compared across different mutualisms. In Plant-Pollinator Interactions: From Specialization to Generalization; Waser, N.M., Ollerton, J., Eds.; University of Chicago Press: Chicago, IL, USA, 2006; pp. 411–435. [Google Scholar]
- Janson, E.M. The Evolutionary Ecology of an Insect-Fungus Interaction: Botryosphaeria dothidea, Dymbiotic with the Goldenrod-galling Midge Asteromyia carbonifera (Diptera: Cecidomyiidae). Ph.D. Thesis, Vanderbilt University, Nashville, TN, USA, 2010. [Google Scholar]
- Cloonan, K.R. Behaviorally Active Substances Affecting Reproductive Success of the Fungus Gnat, Lycoriella ingenua, a Pest of White Button Mushrooms, Agaricus bisporus. Ph.D. Thesis, Pennsylvania State University, Harrisburg, PA, USA, 2017. [Google Scholar]
- Kluth, S.; Kruess, A.; Tscharntke, T. Insects as vectors of plant pathogens: Mutualistic and antagonistic interactions. Oecologia 2002, 133, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, B.; Karlovsky, P.; Vidal, S. Interaction between western corn rootworm (Coleoptera: Chrysomelidae) larvae and root-infecting Fusarium verticillioides. Environ. Entomol. 2010, 39, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Drakulic, J.; Caulfield, J.; Woodcock, C.; Jones, S.P.T.; Linforth, R.; Bruce, T.J.A.; Ray, V. Sharing a host plant (wheat [Triticum aestivum]) increases the fitness of Fusarium graminearum and the severity of Fusarium head blight but reduces the fitness of grain aphids (Sitobion avenae). Appl. Environ. Microbiol. 2015, 81, 3492–3501. [Google Scholar] [CrossRef]
- Hao, Z.-P. Aphids may facilitate the spread of Sclerotinia stem rot in oilseed rape by carrying and depositing ascospores. Mendeley Data 2024, V1. [Google Scholar] [CrossRef]
| Relative Resistant Index (RRI) | Evaluation of Resistance |
|---|---|
| RRI ≤ −1.2 | High resistance (HR) |
| −1.2< RRI ≤ −0.7 | Medium resistance (MR) |
| −0.7 < RRI ≤ 0 | Low resistance (LR) |
| 0 < RRI ≤ 0.9 | Low susceptibility (LS) |
| 0.9 < RRI ≤ 2.0 | Medium susceptibility (MS) |
| RRI > 2.0 | High susceptibility (HS) |
| Cultivar | Relative Resistant Index (RRI) | Evaluation of Resistance | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Xinyou17 | −0.01 | −0.12 | −0.08 | LR |
| Zheping4 | 0.43 | 0.41 | 0.42 | LS |
| Cultivar | Number of Aphids/100 Plants | Incidence of S. sclerotiorum %/100 Plants | Thousand-Seed Weight (g) | Number of Aphids after Insecticide Application/100 Plants | Incidence of S. sclerotiorum Following Insecticide Application %/100 Plants | Thousand-Seed Weight after Insecticide Application (g) |
|---|---|---|---|---|---|---|
| 2019 | ||||||
| Xinyou17 | 875.86 | 15.44 | 3.16 | 32.45 | 5.51 | 3.30 |
| Zheping4 | 5894.40 | 42.04 | 3.29 | 288.97 | 31.27 | 3.30 |
| 2020 | ||||||
| Xinyou17 | 996.67 | 22.15 | 3.05 | 14.76 | 4.39 | 3.11 |
| Zheping4 | 2392.86 | 25.74 | 3.29 | 8.10 | 15.26 | 3.32 |
| 2021 | ||||||
| Xinyou17 | 220.00 | 13.50 | 3.13 | 11.43 | 4.16 | 3.18 |
| Zheping4 | 3168.10 | 37.74 | 3.10 | 13.81 | 21.71 | 3.06 |
| Cultivar × Environment Interaction | p < 0.0001 | p < 0.0001 | p = 0.0485 | p < 0.0001 | p < 0.0001 | p = 0.6061 |
| Variable | Treatment 1 | Cultivar Main Effects | Sclerotinia sclerotiorum Infection Main Effects | Cultivar × S. sclerotiorum Infection Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | Partial η2 | F | p | Partial η2 | F | p | Partial η2 | ||
| Surface-mesophyll (Leaf) | ||||||||||
| Time to the first probe from the start of EPG | Plant | 8.71 | 0.0042 | 0.1028 | 36.96 | 0.0000 | 0.3272 | 6.23 | 0.0147 | 0.0758 |
| Aphid | 8.87 | 0.0039 | 0.1045 | 21.82 | 0.0000 | 0.2231 | 6.47 | 0.0130 | 0.0785 | |
| Number of short probes | Plant | 10.29 | 0.0020 | 0.1193 | 23.04 | 0.0000 | 0.2326 | 9.77 | 0.0025 | 0.1139 |
| Aphid | 7.35 | 0.0083 | 0.0882 | 69.44 | 0.0000 | 0.4775 | 6.84 | 0.0108 | 0.0825 | |
| Time from the beginning of the first probe to the first pd | Plant | 5.04 | 0.0277 | 0.0622 | 163.83 | 0.0000 | 0.6831 | 85.28 | 0.0000 | 0.5288 |
| Aphid | 15.06 | 0.0002 | 0.1654 | 66.93 | 0.0000 | 0.4683 | 2.13 | 0.1484 | 0.0273 | |
| Number of pd | Plant | 7.21 | 0.0089 | 0.0867 | 13.78 | 0.0004 | 0.1535 | 0.02 | 0.8901 | 0.0003 |
| Aphid | 0.62 | 0.4343 | 0.0081 | 66.98 | 0.0000 | 0.4685 | 7.86 | 0.0064 | 0.0937 | |
| Total duration of pd | Plant | 8.00 | 0.0060 | 0.0952 | 5.15 | 0.0261 | 0.0635 | 0.00 | 0.9845 | 0.0000 |
| Aphid | 3.28 | 0.0742 | 0.0413 | 34.69 | 0.0000 | 0.3134 | 2.91 | 0.0921 | 0.0369 | |
| Time from the beginning of that probe to the first E | Plant | 1.24 | 0.2699 | 0.0160 | 80.21 | 0.0000 | 0.5135 | 6.07 | 0.0160 | 0.0740 |
| Aphid | 6.71 | 0.0115 | 0.0812 | 58.13 | 0.0000 | 0.4334 | 15.09 | 0.0002 | 0.1657 | |
| Total C duration with pd | Plant | 13.88 | 0.0004 | 0.1544 | 17.34 | 0.0001 | 0.1857 | 0.06 | 0.8037 | 0.0008 |
| Aphid | 7.35 | 0.0083 | 0.0882 | 4.29 | 0.0418 | 0.0534 | 10.55 | 0.0017 | 0.1219 | |
| Percentage of probing spent in C | Plant | 28.36 | 0.0000 | 0.2718 | 10.76 | 0.0016 | 0.1240 | 0.55 | 0.4615 | 0.0072 |
| Aphid | 8.30 | 0.0052 | 0.0984 | 0.89 | 0.3488 | 0.0116 | 4.35 | 0.0403 | 0.0542 | |
| Phloem | ||||||||||
| Number of E1 periods | Plant | 13.63 | 0.0004 | 0.1521 | 31.74 | 0.0000 | 0.2946 | 0.30 | 0.5836 | 0.0040 |
| Aphid | 3.16 | 0.0794 | 0.0399 | 47.67 | 0.0000 | 0.3855 | 25.90 | 0.0000 | 0.2541 | |
| Total duration of E1 | Plant | 26.94 | 0.0000 | 0.2617 | 4.65 | 0.0342 | 0.0576 | 1.52 | 0.2221 | 0.0195 |
| Aphid | 0.02 | 0.8795 | 0.0003 | 0.97 | 0.3287 | 0.0126 | 24.35 | 0.0000 | 0.2426 | |
| Duration of the E1 followed by the first sustained E2 | Plant | 0.05 | 0.8238 | 0.0007 | 177.64 | 0.0000 | 0.7004 | 11.72 | 0.0010 | 0.1336 |
| Aphid | 3.50 | 0.0654 | 0.0440 | 495.09 | 0.0000 | 0.8669 | 6.90 | 0.0104 | 0.0833 | |
| Relative amount of E1 on E12 | Plant | 38.49 | 0.0000 | 0.3362 | 4.59 | 0.0354 | 0.0569 | 8.91 | 0.0038 | 0.1050 |
| Aphid | 10.24 | 0.0020 | 0.1187 | 2.17 | 0.1448 | 0.0278 | 10.99 | 0.0014 | 0.1263 | |
| Percentage of probing spent in E1 | Plant | 28.05 | 0.0000 | 0.2695 | 0.04 | 0.8423 | 0.0005 | 4.34 | 0.0405 | 0.0541 |
| Aphid | 0.81 | 0.3706 | 0.0106 | 0.30 | 0.5853 | 0.0039 | 17.88 | 0.0001 | 0.1905 | |
| Total duration of E2 periods | Plant | 14.03 | 0.0003 | 0.1558 | 1.21 | 0.2749 | 0.0157 | 6.72 | 0.0114 | 0.0812 |
| Aphid | 5.33 | 0.0237 | 0.0655 | 0.73 | 0.3969 | 0.0095 | 0.67 | 0.4158 | 0.0087 | |
| Duration of the longest E2 | Plant | 46.58 | 0.0000 | 0.3800 | 11.34 | 0.0012 | 0.1298 | 17.37 | 0.0001 | 0.1861 |
| Aphid | 4.66 | 0.0340 | 0.0578 | 5.50 | 0.0216 | 0.0675 | 0.84 | 0.3622 | 0.0109 | |
| phloemian index: percentage of the time of the E2 after the start of the first E2 | Plant | 10.83 | 0.0015 | 0.1247 | 2.45 | 0.1217 | 0.0312 | 1.87 | 0.1753 | 0.0240 |
| Aphid | 1.12 | 0.2933 | 0.0145 | 6.06 | 0.0161 | 0.0739 | 1.71 | 0.1956 | 0.0219 | |
| Relative amount of sE2 on E2 | Plant | 40.36 | 0.0000 | 0.3469 | 45.79 | 0.0000 | 0.3760 | 2.88 | 0.0940 | 0.0365 |
| Aphid | 0.42 | 0.5185 | 0.0055 | 53.30 | 0.0000 | 0.4122 | 23.87 | 0.0000 | 0.2390 | |
| Percentage of probing spent in E2 | Plant | 19.18 | 0.0000 | 0.2015 | 0.89 | 0.3475 | 0.0116 | 0.36 | 0.5492 | 0.0047 |
| Aphid | 20.93 | 0.0000 | 0.2160 | 0.06 | 0.8117 | 0.0008 | 0.11 | 0.7428 | 0.0014 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.-P.; Sheng, L.; Feng, Z.-B.; Fei, W.-X.; Hou, S.-M. Aphids May Facilitate the Spread of Sclerotinia Stem Rot in Oilseed Rape by Carrying and Depositing Ascospores. J. Fungi 2024, 10, 202. https://doi.org/10.3390/jof10030202
Hao Z-P, Sheng L, Feng Z-B, Fei W-X, Hou S-M. Aphids May Facilitate the Spread of Sclerotinia Stem Rot in Oilseed Rape by Carrying and Depositing Ascospores. Journal of Fungi. 2024; 10(3):202. https://doi.org/10.3390/jof10030202
Chicago/Turabian StyleHao, Zhong-Ping, Lei Sheng, Zeng-Bei Feng, Wei-Xin Fei, and Shu-Min Hou. 2024. "Aphids May Facilitate the Spread of Sclerotinia Stem Rot in Oilseed Rape by Carrying and Depositing Ascospores" Journal of Fungi 10, no. 3: 202. https://doi.org/10.3390/jof10030202
APA StyleHao, Z.-P., Sheng, L., Feng, Z.-B., Fei, W.-X., & Hou, S.-M. (2024). Aphids May Facilitate the Spread of Sclerotinia Stem Rot in Oilseed Rape by Carrying and Depositing Ascospores. Journal of Fungi, 10(3), 202. https://doi.org/10.3390/jof10030202






