Biological Control of a Root-Knot Nematode Meloidogyne incognita Infection of Tomato (Solanum lycopersicum L.) by the Oomycete Biocontrol Agent Pythium oligandrum
Abstract
:1. Introduction
2. Methods
2.1. Maintenance and Routine Culturing of P. oligandrum Strains
2.2. Nematicidal Effect of P. oligandrum Culture Filtrates to M. incognita
2.3. Assays for Root Attraction of Nematodes and Nematode Penetration of Roots
2.4. Greenhouse Pot Trial of P. oligandrum Control of RKN Disease in Tomato
2.5. RNA Sequencing and Analysis
2.6. RNA Extraction, cDNA Synthesis, and Real-Time PCR Analysis
2.7. Statistical Analysis
3. Results
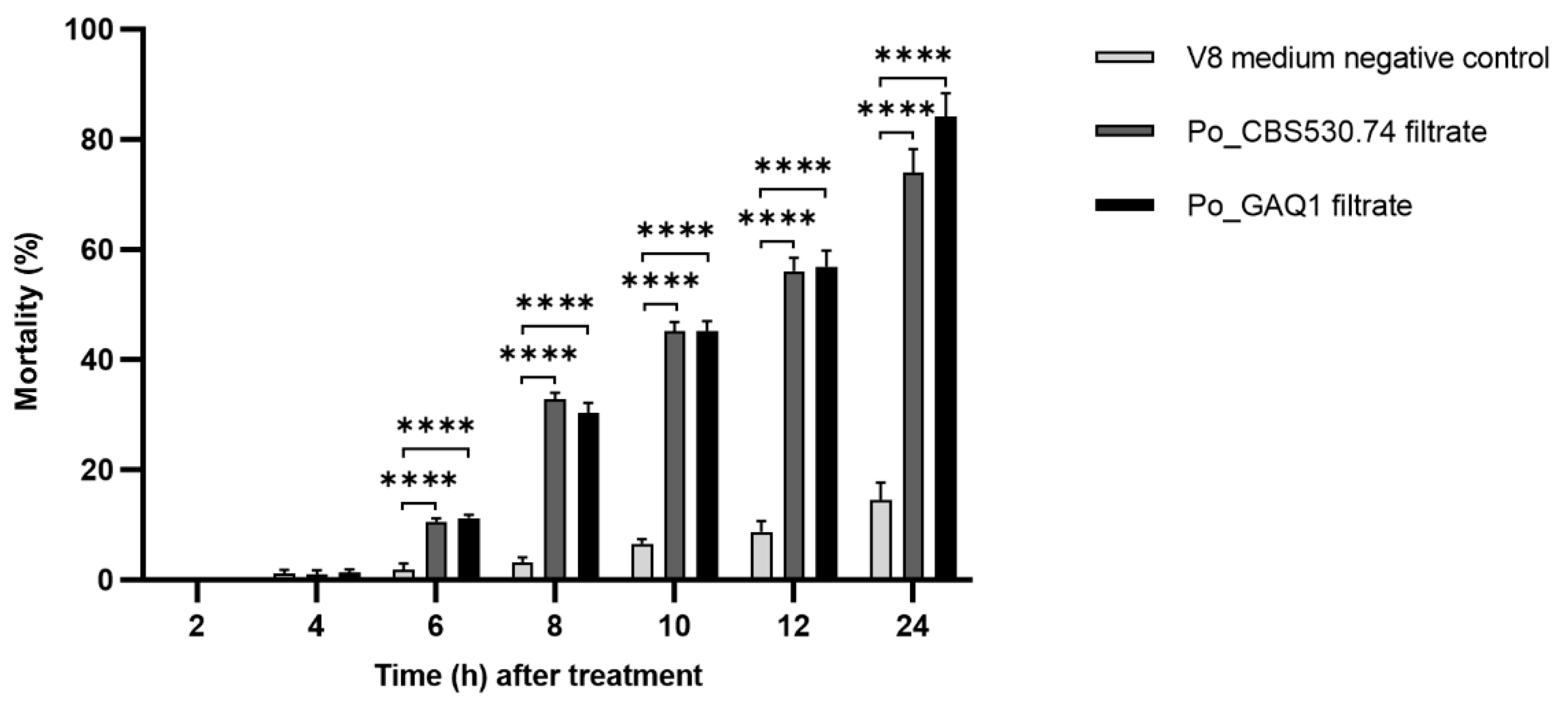
3.1. The P. oligandrum GAQ1 Culture Filtrate Is Lethal to M. incognita
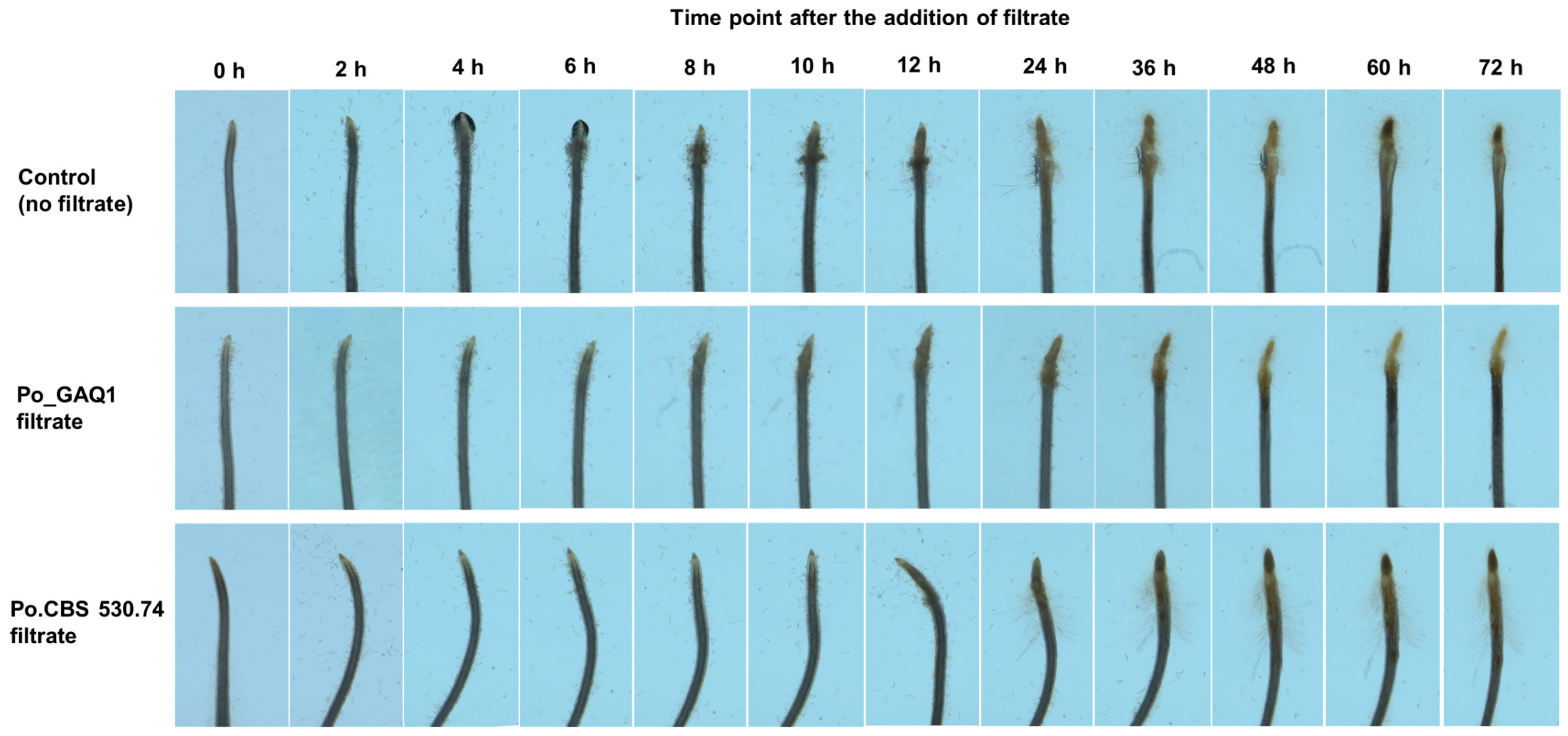
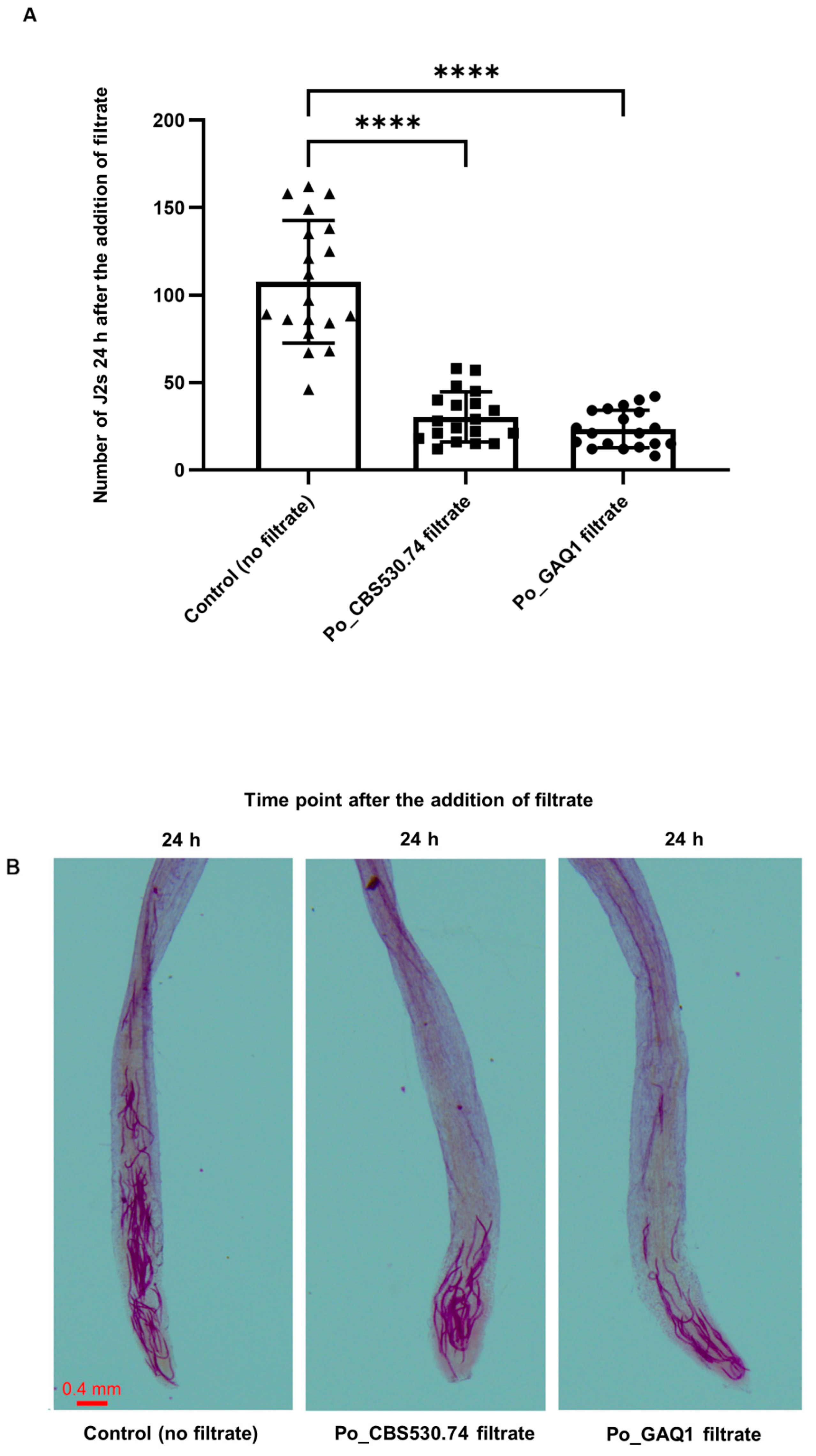
3.2. P. oligandrum Culture Filtrate Inhibits J2s’ Movement toward Tomato Roots
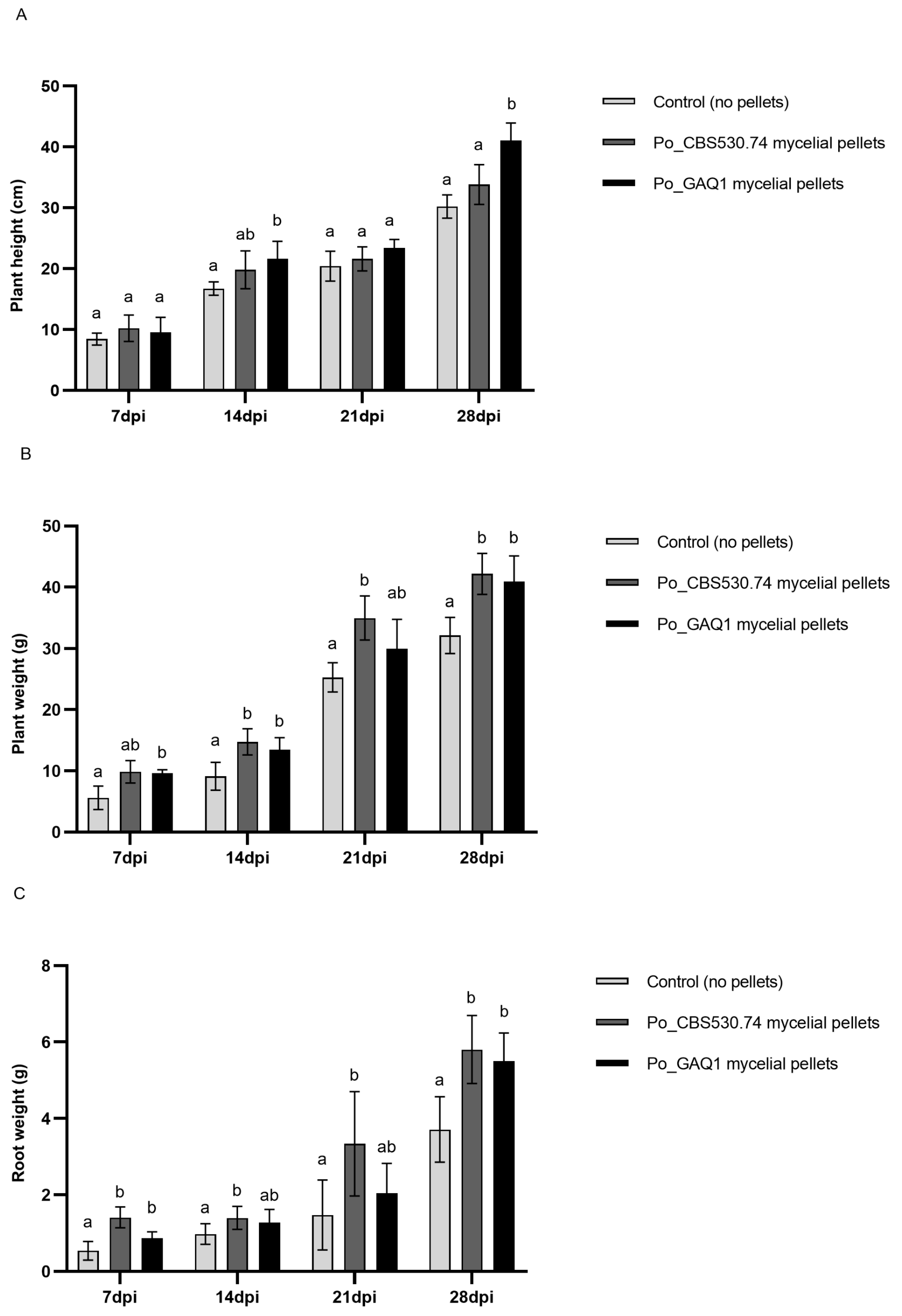
3.3. P. oligandrum Reduces RKN Infestation and Promotes Plant Growth in Pot Trials
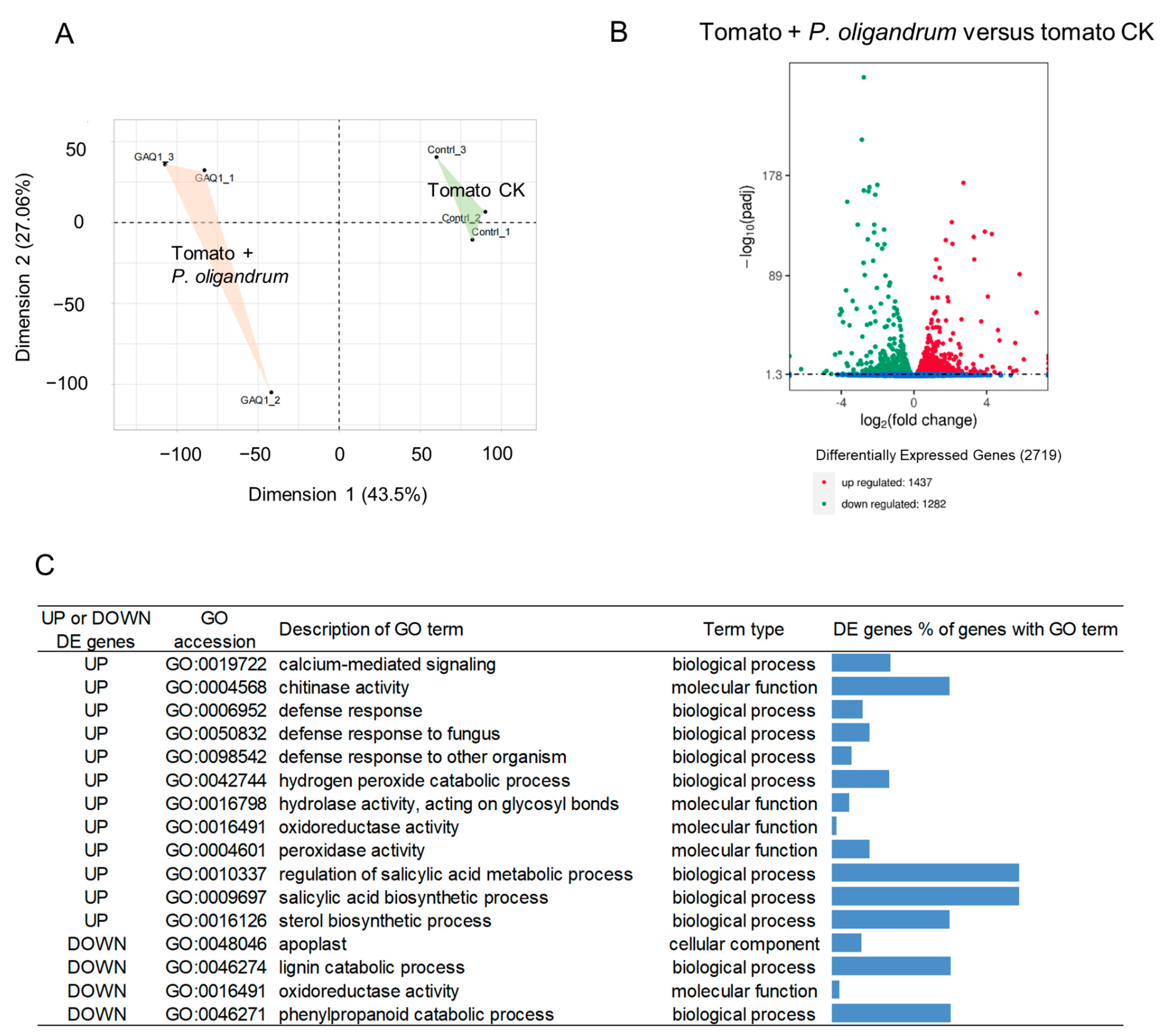
3.4. Inoculation of Tomato Seedling Roots with P. oligandrum GAQ1 Leads to Gene Expression Changes in Tomato Roots
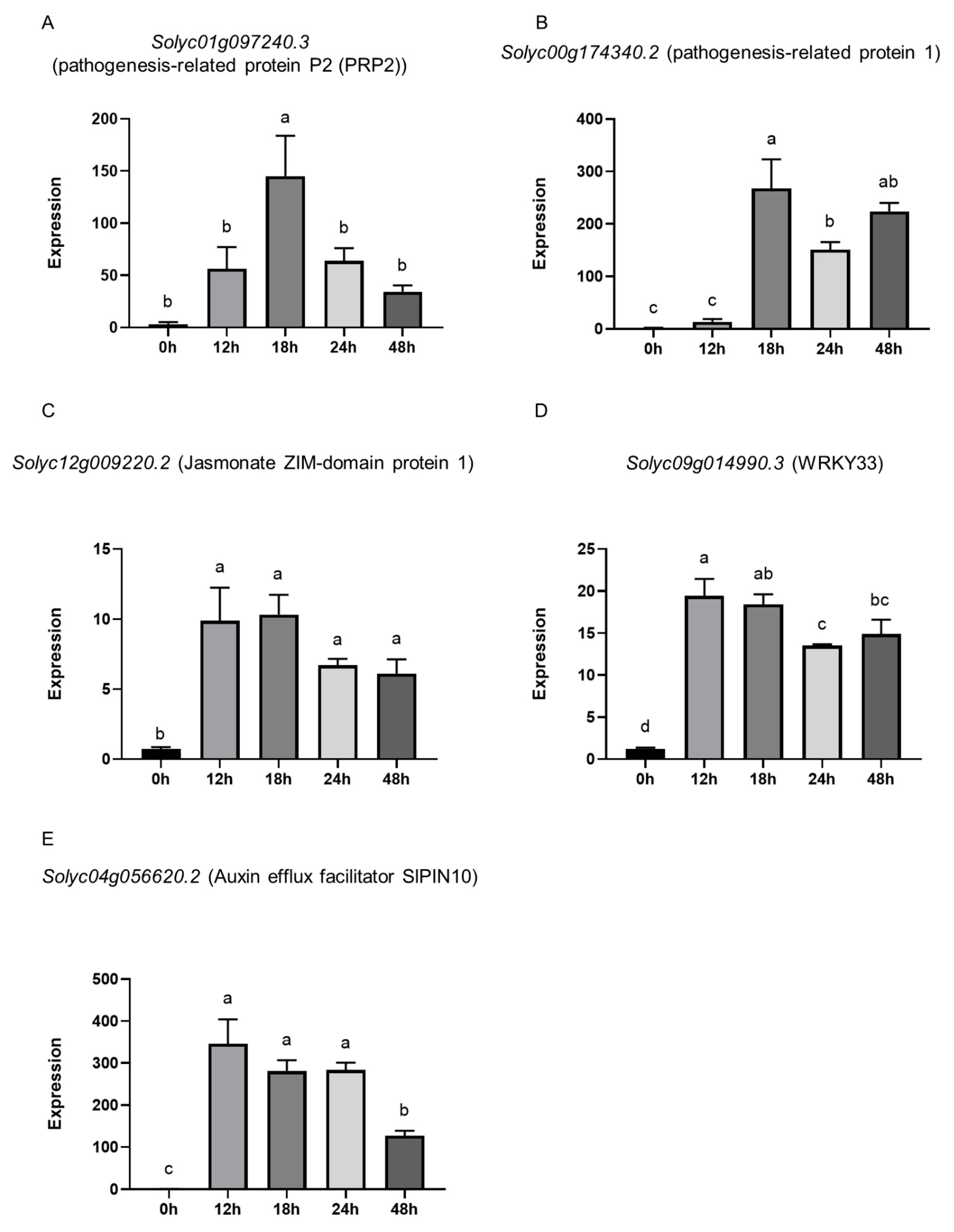
3.5. Presence of P. oligandrum GAQ1 Modulates the Expression of Tomato Defense, Growth, and Signaling-Related Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benhamou, N.; le Floch, G.; Vallance, J.; Gerbore, J.; Grizard, D.; Rey, P. Pythium oligandrum: An example of opportunistic success. Microbiology 2012, 158, 2679–2694. [Google Scholar] [CrossRef] [PubMed]
- Gabrielová, A.; Mencl, K.; Suchánek, M.; Klimeš, R.; Hubka, V.; Kolařík, M. The oomycete Pythium oligandrum can suppress and kill the causative agents of dermatophytoses. Mycopathologia 2018, 183, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Gerbore, J.; Vallance, J.; Yacoub, A.; Delmotte, F.; Grizard, D.; Regnault-Roger, C.; Rey, P. Characterization of Pythium oligandrum populations that colonize the rhizosphere of vines from the Bordeaux region. FEMS Microbiol. Ecol. 2014, 90, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Pellan, L.; Dieye, C.A.T.; Durand, N.; Fontana, A.; Strub, C.; Schorr-Galindo, S. Biocontrol Agents: Toolbox for the Screening of Weapons against Mycotoxigenic Fusarium. J. Fungi 2021, 7, 446. [Google Scholar] [CrossRef] [PubMed]
- Berry, L.A.; Jones, E.E.; Deacon, J.W. Interaction of the mycoparasite Pythium oligandrum with other Pythium species. Biocontrol Sci. Technol. 1993, 3, 247–260. [Google Scholar] [CrossRef]
- Bělonožníková, K.; Hýsková, V.; Chmelík, J.; Kavan, D.; Čeřovská, N.; Ryšlavá, H. Pythium oligandrum in plant protection and growth promotion: Secretion of hydrolytic enzymes, elicitors and tryptamine as auxin precursor. Microbiol. Res. 2022, 258, 126976. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, G.; Rey, P.; Benizri, E.; Benhamou, N.; Tirilly, Y. Impact of auxin-compounds produced by the antagonistic fungus Pythium oligandrum or the minor pathogen Pythium group F on plant growth. Plant Soil 2003, 257, 459–470. [Google Scholar] [CrossRef]
- Luca, I.; Ilie, M.S.; Florea, T.; Olariu-Jurca, A.; Stancu, A.; Dărăbuş, G. The use of Pythium oligandrum in the biological control of roundworm infection in dogs and cats. Pathogens 2022, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Castagnone-Sereno, P.; Danchin, E.G.J.; Perfus-Barbeoch, L.; Abad, P. Diversity and evolution of root-knot nematodes, genus Meloidogyne: New insights from the genomic era. Annu. Rev. Phytopathol. 2013, 51, 203–220. [Google Scholar] [CrossRef]
- Onkendi, E.M.; Kariuki, G.M.; Marais, M.; Moleleki, L.N. The threat of root-knot nematodes (Meloidogyne spp.) in Africa: A review. Plant Pathol. 2014, 63, 727–737. [Google Scholar] [CrossRef]
- Rutter, W.B.; Franco, J.; Gleason, C. Rooting out the mechanisms of root-knot nematode-plant interactions. Annu. Rev. Phytopathol. 2022, 60, 43–76. [Google Scholar] [CrossRef] [PubMed]
- Trudgill, D.L.; Blok, V.C. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.J.; Meng, Y.; Wang, Y.L.; Zhang, T.; Yang, G.F.; Mo, M.H.; Ji, K.F.; Liang, L.M.; Zou, C.G.; Zhang, K.Q. Survival and infectivity of second-stage root-knot nematode Meloidogyne incognita juveniles depend on lysosome-mediated lipolysis. J. Biol. Chem. 2022, 298, 101637. [Google Scholar] [CrossRef] [PubMed]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Shilpa, n.; Sharma, P.; Thakur, V.; Sharma, A.; Rana, R.S.; Kumar, P. A status-quo review on management of root knot nematode in tomato. J. Hortic. Sci. Biotechnol. 2022, 97, 403–416. [Google Scholar] [CrossRef]
- Desaeger, J.; Wram, C.; Zasada, I. New reduced-risk agricultural nematicides—Rationale and review. J. Nematol. 2020, 52, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Shakeel, A.; Waqar, S.; Handoo, Z.A.; Khan, A.A. Microbes vs. nematodes: Insights into biocontrol through antagonistic organisms to control root-knot nematodes. Plants 2023, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, M.M.M.; Askary, T.H. Factors affecting success of biological agents used in controlling the plant-parasitic nematodes. Egypt. J. Biol. Pest Control 2020, 30, 17. [Google Scholar] [CrossRef]
- Yan, Y.; Mao, Q.; Wang, Y.; Zhao, J.; Fu, Y.; Yang, Z.; Peng, X.; Zhang, M.; Bai, B.; Liu, A.; et al. Trichoderma harzianum induces resistance to root-knot nematodes by increasing secondary metabolite synthesis and defense-related enzyme activity in Solanum lycopersicum L. Biol. Control 2021, 158, 104609. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Li, H.; Wang, R.; Zhang, K.-Q.; Xu, J. Fungi–Nematode Interactions: Diversity, Ecology, and Biocontrol Prospects in Agriculture. J. Fungi 2020, 6, 206. [Google Scholar] [CrossRef] [PubMed]
- Daly, P.; Chen, S.; Xue, T.; Li, J.; Sheikh, T.M.M.; Zhang, Q.; Wang, X.; Zhang, J.; Fitzpatrick, D.A.; McGowan, J.; et al. Dual-transcriptomic, microscopic, and biocontrol analyses of the interaction between the bioeffector Pythium oligandrum and the Pythium soft-rot of ginger pathogen Pythium myriotylum. Front. Microbiol. 2021, 12, 765872. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.K.; Vetukuri, R.R.; Grenville-Briggs, L.J. Draft genome sequence of the mycoparasitic oomycete Pythium oligandrum strain CBS 530.74. Genome Announc. 2017, 5, e00346-17. [Google Scholar] [CrossRef] [PubMed]
- Gardener, S.; Jones, J.G. A new solidifying agent for culture media which liquefies on cooling. Microbiology 1984, 130, 731–733. [Google Scholar] [CrossRef]
- Morishita, M.; Barichello, J.M.; Takayama, K.; Chiba, Y.; Tokiwa, S.; Nagai, T. Pluronic F-127 gels incorporating highly purified unsaturated fatty acids for buccal delivery of insulin. Int. J. Pharm. 2001, 212, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- The_Tomato_Genome_Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Hosmani, P.S.; Flores-Gonzalez, M.; Geest, H.v.d.; Maumus, F.; Bakker, L.V.; Schijlen, E.; Haarst, J.v.; Cordewener, J.; Sanchez-Perez, G.; Peters, S.; et al. An improved de novo assembly and annotation of the tomato reference genome using single-molecule sequencing, Hi-C proximity ligation and optical maps. bioRxiv 2019, 767764. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 18. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pattison, R.J.; Catalá, C. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, C.; Wang, H.; Guo, Z. WRKY transcription factors: Evolution, binding, and action. Phytopathol. Res. 2019, 1, 13. [Google Scholar] [CrossRef]
- Bai, Y.; Sunarti, S.; Kissoudis, C.; Visser, R.G.F.; van der Linden, C.G. The role of tomato WRKY genes in plant responses to combined abiotic and biotic stresses. Front. Plant Sci. 2018, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Wager, A.; Browse, J. Social network: JAZ protein interactions expand our knowledge of jasmonate signaling. Front. Plant Sci. 2012, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Khoja, S.; Eltayef, K.M.; Baxter, I.; Myrta, A.; Bull, J.C.; Butt, T. Volatiles of the entomopathogenic fungus, Metarhizium brunneum, attract and kill plant parasitic nematodes. Biol. Control 2021, 152, 104472. [Google Scholar] [CrossRef]
- Cao, H.; Jiao, Y.; Yin, N.; Li, Y.; Ling, J.; Mao, Z.; Yang, Y.; Xie, B. Analysis of the activity and biological control efficacy of the Bacillus subtilis strain Bs-1 against Meloidogyne incognita. Crop Prot. 2019, 122, 125–135. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, R.; Ding, M.; Liu, Y.; Li, L. Biocontrol of the root-knot nematode Meloidogyne incognita by a nematicidal bacterium Pseudomonas simiae MB751 with cyclic dipeptide. Pest. Manag. Sci. 2021, 77, 4365–4374. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liang, L.; Li, J.; Zhang, K.Q. Nematicidal enzymes from microorganisms and their applications. Appl. Microbiol. Biotechnol. 2013, 97, 7081–7095. [Google Scholar] [CrossRef] [PubMed]
- Faure, C.; Veyssiere, M.; Boelle, B.; San Clemente, H.; Bouchez, O.; Lopez-Roques, C.; Chaubet, A.; Martinez, Y.; Bezouska, K.; Suchanek, M.; et al. Long-read genome sequence of the sugar beet rhizosphere mycoparasite Pythium oligandrum. G3 Genes Genomes Genet. 2020, 10, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Andersen, C.B.; Vetukuri, R.R.; Dou, D.; Grenville-Briggs, L.J. Horizontal gene transfer and tandem duplication shape the unique cazyme complement of the mycoparasitic oomycetes Pythium oligandrum and Pythium periplocum. Front. Microbiol. 2020, 11, 581698. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; You, J.; Wang, Y.; Long, Y.; Wang, S.; Pan, F.; Yu, Z. Biocontrol efficacy of Bacillus velezensis strain YS-AT-DS1 against the root-knot nematode Meloidogyne incognita in tomato plants. Front. Microbiol. 2022, 13, 1035748. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yuen, G.; Wang, Y.; Wei, L.; Ji, G. Evaluation of bacterial biological control agents for control of root-knot nematode disease on tomato. Crop Prot. 2016, 84, 8–13. [Google Scholar] [CrossRef]
- Benhamou, N.; Rey, P.; Chérif, M.; Hockenhull, J.; Tirilly, Y. Treatment with the mycoparasite Pythium oligandrum triggers induction of defense-related reactions in tomato roots when challenged with Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology 1997, 87, 108–122. [Google Scholar] [CrossRef]
- Fujimoto, T.; Mizukubo, T.; Abe, H.; Seo, S. Sclareol induces plant resistance to root-knot nematode partially through ethylene-dependent enhancement of lignin accumulation. Mol. Plant. Microbe Interact. 2015, 28, 398–407. [Google Scholar] [CrossRef]
- Rey, P.; Benhamou, N.; Wulff, E.; Tirilly, Y. Interactions between tomato (Lycopersicon esculentum) root tissues and the mycoparasite Pythium oligandrum. Physiol. Mol. Plant Pathol. 1998, 53, 105–122. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Bělonožníková, K.; Vaverová, K.; Vaněk, T.; Kolařík, M.; Hýsková, V.; Vaňková, R.; Dobrev, P.; Křížek, T.; Hodek, O.; Čokrtová, K.; et al. Novel insights into the effect of Pythium strains on rapeseed metabolism. Microorganisms 2020, 8, 1472. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.G.; Dean, A.M.; Mitchell-Olds, T. Rapid evolution in plant chitinases: Molecular targets of selection in plant-pathogen coevolution. Proc. Natl. Acad. Sci. USA 2000, 97, 5322–5327. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Yang, L.; Qiu, F.; Li, Y.; Zheng, Y.; Liu, S.; Song, L.; Liang, W. Enhancing tomato resistance by exploring early defense events against Fusarium wilt disease. Phytopathol. Res. 2023, 5, 24. [Google Scholar] [CrossRef]
- Wang, A.Y.; Lou, B.G.; Xu, T.; Lin, C. Defense responses in tomato fruit induced by oligandrin against Botrytis cinerea. Afr. J. Biotechnol. 2011, 10, 4596–4601. [Google Scholar]
- Yang, K.; Dong, X.; Li, J.; Wang, Y.; Cheng, Y.; Zhai, Y.; Li, X.; Wei, L.; Jing, M.; Dou, D. Type 2 Nep1-like proteins from the biocontrol oomycete Pythium oligandrum suppress Phytophthora capsici infection in solanaceous plants. J. Fungi 2021, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.M.M.; Zhou, D.; Ali, H.; Hussain, S.; Wang, N.; Chen, S.; Zhao, Y.; Wen, X.; Wang, X.; Zhang, J.; et al. Volatile organic compounds emitted by the biocontrol agent Pythium oligandrum contribute to ginger plant growth and disease resistance. Microbiol. Spectr. 2023, 11, e01510–e01523. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.M.M.; Zhou, D.; Haider, M.S.; Hussain, S.; Wang, N.; Chen, S.; Zhao, Y.; Wen, X.; Feng, H.; Wang, X.; et al. Volatile organic compounds from Pythium oligandrum play a role in its parasitism on plant-pathogenic Pythium myriotylum. Appl. Environ. Microbiol. 2023, 89, e0203622. [Google Scholar] [CrossRef]
- Luca, I.; Imre, M.; Ilie, M.S.; Oprescu, I.; Dărăbuş, G. The biological potential of a product containing Pythium oligandrum against Uncinaria stenocephala (Railliet, 1884) larvae. J. Hell. Vet. Med. Soc. 2022, 73, 3651–3656. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Li, W.; Li, M.; Ru, N.; Chen, S.; Jiu, M.; Feng, H.; Wei, L.; Daly, P.; Zhou, D. Biological Control of a Root-Knot Nematode Meloidogyne incognita Infection of Tomato (Solanum lycopersicum L.) by the Oomycete Biocontrol Agent Pythium oligandrum. J. Fungi 2024, 10, 265. https://doi.org/10.3390/jof10040265
Xue Y, Li W, Li M, Ru N, Chen S, Jiu M, Feng H, Wei L, Daly P, Zhou D. Biological Control of a Root-Knot Nematode Meloidogyne incognita Infection of Tomato (Solanum lycopersicum L.) by the Oomycete Biocontrol Agent Pythium oligandrum. Journal of Fungi. 2024; 10(4):265. https://doi.org/10.3390/jof10040265
Chicago/Turabian StyleXue, Yuwei, Weishan Li, Mengnan Li, Ningchen Ru, Siqiao Chen, Min Jiu, Hui Feng, Lihui Wei, Paul Daly, and Dongmei Zhou. 2024. "Biological Control of a Root-Knot Nematode Meloidogyne incognita Infection of Tomato (Solanum lycopersicum L.) by the Oomycete Biocontrol Agent Pythium oligandrum" Journal of Fungi 10, no. 4: 265. https://doi.org/10.3390/jof10040265
APA StyleXue, Y., Li, W., Li, M., Ru, N., Chen, S., Jiu, M., Feng, H., Wei, L., Daly, P., & Zhou, D. (2024). Biological Control of a Root-Knot Nematode Meloidogyne incognita Infection of Tomato (Solanum lycopersicum L.) by the Oomycete Biocontrol Agent Pythium oligandrum. Journal of Fungi, 10(4), 265. https://doi.org/10.3390/jof10040265







