Just around the Corner: Advances in the Optimization of Yeasts and Filamentous Fungi for Lactic Acid Production
Abstract
:1. Introduction
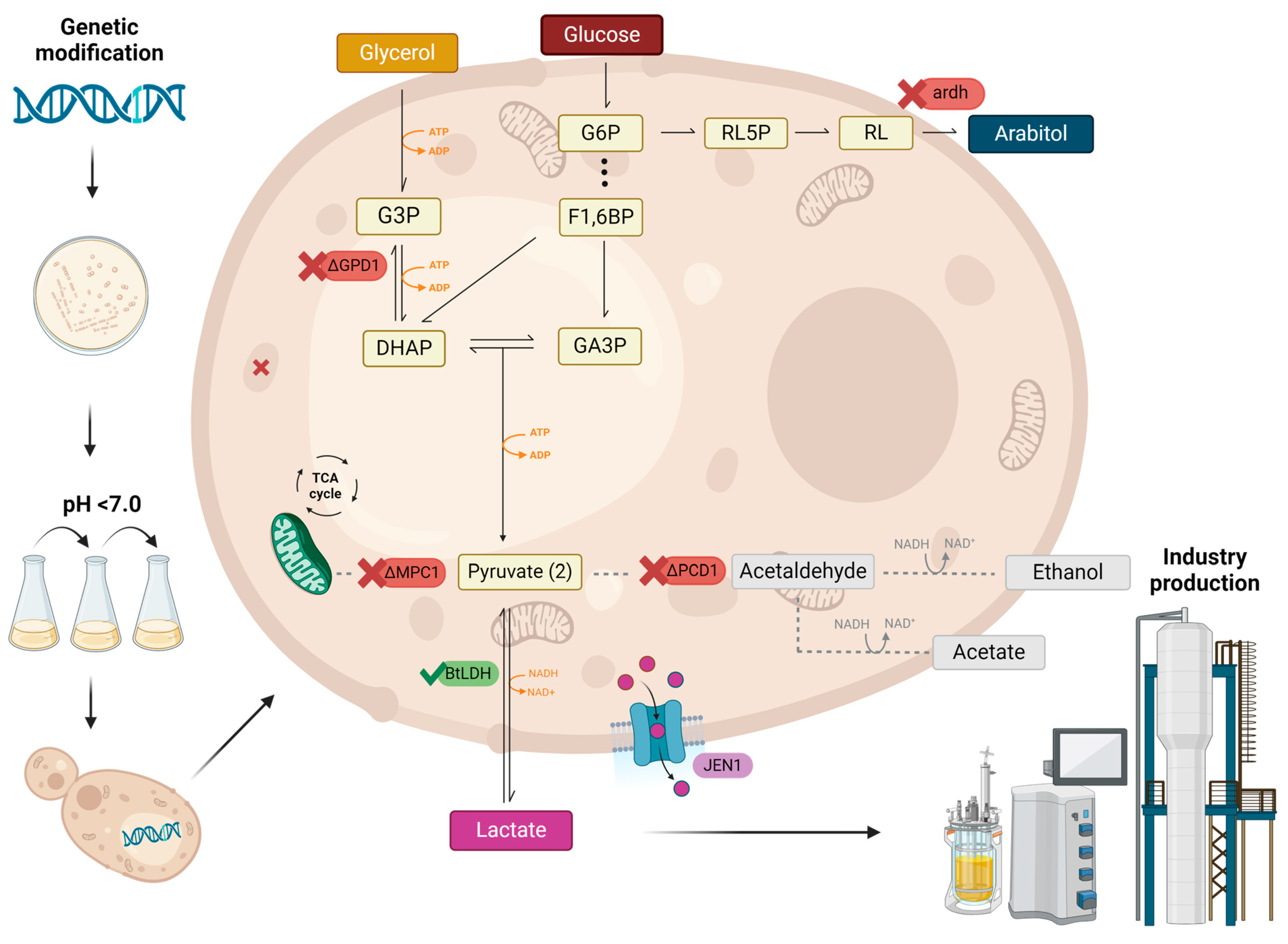
2. Recent Metabolic Engineering Approaches to Improve Lactic Acid Production in Yeast
3. Common Metabolic Engineering Strategies
| Isomer (L, D) | Host | LDH | Complementary Modifications | Buffering Conditions | Titer g/L | Productivity (g∙L−1∙h−1) | Yield (g/g) | Carbon Source | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| L | Aspergillus oryzae | Bos taurus | Disruption of native LDH gene | 3% CaCO3 pH 6.0 | 30 | 100 g/L of starch (dextrin or maltose) | [63] | ||
| L | A. oryzae | B. taurus | 3% CaCO3 | 50.1 | 0.29 | 0.51 | 100 g/L glucose | [64] | |
| L | A. oryzae | B. taurus | ∆pdcA | 56.4 | 0.33 | 0.58 | |||
| L | A. oryzae | B. taurus | ∆mpcA | 65.4 | 0.45 | 0.67 | |||
| L | A. oryzae | B. taurus | ∆pcdA/mpcA | 81.2 | 0.67 | 0.81 | |||
| L | A. oryzae | Lactococcus lactis | ∆pcdA/mpcA without BtLDH | 90.1 | 0.91 | ||||
| L | Aspergillus niger | Mus musculus (11 copies) | Non-neutralized medium | 7.7 | 60 g/L glucose | [65] | |||
| L | Candida glycerinogenes | Rhizopus oryzae | LDH gene from R. oryzae was expressed under pH-inducible promoter (PCggmt1) | pH 5.5 | 3.9 | 100 g/L glucose | [38] | ||
| pH 2.5 | 12.3 | ||||||||
| L | Candida sonorensis | Rhizopus oryzae | pdc1Δ::RoLDH pdc2Δ | CaCO3 | 78 | 0.81 | 100 g/L glucose | [43] | |
| L | C. sonorensis | Bacillus megaterium | pdc1Δ::BmLDH pdc2Δ | 84 | 0.85 | ||||
| L | C. sonorensis | Lactobacillus helveticus | pdc1Δ::LhLDH pdc2Δ | 92 | 0.94 | ||||
| L | Kluyveromyces marxianus KM5 | Lactobacillus acidophilus and Staphylococcus epidermidis | Non-neutralized medium | 16 | 50 g/L glucose | [28] | |||
| 3.5% CaCO3 | 24 | 0.48 | |||||||
| L | K. marxianus KM5 | L. acidophilus and B. taurus | Non-neutralized medium | 14.8 | |||||
| 3.5% CaCO3 | 21.2 | ||||||||
| L | K. marxianus BY25571 | Lactiplantibacillus plantarum | ∆pdc1 | Non-neutralized medium | 10.5 | 0.65 | 100 g/L glucose | [66] | |
| D | K. marxianus BY25571 | L. plantarum | ∆pdc1 | 8.9 | 0.66 | ||||
| L | K. marxianus BY25571 | L. plantarum | ∆pdc1 | 3% CaCO3 | 46.3 | 0.80 | |||
| D | K. marxianus BY25571 | L. plantarum | ∆pdc1 | 40.0 | 0.78 | ||||
| L | K. marxianus BY25571 | L. plantarum | ∆pdc1 and ∆cyb2 | NaOH pH 6 | 130 | 2 | 0.98 | 230 g/L Jerusalem artichoke | |
| D | K. marxianus BY25571 | L. plantarum | ∆pdc1 and ∆dld1 | 122 | 2 | 0.95 | |||
| L | K. marxianus YKX001 | B. megaterium | NaOH pH 5.5 | 47.37 | 0.99 | 0.5 | 80 g/L glucose and 20 g/L xylose | [9] | |
| L | K. marxianus YKX001 | Plasmodium falciparum | 50 | 1.04 | 0.55 | ||||
| L | K. marxianus YKX001 | P. falciparum and B. megaterium | Expression of Jen1 from S. cerevisiae, overexpression of native PFK, and ∆dld1 | 103 | 1.44 | 180 g/L corncob residue | |||
| L | Komagataellla phaffii GLJ | B. taurus | Expression of Jen1 from S. cerevisiae | NH4OH pH 5 | 20 | 0.41 | 0.47 | 40 g/L glycerol | [22] |
| L | K. phaffii GLS | B. taurus | Overexpression of native Jen1 | ~28 | 0.67 | 0.67 | 40 g/L glycerol | ||
| L | K. phaffii GLp | B. taurus | ∆pdc1 | 30 | 0.15 | 0.65 | 80 g/L glycerol | ||
| L | K. phaffii GLpard | B. taurus | ∆pdc1 and ∆ardh | 30 | 0.85 | 60 g/L glycerol | [39] | ||
| L | K. phaffii GLpm | B. taurus | ∆pdc1 and ∆mpc1 | 10.25 | 0.15 | 0.27 | 40 g/L glycerol | [40] | |
| D | K. phaffii | Leuconostoc mesenteroides (4 copies) | 3.48 | 0.04 | 0.22 | Methanol | |||
| L | K. phaffii | L. plantarum | Parental strain harbors peroxisomal a CO2-fixation pathway. ∆cyb2 | 2M NaOH | 0.2 | 0.85 mg/g/h | CO2 | [67] | |
| L | O. polymorpha NCYC495 leu1.1 | L. helveticus | PMOX-driven LDH expression, nitrogen source optimization, and adaptive evolution | 3.8 | 0.03 | 0.08 | Methanol | [68] | |
| D | Pichia kudriavzevii NG7 | L. plantarum | ∆pdc1 and adaptive evolution (6% LA) | pH 3.6 | 135 | 3.66 | 0.75 | 100 g/L glucose | [69] |
| pH 4.7 | 154 | 4.16 | 0.72 | ||||||
| L | P. kudriavzevii E1 | Weizmannia coagulans 2–6 and B. taurus | ∆pdc1 and ∆dld | Non-neutralized medium | 74.57 | 0.93 | Glucose | [70] | |
| L | Saccharomyces cerevisiae SP4 | Pediococcus sinensis (3 copies) | ∆pdc1, ∆cyb2, ∆gpd1, ∆nde1 | 26.6 | 0.34 | 80 g/L glucose | [30] | ||
| L | S. cerevisiae SP5 | P. sinensis (4 copies) | ∆pdc1, ∆cyb2, ∆gpd1, ∆trp1, ∆nde1 | 35.8 | 0.46 | ||||
| L | S. cerevisiae SP6 | P. sinensis (4 copies) | ∆pdc1, ∆cyb2, ∆gpd1, ∆trp1, ∆nde1/nde2 | 36.4 | 0.46 | ||||
| L | S. cerevisiae SP7 | P. sinensis (5 copies) | ∆pdc1, ∆cyb2, ∆gpd1, ∆trp1, ∆nde1/nde2 | 37.8 | 0.48 | ||||
| Ca(OH)2 pH 3.5 | 117 | 0.58 | Fed-batch glucose | ||||||
| L | S. cerevisiae EJ4L | R. oryzae | cdt-1, gh1-1, XYL1, XYL2, XYL3; ∆ald6, ∆pho13 | NaOH pH 6 | 83 | 0.42 | 0.66 | 10 g/L glucose 40 g/L xylose 80 g/L cellobiose | [71] |
| 35 g/L CaCO3 | 23.77 | 0.58 | 0.17 | 41 g/L lactose | [72] | ||||
| L | S. cerevisiae SP1130 | B. taurus and P. sinensis japonica | ∆pdc1, ∆cyb2, ∆gpd1, ∆adh1 Expression of mhpF and eutE from E. coli | Ca(OH)2 pH 4.7 | 142 | 3.55 | 0.89 | Fed-batch glucose | [60] |
| D | S. cerevisiae JHY5330 | L. mesenteroides subsp. Mesenteroides | ∆pdc1, ∆adh1, ∆gpd1/2, ∆dld1, ∆jen1. Overexpression of HAA1 | Non-neutralized medium | 48.9 | 0.41 | 0.79 | 70 g/L glucose | [73] |
| CaCO3 | 112 | 2.20 | 0.80 | Fed-batch glucose | |||||
| D | S. cerevisiae JHY5730 | L. mesenteroides | ∆adh1-5, ∆gpd1/2, ∆dld1, ∆pdc1, and adaptative evolution (4% LA). | NaOH pH 3.5 | 82.6 | 1.50 | 0.83 | Fed-batch glucose | [17] |
| L | S. cerevisiae IBB14LA1_5 | P. falciparum | Integration of XR, XDH and XK genes and ∆pdc1. | Non-neutralized medium | 2.6 | 0.04 | 0.18 | xylose | [74] |
| D | S. cerevisiae YIP-J-C-D-A1 | Escherichia coli (3 copies inserted in transposon locus Ty1) | ∆pcd1/6, ∆adh1, ∆dld1, ∆cyb2, and ∆Jen1. | Ca(OH)2 | 80 | 1.10 | 0.60 | Fed-batch glucose | [75] |
| D | S. cerevisiae YIP-I-J-C-D-A1 | E. coli (3 copies) | YIP-J-C-D-A1 plus expression of IoGAS1. | Non-neutralized medium | 85.3 | 1.20 | 0.71 | Fed-batch glucose | [76] |
| D | S. cerevisiae YIP-A15G12 | YIP-I-J-C-D-A1 ∆adh5 ∆gpd2 ∆gpd1 ∆adh3 ∆adh4 | 92.0 | 1.21 | 0.70 | ||||
| L | S. cerevisiae SR8L | L. acidophilus | XYL1, XYL2, XYL3; ∆ald6, ∆pho13 | CaCO3 | 13.4 | 0.67 | 20 g/L xylose | [77] | |
| 11.2 | 0.11 | Acid-treated spent coffee grounds | |||||||
| L | S. cerevisiae BK01 | L. acidophilus | Adaptative evolution (8% LA) | Non-neutralized medium | 119 | 0.72 | 200 g/L glucose | [78] | |
| L | S. cerevisiae PK27 | Lactobacillus lactis, Rhizopus oryzae | Adaptative evolution (7% LA) | Non-neutralized medium | 37.94 | 0.66 | 0.37 | 80 g/L glucose | [55] |
| L | S. cerevisiae NO.2-100 | L. casei, R. oryzae, and B. taurus | ∆pdc1,5,6 and ∆adh1. Expression of ALD from E. coli and overexpression of Jen1. Adaptative evolution (6% LA) | 50 g/L CaCO3 | 121.5 | 1.69 | 0.81 | 90 g/L glucose | [48] |
4. Membrane Transporters
5. Improving Acidity Tolerance in Yeast and Its Influence on Lactic Acid Production
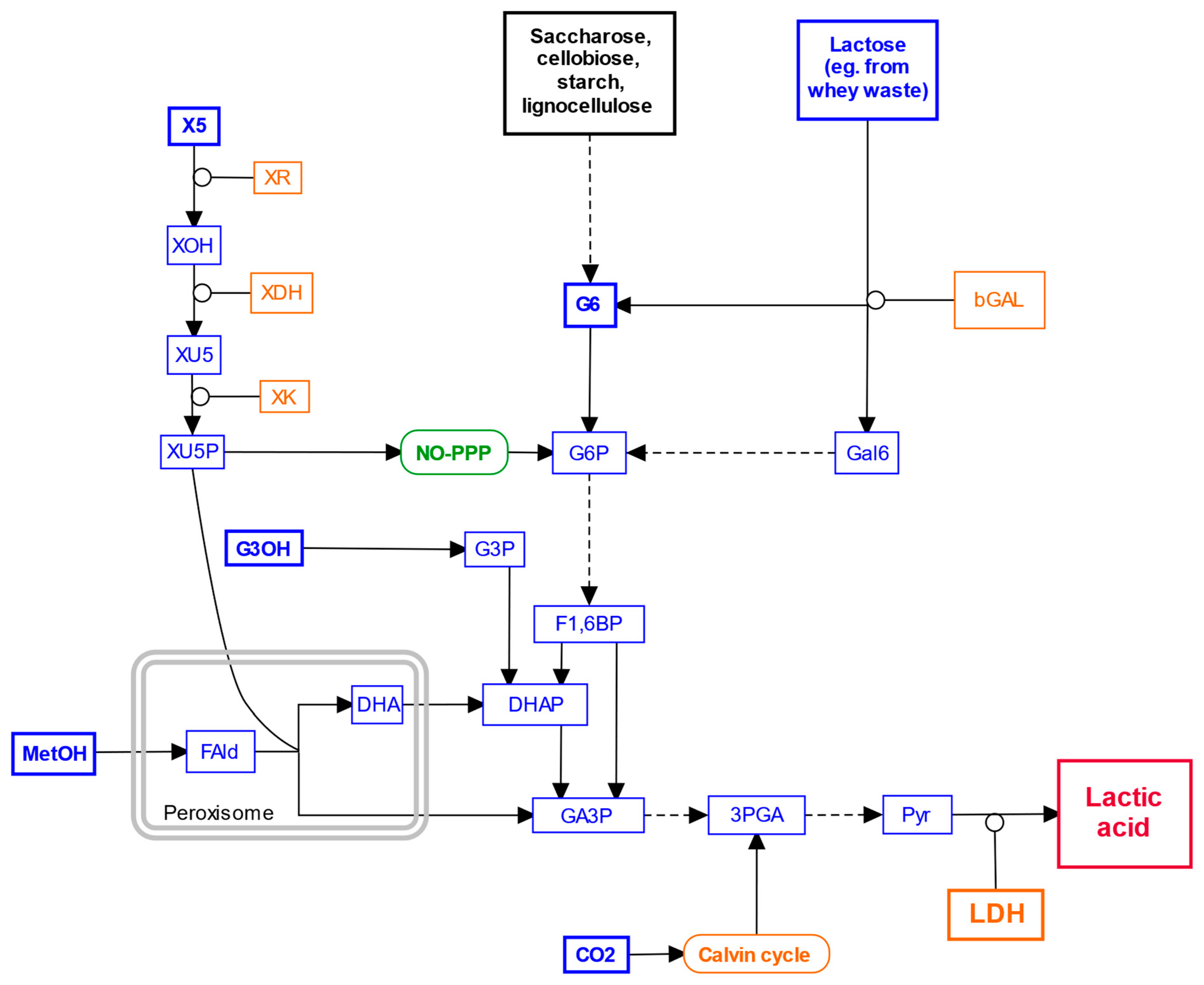
6. Exploring Alternative Carbon Sources
7. Gene Editing for Lactic Acid Production
8. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, C.; Ma, C.; Xu, P. Biotechnological Routes Based on Lactic Acid Production from Biomass. Biotechnol. Adv. 2011, 29, 930–939. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M. Lactic Acid: Recent Advances in Products, Processes and Technologies—A Review. J. Chem. Tech. Biotech. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; Oliveira, R.P.D.S. Lactic Acid Properties, Applications and Production: A Review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Shibata, K.; Sonomoto, K. Isolation and Characterisation of Lactic Acid Bacterium for Effective Fermentation of Cellobiose into Optically Pure Homo L-(+)-Lactic Acid. Appl. Microbiol. Biotechnol. 2011, 89, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative Production of Lactic Acid from Biomass: An Overview on Process Developments and Future Perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef]
- Research, G.V. Lactic Acid and Poly Lactic Acid (PLA) Market Analysis by Application (Packaging, Agriculture, Transport, Electronics, Textiles) and Segment Forecasts to 2020; Grand View Research: San Francisco, CA, USA, 2014; ISBN 978-1-68038-126-9. [Google Scholar]
- Djukić-Vuković, A.; Mladenović, D.; Ivanović, J.; Pejin, J.; Mojović, L. Towards Sustainability of Lactic Acid and Poly-Lactic Acid Polymers Production. Renew. Sustain. Energy Rev. 2019, 108, 238–252. [Google Scholar] [CrossRef]
- Miller, C.; Fosmer, A.; Rush, B.; McMullin, T.; Beacom, D.; Suominen, P. Industrial Production of Lactic Acid. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 179–188. ISBN 978-0-08-088504-9. [Google Scholar]
- Kong, X.; Zhang, B.; Hua, Y.; Zhu, Y.; Li, W.; Wang, D.; Hong, J. Efficient L-Lactic Acid Production from Corncob Residue Using Metabolically Engineered Thermo-Tolerant Yeast. Bioresour. Technol. 2019, 273, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in Research and Development of Bioplastic for Food Packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Comp. Rev. Food Sci. Food Safe 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Park, H.-S.; Hong, C.-K. Relationship between the Stereocomplex Crystallization Behavior and Mechanical Properties of PLLA/PDLA Blends. Polymers 2021, 13, 1851. [Google Scholar] [CrossRef] [PubMed]
- Parra-Ramírez, D.; Martinez, A.; Cardona, C.A. Lactic Acid Production from Glucose and Xylose Using the Lactogenic Escherichia Coli Strain JU15: Experiments and Techno-Economic Results. Bioresour. Technol. 2019, 273, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Willem Schepers, A.; Thibault, J.; Lacroix, C. Lactobacillus Helveticus Growth and Lactic Acid Production during pH-Controlled Batch Cultures in Whey Permeate/Yeast Extract Medium. Part II: Kinetic Modeling and Model Validation. Enzym. Microb. Technol. 2002, 30, 187–194. [Google Scholar] [CrossRef]
- Mazumdar, S.; Blankschien, M.D.; Clomburg, J.M.; Gonzalez, R. Efficient Synthesis of L-Lactic Acid from Glycerol by Metabolically Engineered Escherichia Coli. Microb. Cell Fact. 2013, 12, 7. [Google Scholar] [CrossRef]
- Baek, S.-H.; Kwon, E.Y.; Bae, S.-J.; Cho, B.-R.; Kim, S.-Y.; Hahn, J.-S. Improvement of D-Lactic Acid Production in Saccharomyces cerevisiae under Acidic Conditions by Evolutionary and Rational Metabolic Engineering. Biotechnol. J. 2017, 12, 1700015. [Google Scholar] [CrossRef]
- Lin, H.-T.V.; Huang, M.-Y.; Kao, T.-Y.; Lu, W.-J.; Lin, H.-J.; Pan, C.-L. Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation. Fermentation 2020, 6, 37. [Google Scholar] [CrossRef]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. 16 Years Research on Lactic Acid Production with Yeast—Ready for the Market? Biotechnol. Genet. Eng. Rev. 2010, 27, 229–256. [Google Scholar] [CrossRef]
- Turner, T.L.; Zhang, G.-C.; Kim, S.R.; Subramaniam, V.; Steffen, D.; Skory, C.D.; Jang, J.Y.; Yu, B.J.; Jin, Y.-S. Lactic Acid Production from Xylose by Engineered Saccharomyces cerevisiae without PDC or ADH Deletion. Appl. Microbiol. Biotechnol. 2015, 99, 8023–8033. [Google Scholar] [CrossRef]
- Ishida, N.; Saitoh, S.; Tokuhiro, K.; Nagamori, E.; Matsuyama, T.; Kitamoto, K.; Takahashi, H. Efficient Production of L-Lactic Acid by Metabolically Engineered Saccharomyces cerevisiae with a Genome-Integrated L-Lactate Dehydrogenase Gene. Appl. Env. Microbiol. 2005, 71, 1964–1970. [Google Scholar] [CrossRef]
- de Lima, P.B.A.; Mulder, K.C.L.; Melo, N.T.M.; Carvalho, L.S.; Menino, G.S.; Mulinari, E.; de Castro, V.H.; Dos Reis, T.F.; Goldman, G.H.; Magalhães, B.S.; et al. Novel Homologous Lactate Transporter Improves L-Lactic Acid Production from Glycerol in Recombinant Strains of Pichia pastoris. Microb. Cell Fact. 2016, 15, 158. [Google Scholar] [CrossRef]
- Branduardi, P.; Sauer, M.; De Gioia, L.; Zampella, G.; Valli, M.; Mattanovich, D.; Porro, D. Lactate Production Yield from Engineered Yeasts Is Dependent from the Host Background, the Lactate Dehydrogenase Source and the Lactate Export. Microb. Cell Fact. 2006, 5, 4. [Google Scholar] [CrossRef]
- Adachi, E.; Torigoe, M.; Sugiyama, M.; Nikawa, J.-I.; Shimizu, K. Modification of Metabolic Pathways of Saccharomyces cerevisiae by the Expression of Lactate Dehydrogenase and Deletion of Pyruvate Decarboxylase Genes for the Lactic Acid Fermentation at Low pH Value. J. Ferment. Bioeng. 1998, 86, 284–289. [Google Scholar] [CrossRef]
- Valli, M.; Sauer, M.; Branduardi, P.; Borth, N.; Porro, D.; Mattanovich, D. Improvement of Lactic Acid Production in Saccharomyces Cerevisiae by Cell Sorting for High Intracellular pH. Appl. Environ. Microbiol. 2006, 72, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Sauer, M.; Porro, D.; Branduardi, P. Effect of HXT 1 and HXT 7 Hexose Transporter Overexpression on Wild-Type and Lactic Acid Producing Saccharomyces cerevisiae Cells. Microb. Cell Fact. 2010, 9, 15. [Google Scholar] [CrossRef]
- Pacheco, A.; Talaia, G.; Sá-Pessoa, J.; Bessa, D.; Gonçalves, M.J.; Moreira, R.; Paiva, S.; Casal, M.; Queirós, O. Lactic Acid Production in Saccharomyces cerevisiae Is Modulated by Expression of the Monocarboxylate Transporters Jen1 and Ady2. FEMS Yeast Res. 2012, 12, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; In, J.H.; Park, J.-B.; Shin, J.; Park, J.H.; Sung, B.H.; Sohn, J.-H.; Seo, J.-H.; Park, J.-B.; Kim, S.R.; et al. Co-Expression of Two Heterologous Lactate Dehydrogenases Genes in Kluyveromyces marxianus for L-Lactic Acid Production. J. Biotechnol. 2017, 241, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Peetermans, A.; Foulquié-Moreno, M.R.; Thevelein, J.M. Mechanisms Underlying Lactic Acid Tolerance and Its Influence on Lactic Acid Production in Saccharomyces cerevisiae. Microb. Cell 2021, 8, 111–130. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, C.D.; Lee, S.H.; Park, Y.K.; Cho, K.M. Engineering Cellular Redox Balance in Saccharomyces cerevisiae for Improved Production of L-Lactic Acid. Biotech. Bioeng. 2015, 112, 751–758. [Google Scholar] [CrossRef]
- Braga, M.; Ferreira, P.M.; Almeida, J.R.M. Screening Method to Prioritize Relevant Bio-based Acids and Their Biochemical Processes Using Recent Patent Information. Biofuels Bioprod. Bioref. 2021, 15, 231–249. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Shang, N.; Li, P. Microbial Fermentation Processes of Lactic Acid: Challenges, Solutions, and Future Prospects. Foods 2023, 12, 2311. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic Acid Production—Producing Microorganisms and Substrates Sources-State of Art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Bianchi, M.M.; Brambilla, L.; Protani, F.; Liu, C.L.; Lievense, J.; Porro, D. Efficient Homolactic Fermentation by Kluyveromyces Lactis Strains Defective in Pyruvate Utilization and Transformed with the Heterologous LDH Gene. Appl. Environ. Microbiol. 2001, 67, 5621–5625. [Google Scholar] [CrossRef]
- Bianchi, M.M.; Tizzani, L.; Destruelle, M.; Frontali, L.; Wésolowski-Louvel, M. The “petite-Negative” Yeast Kluyveromyces Lactis Has a Single Gene Expressing Pyruvate Decarboxylase Activity. Mol. Microbiol. 1996, 19, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Branduardi, P.; Valli, M.; Brambilla, L.; Sauer, M.; Alberghina, L.; Porro, D. The Yeast Zygosaccharomyces Bailii: A New Host for Heterologous Protein Production, Secretion and for Metabolic Engineering Applications. FEMS Yeast Res. 2004, 4, 493–504. [Google Scholar] [CrossRef]
- Porro, D.; Bianchi, M.M.; Brambilla, L.; Menghini, R.; Bolzani, D.; Carrera, V.; Lievense, J.; Liu, C.L.; Ranzi, B.M.; Frontali, L.; et al. Replacement of a Metabolic Pathway for Large-Scale Production of Lactic Acid from Engineered Yeasts. Appl. Environ. Microbiol. 1999, 65, 4211–4215. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; He, Q.; Liu, G.; Lu, X.; Zong, H.; Chen, W.; Zhuge, B. Identification and Application of Novel Low pH-Inducible Promoters for Lactic Acid Production in the Tolerant Yeast Candida Glycerinogenes. J. Biosci. Bioeng. 2019, 128, 8–12. [Google Scholar] [CrossRef]
- Melo, N.T.M.; Mulder, K.C.L.; Nicola, A.M.; Carvalho, L.S.; Menino, G.S.; Mulinari, E.; Parachin, N.S. Effect of Pyruvate Decarboxylase Knockout on Product Distribution Using Pichia Pastoris (Komagataella phaffii) Engineered for Lactic Acid Production. Bioeng 2018, 5, 17. [Google Scholar] [CrossRef]
- Tamires Moreira Melo, N.; Pontes, G.C.; Procópio, D.P.; Cunha, G.C.d.G.E.; Eliodório, K.P.; Paes, H.C.; Basso, T.O.; Parachin, N.S. Evaluation of Product Distribution in Chemostat and Batch Fermentation in Lactic Acid-Producing Komagataella phaffii Strains Utilizing Glycerol as Substrate. Microorganisms 2020, 8, 781. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Junqueira, A.C.; Moreira Melo, N.T.; Skorupa Parachin, N.; Costa Paes, H. Loss of a Functional Mitochondrial Pyruvate Carrier in Komagataella phaffii Does Not Improve Lactic Acid Production from Glycerol in Aerobic Cultivation. Microorganisms 2023, 11, 483. [Google Scholar] [CrossRef]
- Ilmén, M.; Koivuranta, K.; Ruohonen, L.; Suominen, P.; Penttilä, M. Efficient Production of L-Lactic Acid from Xylose by Pichia stipitis. Appl. Environ. Microbiol. 2007, 73, 117–123. [Google Scholar] [CrossRef]
- Ilmén, M.; Koivuranta, K.; Ruohonen, L.; Rajgarhia, V.; Suominen, P.; Penttilä, M. Production of L-Lactic Acid by the Yeast Candida Sonorensis Expressing Heterologous Bacterial and Fungal Lactate Dehydrogenases. Microb. Cell Fact. 2013, 12, 53. [Google Scholar] [CrossRef]
- Tokuhiro, K.; Ishida, N.; Nagamori, E.; Saitoh, S.; Onishi, T.; Kondo, A.; Takahashi, H. Double Mutation of the PDC1 and ADH1 Genes Improves Lactate Production in the Yeast Saccharomyces cerevisiae Expressing the Bovine Lactate Dehydrogenase Gene. Appl. Microbiol. Biotechnol. 2009, 82, 883–890. [Google Scholar] [CrossRef]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, B.P.; DeVeaux, L.C.; Christopher, L.P. Metabolic Engineering as a Tool for Enhanced Lactic Acid Production. Trends Biotechnol. 2014, 32, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Dequin, S.; Barre, P. Mixed Lactic Acid–Alcoholic Fermentation by Saccharomyes cerevisiae Expressing the Lactobacillus casei L(+)–LDH. Nat. Biotechnol. 1994, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Luo, R.; Li, Y.; Chen, X. Metabolic Engineering and Adaptive Evolution for Efficient Production of L-Lactic Acid in Saccharomyces cerevisiae. Microbiol. Spectr. 2022, 10, e0227722. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A Review on the Current Developments in Continuous Lactic Acid Fermentations and Case Studies Utilising Inexpensive Raw Materials. Process Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Yamada, R.; Ogura, K.; Kimoto, Y.; Ogino, H. Toward the Construction of a Technology Platform for Chemicals Production from Methanol: D-Lactic Acid Production from Methanol by an Engineered Yeast Pichia Pastoris. World J. Microbiol. Biotechnol. 2019, 35, 37. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, R.; Yamada, R.; Matsumoto, T.; Yoshihara, S.; Tokumoto, H.; Ogino, H. Construction of Lactic Acid-Tolerant Saccharomyces cerevisiae by Using CRISPR-Cas-Mediated Genome Evolution for Efficient D-Lactic Acid Production. Appl. Microbiol. Biotechnol. 2020, 104, 9147–9158. [Google Scholar] [CrossRef]
- Ishida, N.; Saitoh, S.; Onishi, T.; Tokuhiro, K.; Nagamori, E.; Kitamoto, K.; Takahashi, H. The Effect of Pyruvate Decarboxylase Gene Knockout in Saccharomyces cerevisiae on L-Lactic Acid Production. Biosci. Biotechnol. Biochem. 2006, 70, 1148–1153. [Google Scholar] [CrossRef]
- Kozak, B.U.; van Rossum, H.M.; Benjamin, K.R.; Wu, L.; Daran, J.-M.G.; Pronk, J.T.; van Maris, A.J.A. Replacement of the Saccharomyces cerevisiae Acetyl-CoA Synthetases by Alternative Pathways for Cytosolic Acetyl-CoA Synthesis. Metab. Eng. 2014, 21, 46–59. [Google Scholar] [CrossRef]
- Klein, M.; Swinnen, S.; Thevelein, J.M.; Nevoigt, E. Glycerol Metabolism and Transport in Yeast and Fungi: Established Knowledge and Ambiguities. Environ. Microbiol. 2017, 19, 878–893. [Google Scholar] [CrossRef]
- Li, F.; Wei, X.; Sun, Q.; Guo, Y.; Liu, J. Production of L-Lactic Acid in Saccharomyces cerevisiae Through Metabolic Engineering and Rational Cofactor Engineering. Sugar Technol. 2022, 24, 1272–1283. [Google Scholar] [CrossRef]
- Shu, C.; Guo, C.; Luo, S.; Jiang, S.; Zheng, Z. Influence of Altered NADH Metabolic Pathway on the Respiratory-Deficient Mutant of Rhizopus oryzae and Its L-Lactate Production. Appl. Biochem. Biotechnol. 2015, 176, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, G.G. An NAD+-Independent l-Lactate Dehydrogenase from Rhizopus oryzae. Biochim. Et. Biophys. Acta (BBA) Enzymol. 1971, 250, 25–34. [Google Scholar] [CrossRef]
- Blazy, B.; Bardet, M.; Baudras, A. A Standard High-Yield Purification of L-(+)-Lactate: Cytochrome c Oxidoreductase (Cytochrome B2) from the Yeast Hansenula anomala. Anal. Biochem. 1978, 88, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Lodi, T.; Ferrero, I. Isolation of the DLD Gene of Saccharomyces cerevisiae Encoding the Mitochondrial Enzyme D-Lactate Ferricytochrome c Oxidoreductase. Mol. Genet. Genom. 1993, 238, 315–324. [Google Scholar] [CrossRef]
- Song, J.-Y.; Park, J.-S.; Kang, C.D.; Cho, H.-Y.; Yang, D.; Lee, S.; Cho, K.M. Introduction of a Bacterial Acetyl-CoA Synthesis Pathway Improves Lactic Acid Production in Saccharomyces cerevisiae. Metab. Eng. 2016, 35, 38–45. [Google Scholar] [CrossRef]
- Cavelius, P.; Engelhart-Straub, S.; Mehlmer, N.; Lercher, J.; Awad, D.; Brück, T. The Potential of Biofuels from First to Fourth Generation. PLoS Biol. 2023, 21, e3002063. [Google Scholar] [CrossRef]
- Gosalawit, C.; Khunnonkwao, P.; Jantama, K. Genome Engineering of Kluyveromyces marxianus for High D-(−)-Lactic Acid Production under Low pH Conditions. Appl. Microbiol. Biotechnol. 2023, 107, 5095–5105. [Google Scholar] [CrossRef] [PubMed]
- Wakai, S.; Yoshie, T.; Asai-Nakashima, N.; Yamada, R.; Ogino, C.; Tsutsumi, H.; Hata, Y.; Kondo, A. L-Lactic Acid Production from Starch by Simultaneous Saccharification and Fermentation in a Genetically Engineered Aspergillus oryzae Pure Culture. Bioresour. Technol. 2014, 173, 376–383. [Google Scholar] [CrossRef]
- Zhang, S.; Wakai, S.; Sasakura, N.; Tsutsumi, H.; Hata, Y.; Ogino, C.; Kondo, A. Pyruvate Metabolism Redirection for Biological Production of Commodity Chemicals in Aerobic Fungus Aspergillus oryzae. Metab. Eng. 2020, 61, 225–237. [Google Scholar] [CrossRef]
- Dave, K.K.; Punekar, N.S. Expression of Lactate Dehydrogenase in Aspergillus Niger for L-Lactic Acid Production. PLoS ONE 2015, 10, e0145459. [Google Scholar] [CrossRef]
- Bae, J.-H.; Kim, H.-J.; Kim, M.-J.; Sung, B.H.; Jeon, J.-H.; Kim, H.-S.; Jin, Y.-S.; Kweon, D.-H.; Sohn, J.-H. Direct Fermentation of Jerusalem Artichoke Tuber Powder for Production of L-Lactic Acid and D-Lactic Acid by Metabolically Engineered Kluyveromyces marxianus. J. Biotechnol. 2018, 266, 27–33. [Google Scholar] [CrossRef]
- Baumschabl, M.; Ata, Ö.; Mitic, B.M.; Lutz, L.; Gassler, T.; Troyer, C.; Hann, S.; Mattanovich, D. Conversion of CO2 into Organic Acids by Engineered Autotrophic Yeast. Proc. Natl. Acad. Sci. USA 2022, 119, e2211827119. [Google Scholar] [CrossRef]
- Wefelmeier, K.; Schmitz, S.; Haut, A.M.; Otten, J.; Jülich, T.; Blank, L.M. Engineering the Methylotrophic Yeast Ogataea Polymorpha for Lactate Production from Methanol. Front. Bioeng. Biotechnol. 2023, 11, 1223726. [Google Scholar] [CrossRef]
- Park, H.J.; Bae, J.; Ko, H.; Lee, S.; Sung, B.H.; Han, J.; Sohn, J. Low-pH Production of D-Lactic Acid Using Newly Isolated Acid Tolerant Yeast Pichia kudriavzevii NG7. Biotech. Bioeng. 2018, 115, 2232–2242. [Google Scholar] [CrossRef]
- Zhang, B.; Li, R.; Yu, L.; Wu, C.; Liu, Z.; Bai, F.; Yu, B.; Wang, L. L-Lactic Acid Production via Sustainable Neutralizer-Free Route by Engineering Acid-Tolerant Yeast Pichia Kudriavzevii. J. Agric. Food Chem. 2023, 71, 11131–11140. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.L.; Zhang, G.-C.; Oh, E.J.; Subramaniam, V.; Adiputra, A.; Subramaniam, V.; Skory, C.D.; Jang, J.Y.; Yu, B.J.; Park, I.; et al. Lactic Acid Production from Cellobiose and Xylose by Engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2016, 113, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.L.; Kim, E.; Hwang, C.; Zhang, G.-C.; Liu, J.-J.; Jin, Y.-S. Short Communication: Conversion of Lactose and Whey into Lactic Acid by Engineered Yeast. J. Dairy Sci. 2017, 100, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-H.; Kwon, E.Y.; Kim, Y.H.; Hahn, J.-S. Metabolic Engineering and Adaptive Evolution for Efficient Production of D-Lactic Acid in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Novy, V.; Brunner, B.; Müller, G.; Nidetzky, B. Toward “Homolactic” Fermentation of Glucose and Xylose by Engineered Saccharomyces cerevisiae Harboring a Kinetically Efficient l-Lactate Dehydrogenase within Pdc1-Pdc5 Deletion Background. Biotechnol. Bioeng. 2017, 114, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yang, M.; Mu, T.; Wu, F.; Hao, X.; Chen, R.; Sharshar, M.M.; Thygesen, A.; Wang, Q.; Xing, J. Systematically Redesigning and Optimizing the Expression of D-Lactate Dehydrogenase Efficiently Produces High-Optical-Purity D-Lactic Acid in Saccharomyces cerevisiae. Biochem. Eng. J. 2019, 144, 217–226. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, M.; Hao, X.; Sharshar, M.M.; Wang, Q.; Xing, J. Improvement of D-LACTIC Acid Production at Low pH through Expressing Acid-resistant Gene IoGAS1 in Engineered Saccharomyces cerevisiae. J. Chem. Technol. Biotech. 2021, 96, 732–742. [Google Scholar] [CrossRef]
- Kim, J.-W.; Jang, J.H.; Yeo, H.J.; Seol, J.; Kim, S.R.; Jung, Y.H. Lactic Acid Production from a Whole Slurry of Acid-Pretreated Spent Coffee Grounds by Engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019, 189, 206–216. [Google Scholar] [CrossRef]
- Jang, B.-K.; Ju, Y.; Jeong, D.; Jung, S.-K.; Kim, C.-K.; Chung, Y.-S.; Kim, S.-R. L-Lactic Acid Production Using Engineered Saccharomyces cerevisiae with Improved Organic Acid Tolerance. J. Fungi 2021, 7, 928. [Google Scholar] [CrossRef]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Wolf, C.R.; Silva, E.C.; Lima, G.E.S.; De Lira Silva, L.; Serra, T.M.; Cauduro, F.; De Oliveira, L.G. Biodiesel from Castor Oil: A Comparison of Ethanolysis versus Methanolysis. Energy Fuels 2006, 20, 2262–2265. [Google Scholar] [CrossRef]
- Anastácio, G.S.; Santos, K.O.; Suarez, P.A.Z.; Torres, F.A.G.; De Marco, J.L.; Parachin, N.S. Utilization of Glycerin Byproduct Derived from Soybean Oil Biodiesel as a Carbon Source for Heterologous Protein Production in Pichia pastoris. Bioresour. Technol. 2014, 152, 505–510. [Google Scholar] [CrossRef]
- Baumann, K.; Carnicer, M.; Dragosits, M.; Graf, A.B.; Stadlmann, J.; Jouhten, P.; Maaheimo, H.; Gasser, B.; Albiol, J.; Mattanovich, D.; et al. A Multi-Level Study of Recombinant Pichia pastoris in Different Oxygen Conditions. BMC Syst. Biol. 2010, 4, 141. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, K.H.; Park, K.H.; Jang, J.; Hahn, J.-S. Activation of Haa1 and War1 Transcription Factors by Differential Binding of Weak Acid Anions in Saccharomyces cerevisiae. Nucleic Acids Res. 2019, 47, 1211–1224. [Google Scholar] [CrossRef]
- Abbott, D.A.; Suir, E.; Van Maris, A.J.A.; Pronk, J.T. Physiological and Transcriptional Responses to High Concentrations of Lactic Acid in Anaerobic Chemostat Cultures of Saccharomyces cerevisiae. Appl. Env. Microbiol. 2008, 74, 5759–5768. [Google Scholar] [CrossRef]
- Kawahata, M.; Masaki, K.; Fujii, T.; Iefuji, H. Yeast Genes Involved in Response to Lactic Acid and Acetic Acid: Acidic Conditions Caused by the Organic Acids in Saccharomyces cerevisiae Cultures Induce Expression of Intracellular Metal Metabolism Genes Regulated by Aft1p. FEMS Yeast Res. 2006, 6, 924–936. [Google Scholar] [CrossRef]
- Suzuki, T.; Sakamoto, T.; Sugiyama, M.; Ishida, N.; Kambe, H.; Obata, S.; Kaneko, Y.; Takahashi, H.; Harashima, S. Disruption of Multiple Genes Whose Deletion Causes Lactic-Acid Resistance Improves Lactic-Acid Resistance and Productivity in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2013, 115, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The Emergence of Adaptive Laboratory Evolution as an Efficient Tool for Biological Discovery and Industrial Biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dato, L.; Berterame, N.M.; Ricci, M.A.; Paganoni, P.; Palmieri, L.; Porro, D.; Branduardi, P. Changes in SAM2 Expression Affect Lactic Acid Tolerance and Lactic Acid Production in Saccharomyces cerevisiae. Microb. Cell Fact. 2014, 13, 147. [Google Scholar] [CrossRef]
- Kuanyshev, N.; Ami, D.; Signori, L.; Porro, D.; Morrissey, J.P.; Branduardi, P. Assessing Physio-Macromolecular Effects of Lactic Acid on Zygosaccharomyces bailii Cells during Microaerobic Fermentation. FEMS Yeast Res. 2016, 16, fow058. [Google Scholar] [CrossRef] [PubMed]
- Dusselier, M.; Van Wouwe, P.; Dewaele, A.; Makshina, E.; Sels, B.F. Lactic Acid as a Platform Chemical in the Biobased Economy: The Role of Chemocatalysis. Energy Environ. Sci. 2013, 6, 1415. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. A Review on the Lactic Acid Fermentation from Low-Cost Renewable Materials: Recent Developments and Challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Tamakawa, H.; Ikushima, S.; Yoshida, S. Efficient Production of L-Lactic Acid from Xylose by a Recombinant Candida Utilis Strain. J. Biosci. Bioeng. 2012, 113, 73–75. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef]
- Alimardani-Theuil, P.; Gainvors-Claisse, A.; Duchiron, F. Yeasts: An Attractive Source of Pectinases—From Gene Expression to Potential Applications: A Review. Process Biochem. 2011, 46, 1525–1537. [Google Scholar] [CrossRef]
- Kim, S.R.; Skerker, J.M.; Kang, W.; Lesmana, A.; Wei, N.; Arkin, A.P.; Jin, Y.-S. Rational and Evolutionary Engineering Approaches Uncover a Small Set of Genetic Changes Efficient for Rapid Xylose Fermentation in Saccharomyces cerevisiae. PLoS ONE 2013, 8, e57048. [Google Scholar] [CrossRef]
- Varriale, L.; Ulber, R. Fungal-Based Biorefinery: From Renewable Resources to Organic Acids. ChemBioEng Rev. 2023, 10, 272–292. [Google Scholar] [CrossRef]
- Saito, K.; Hasa, Y.; Abe, H. Production of Lactic Acid from Xylose and Wheat Straw by Rhizopus oryzae. J. Biosci. Bioeng. 2012, 114, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C.; Dulf, F.V.; Pop, O.L.; Socaciu, C. L-(+)-Lactic Acid Production by Pellet-Form Rhizopus oryzae NRRL 395on Biodiesel Crude Glycerol. Microb. Cell Fact. 2013, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liao, W.; Chen, S. Co-Production of Lactic Acid and Chitin Using a Pelletized Filamentous Fungus Rhizopus oryzae Cultured on Cull Potatoes and Glucose. J. Appl. Microbiol. 2008, 105, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, A.; Konishi, R.; Otomo, C.; Kishida, M.; Takayama, S.; Matsumoto, T.; Tanaka, T.; Kondo, A. Metabolic Engineering of Schizosaccharomyces Pombe via CRISPR-Cas9 Genome Editing for Lactic Acid Production from Glucose and Cellobiose. Metab. Eng. Commun. 2017, 5, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, R.; Yamada, R.; Ogino, H. Improved Stress Tolerance of Saccharomyces cerevisiae by CRISPR-Cas-Mediated Genome Evolution. Appl. Biochem. Biotechnol. 2019, 189, 810–821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, N.T.M.; de Oliveira Junqueira, A.C.; Lima, L.F.; de Oliveira, K.B.S.; dos Reis, M.C.G.; Franco, O.L.; Paes, H.C. Just around the Corner: Advances in the Optimization of Yeasts and Filamentous Fungi for Lactic Acid Production. J. Fungi 2024, 10, 207. https://doi.org/10.3390/jof10030207
Melo NTM, de Oliveira Junqueira AC, Lima LF, de Oliveira KBS, dos Reis MCG, Franco OL, Paes HC. Just around the Corner: Advances in the Optimization of Yeasts and Filamentous Fungi for Lactic Acid Production. Journal of Fungi. 2024; 10(3):207. https://doi.org/10.3390/jof10030207
Chicago/Turabian StyleMelo, Nadielle Tamires Moreira, Ana Caroline de Oliveira Junqueira, Letícia Ferreira Lima, Kamila Botelho Sampaio de Oliveira, Micaela Cristiane Gomes dos Reis, Octávio Luiz Franco, and Hugo Costa Paes. 2024. "Just around the Corner: Advances in the Optimization of Yeasts and Filamentous Fungi for Lactic Acid Production" Journal of Fungi 10, no. 3: 207. https://doi.org/10.3390/jof10030207
APA StyleMelo, N. T. M., de Oliveira Junqueira, A. C., Lima, L. F., de Oliveira, K. B. S., dos Reis, M. C. G., Franco, O. L., & Paes, H. C. (2024). Just around the Corner: Advances in the Optimization of Yeasts and Filamentous Fungi for Lactic Acid Production. Journal of Fungi, 10(3), 207. https://doi.org/10.3390/jof10030207






