Impacts of Managed Vegetation Restoration on Arbuscular Mycorrhizal Fungi and Diazotrophs in Karst Ecosystems
Abstract
1. Introduction
2. Materials and Methods
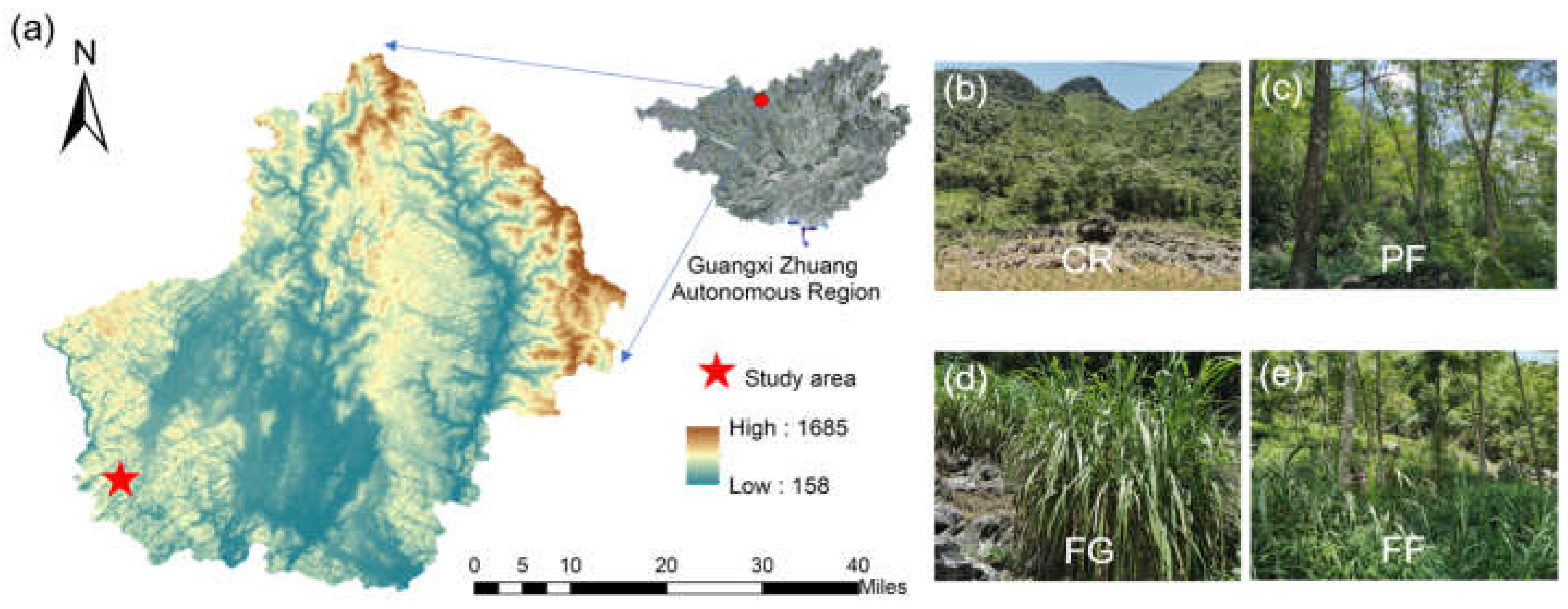
2.1. Study Area
2.2. Soil Sampling
2.3. Soil Analysis
2.4. DNA Extraction and Amplicon Sequencing
2.5. Sequence Analysis
2.6. Statistical Analysis
3. Results
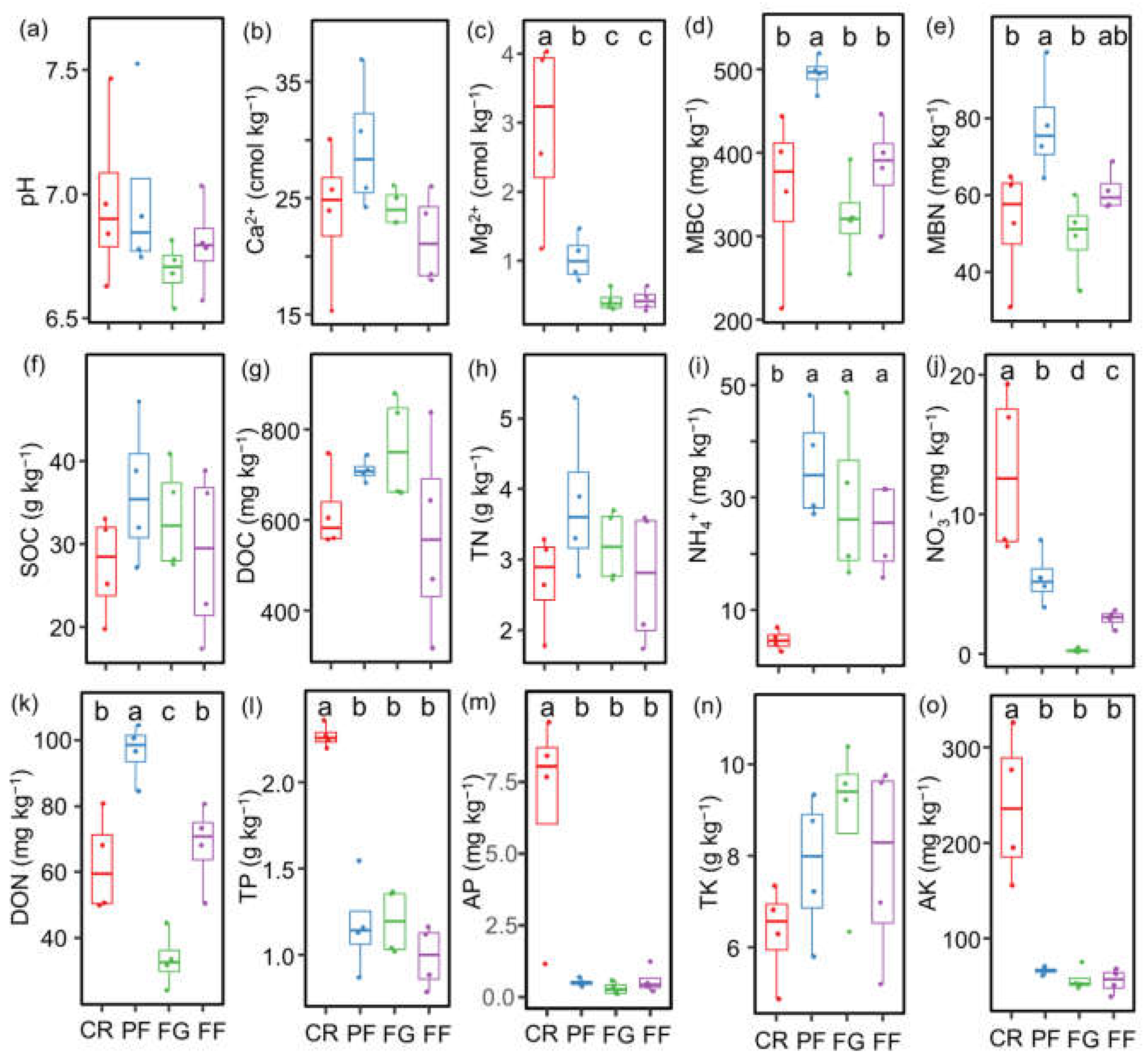
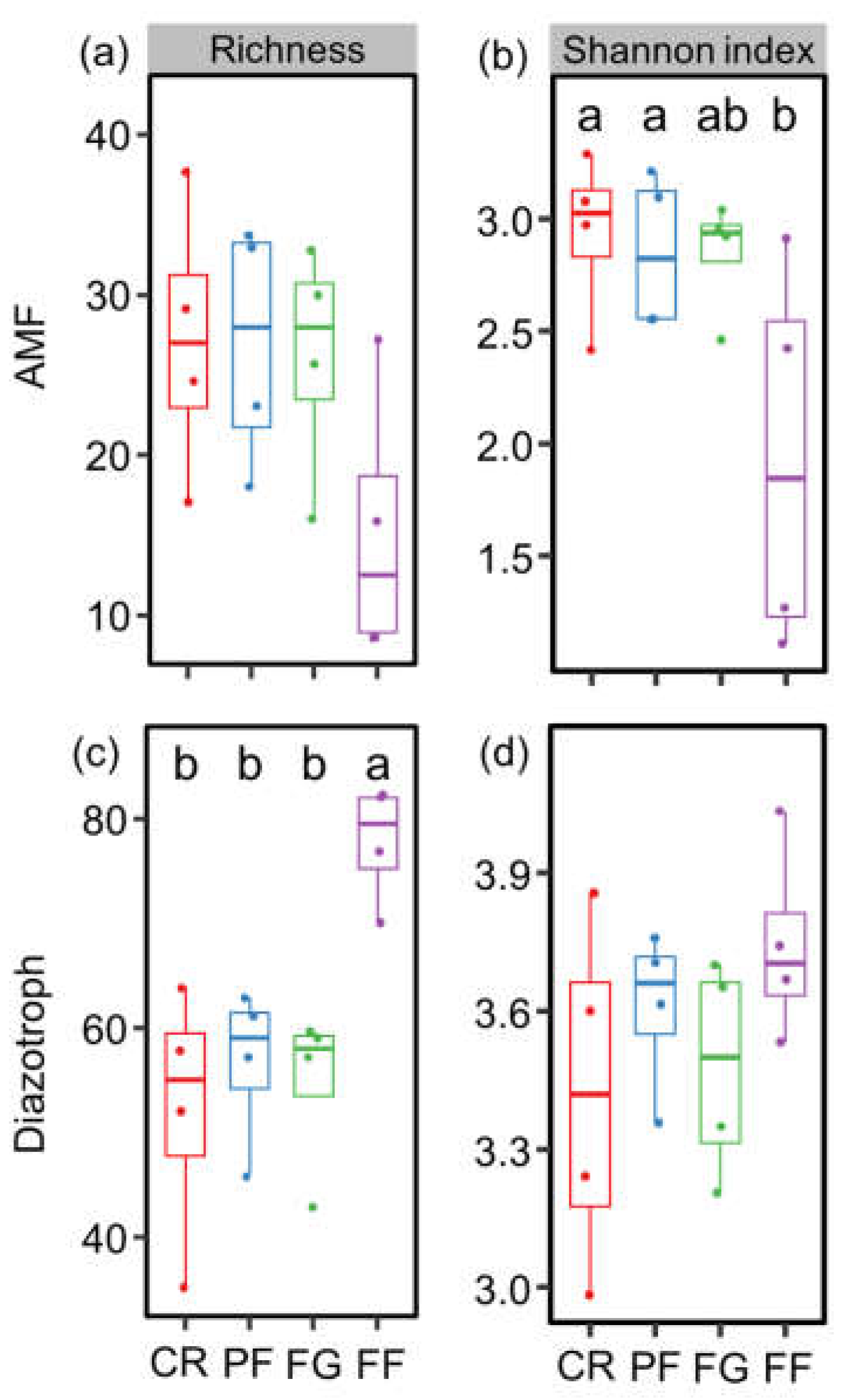
3.1. Soil Properties and the Diversity of AMF and Diazotrophs
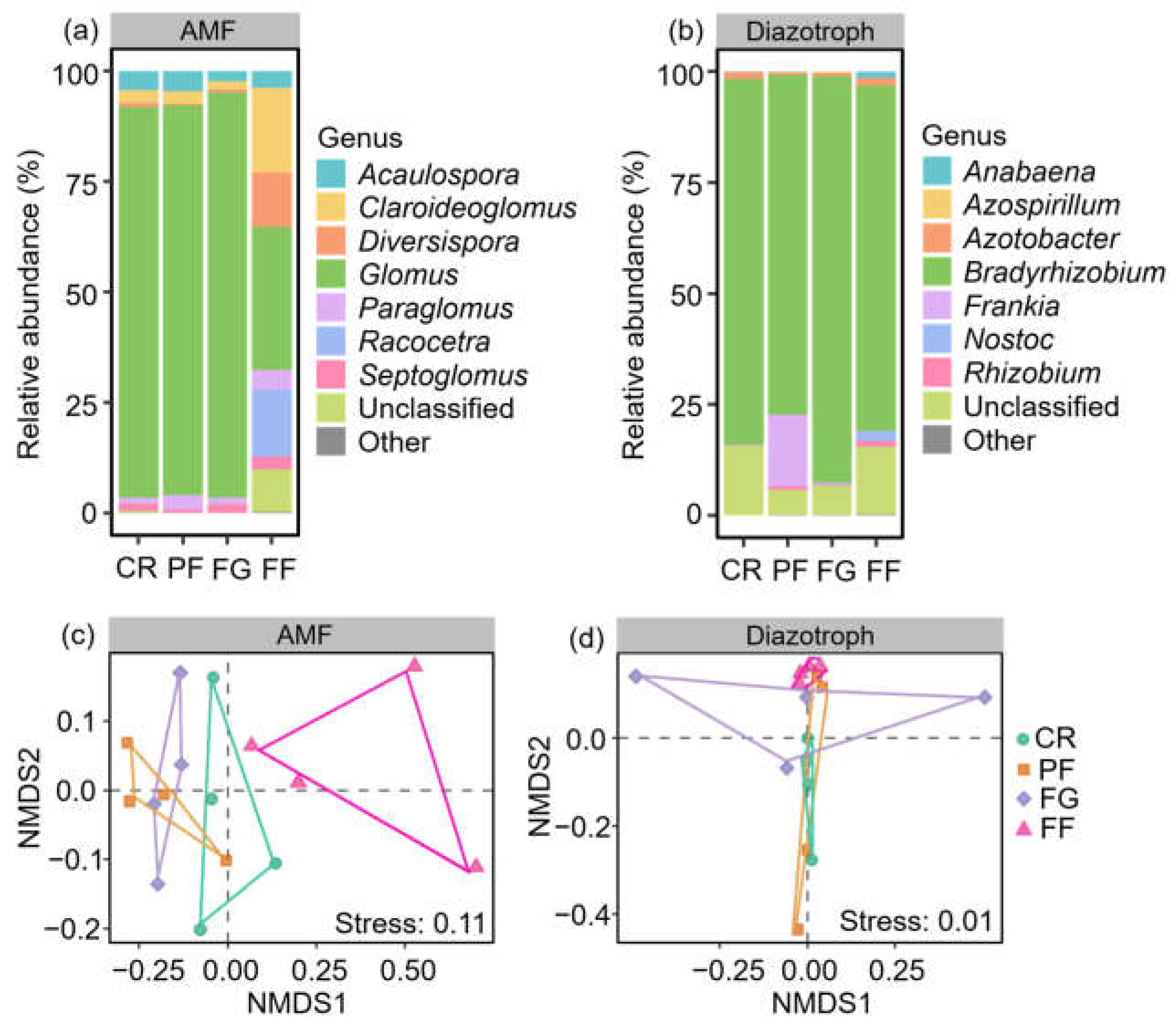
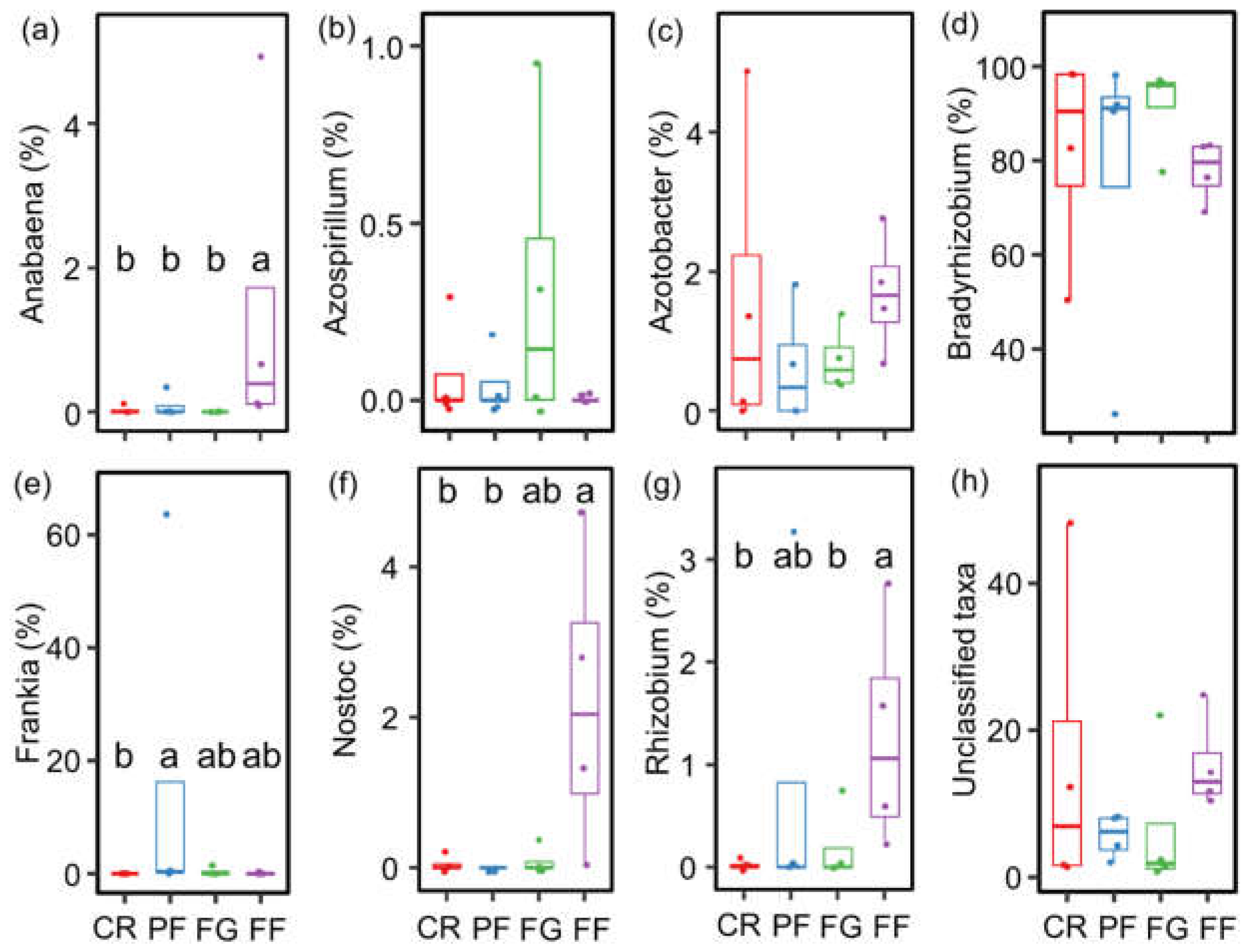
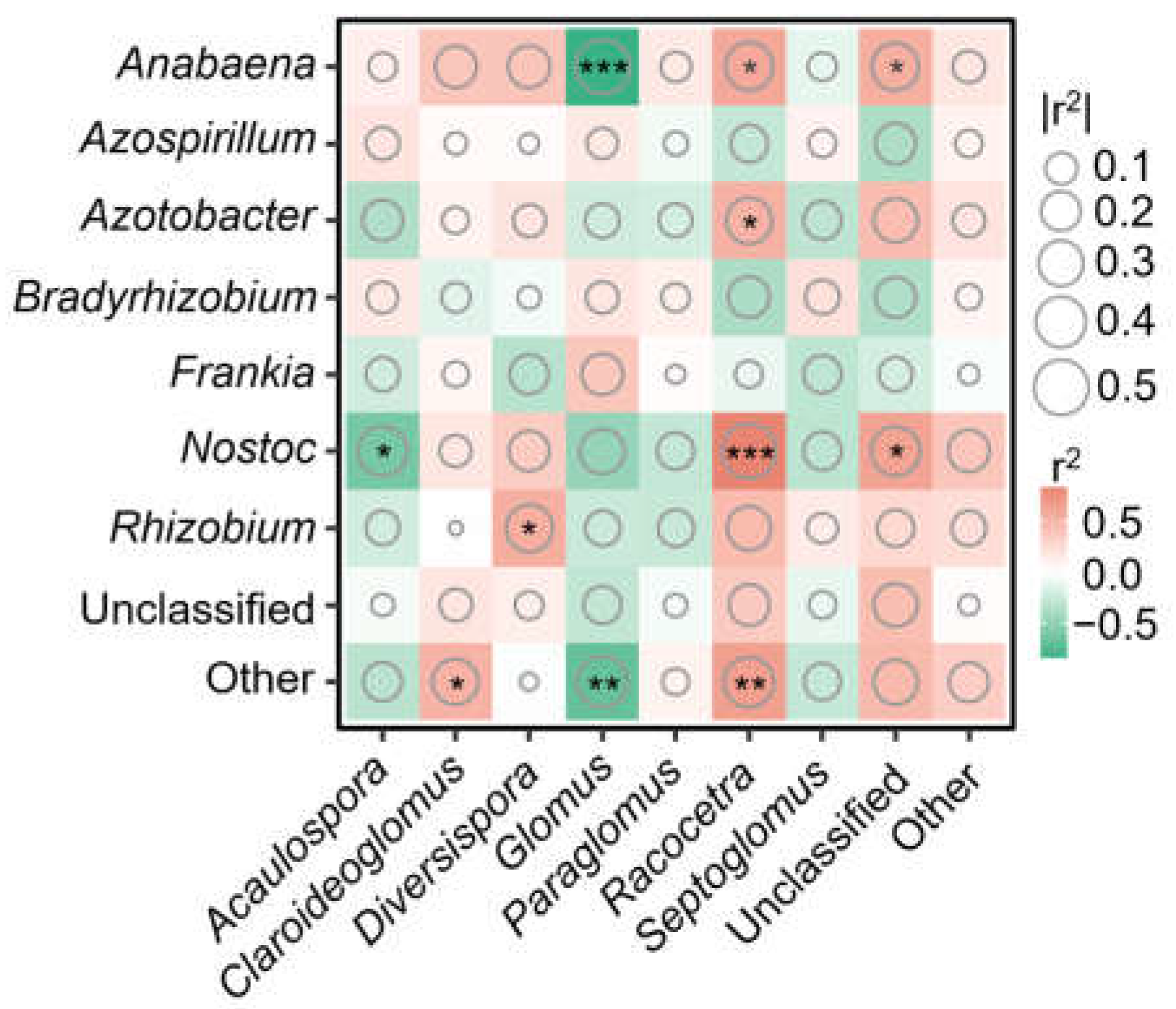
3.2. The community Compositions of AMF and Diazotrophs
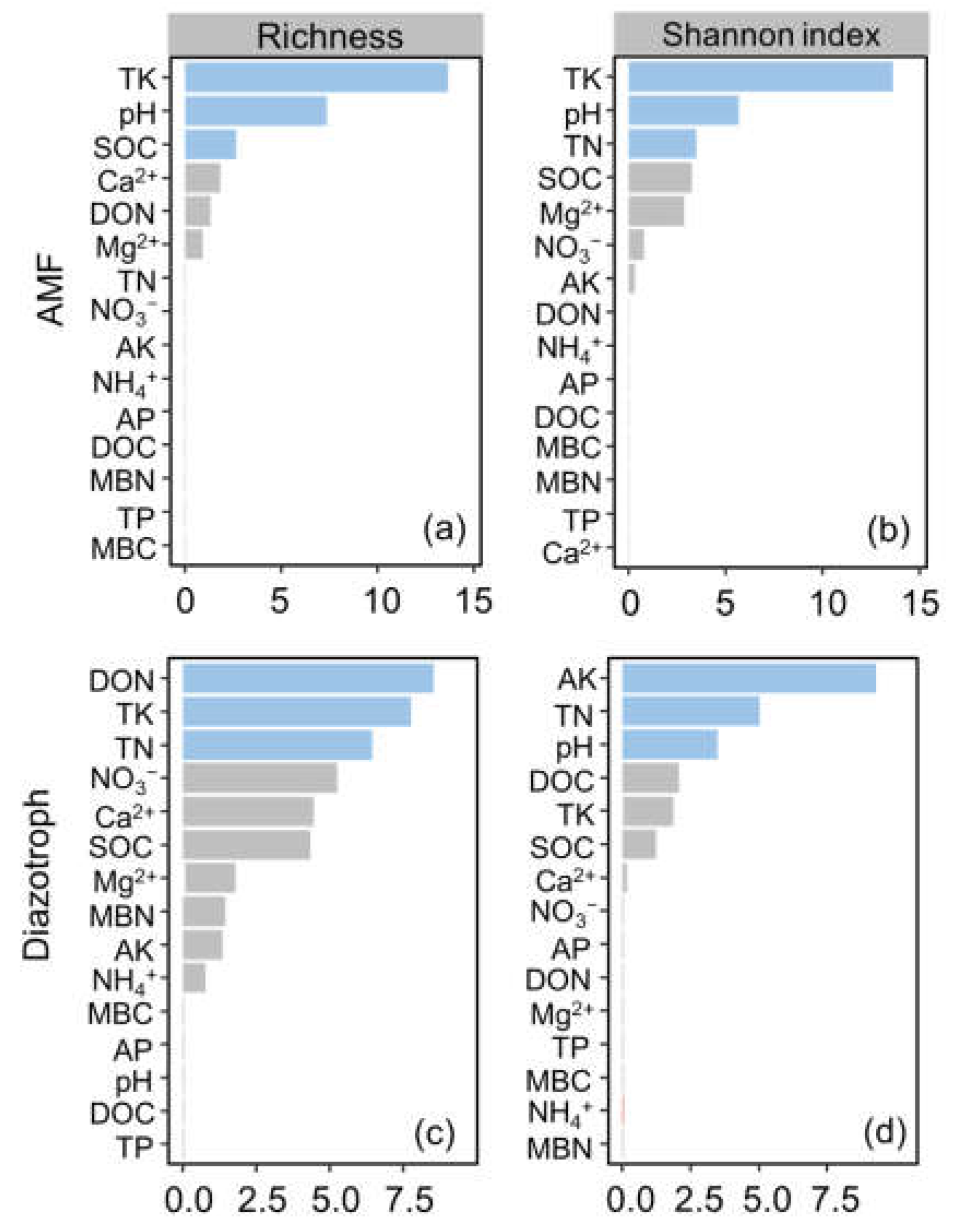
3.3. Drivers of AMF and Diazotroph Abundance and Diversity
4. Discussion
4.1. The Effect of Managed Vegetation Restoration on AMF and Diazotroph Diversity
4.2. Effect of Managed Vegetation Restoration on AMF and Diazotroph Community Compositions
4.3. Implications for Future Managed Vegetation Restoration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Der Heijden, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 489–512. [Google Scholar] [CrossRef]
- Xiao, D.; He, X.; Xu, Z.; Bai, S.H.; Zhang, W.; Hu, P.; Chen, M.; Wang, K. Strong cooperations among diazotroph and arbuscular mycorrhizal fungi taxa promote free-living nitrogen fixation at soil-rock mixing layer. Geoderma 2023, 437, 116600. [Google Scholar] [CrossRef]
- Xiao, D.; Chen, Y.; He, X.; Xu, Z.; Bai, S.H.; Zhang, W.; Cheng, M.; Hu, P.; Wang, K. Temperature and precipitation significantly influence the interactions between arbuscular mycorrhizal fungi and diazotrophs in karst ecosystems. For. Ecol. Manag. 2021, 497, 119464. [Google Scholar] [CrossRef]
- Xu, X.; Qiu, Y.; Zhang, K.; Yang, F.; Chen, M.; Luo, X.; Yan, X.; Wang, P.; Zhang, Y.; Chen, H.; et al. Climate warming promotes deterministic assembly of arbuscular mycorrhizal fungal communities. Glob. Change Biol. 2022, 28, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Gaby, J.C.; Rishishwar, L.; Valderrama-Aguirre, L.C.; Green, S.J.; Valderrama-Aguirre, A.; Jordan, I.K.; Kostka, J.E. Diazotroph community characterization via a high-throughput nifH amplicon sequencing and analysis pipeline. Appl. Environ. Microbiol. 2018, 84, e01512–e01517. [Google Scholar] [CrossRef]
- Liang, Y.; He, X.; Chen, C.; Feng, S.; Liu, L.; Chen, X.; Zhao, Z.; Su, Y. Influence of plant communities and soil properties during natural vegetation restoration on arbuscular mycorrhizal fungal communities in a karst region. Ecol. Eng. 2015, 82, 57–65. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, D.; Wang, Y.; Diao, L.; Cheng, X.; Luo, Y.; An, S.; Yang, W. Shift in soil microbial communities along ~160 years of natural vegetation restoration on the Loess Plateau of China. Appl. Soil Ecol. 2022, 173, 104394. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.I.; van Bodegom, P.M.; Merckx, V.S.; Soudzilovskaia, N. Environmental drivers for cheaters of arbuscular mycorrhizal symbiosis in tropical rainforests. New Phytol. 2019, 223, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Li, K.; Zhang, W.; Wang, H.; Liu, H.; Zhu, X.; Wu, H.; Zhang, X.; Lin, Q.; Sun, X.; et al. Differentiations of determinants for the community compositions of bacteria, fungi, and nitrogen fixers in various steppes. Ecol. Evol. 2019, 9, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Erlacher, A.; Ramadan, E.M.; El-Arabi, T.F.; Müller, H.; Bragina, A.; Berg, G. Comparisons of diazotrophic communities in native and agricultural desert ecosystems reveal plants as important drivers in diversity. FEMS Microbiol. Ecol. 2016, 92, fiv166. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Pan, F.; He, X.; Chen, X.; Su, Y. Effect of vegetation types on soil arbuscular mycorrhizal fungi and nitrogen-fixing bacterial communities in a karst region. Environ. Sci. Pollut. Res. 2016, 23, 18482–18491. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Menge, D.N.; Reed, S.C.; Cleveland, C.C. Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130119. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Liu, D.; Ledgard, S.; Luo, J.; Fan, J.; Yuan, J.; Chen, Z.; Ding, W. Long-term application of lime or pig manure rather than plant residues suppressed diazotroph abundance and diversity and altered community structure in an acidic Ultisol. Soil Biol. Biochem. 2018, 123, 218–228. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Millner, P.D. Biodiversity of arbuscular mycorrhizal fungi in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 77–93. [Google Scholar] [CrossRef]
- Kabir, Z.; Koide, R.T. The effect of dandelion or a cover crop on mycorrhiza inoculum potential, soil aggregation and yield of maize. Agric. Ecosyst. Environ. 2000, 78, 167–174. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Sheng, M.; Tian, J. Ecological stoichiometry and environmental influencing factors of soil nutrients in the karst rocky desertification ecosystem, southwest China. Glob. Ecol. Conserv. 2018, 16, e00449. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Li, S.L.; Liu, C.Q.; Chen, J.A.; Wang, S.J. Karst ecosystem and environment: Characteristics, evolution processes, and sustainable development. Agric. Ecosyst. Environ. 2021, 306, 107173. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, W.; Hu, P.; Xiao, D.; Yang, R.; Ye, Y.; Wang, K. The formation of large macroaggregates induces soil organic carbon sequestration in short-term cropland restoration in a typical karst area. Sci. Total Environ. 2021, 801, 149588. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, X.; Green, S.; Dungait, J.A.J.; Wen, X.; Tang, Y.; Guo, Z.; Yang, Y.; Sun, X.; Quine, T.A. Nitrogen functional gene activity in soil profiles under progressive vegetative recovery after abandonment of agriculture at the Puding Karst Critical Zone Observatory, SW China. Soil Biol. Biochem. 2018, 125, 93–102. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Xiao, L.; Yang, R.; Xiao, D.; Zhao, J.; Wang, W.; Chen, H.; Wang, K. Moss-dominated biological soil crusts modulate soil nitrogen following vegetation restoration in a subtropical karst region. Geoderma 2019, 352, 70–79. [Google Scholar] [CrossRef]
- Wang, J.; Wen, X.; Lyu, S.; Zhang, X.; Li, S.; Guo, Q. Vegetation recovery alters soil N status in subtropical karst plateau area: Evidence from natural abundance δ15N and δ18O. Plant Soil 2021, 460, 609–623. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, M.; Mao, Q.; Xiao, K.; Wang, K.; Li, D. Cropland conversion changes the status of microbial resource limitation in degraded karst soil. Geoderma 2019, 352, 197–203. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P.W. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef]
- Sato, K.; Suyama, Y.; Saito, M.; Sugawara, K. A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl. Sci. 2005, 51, 179–181. [Google Scholar] [CrossRef]
- Xiao, D.; Hong, T.; Chen, M.; He, X.; Wang, K. Assessing the effect of slope position on the community assemblage of soil diazotrophs and root arbuscular mycorrhizal fungi. J. Fungi 2023, 9, 394. [Google Scholar] [CrossRef]
- Tang, Y.; Tian, J.; Li, X.; Yao, M.; Wang, S.; Kuzyakov, Y.; Dungait, J.A. Higher free-living N2 fixation at rock-soil interfaces than topsoils during vegetation recovery in karst soils. Soil Biol. Biochem. 2021, 159, 108286. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.; Xiao, D.; Chen, M.; Cheng, M.; Wang, Z. Impacts of lithology and slope position on arbuscular mycorrhizal fungi communities in a karst forest soil. J. Fungi 2023, 9, 1133. [Google Scholar] [CrossRef] [PubMed]
- Barger, N.N.; Weber, B.; Garcia-Pichel, F.; Zaady, E.; Belnap, J. Patterns and controls on nitrogen cycling of biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer: Cham, Switzerland, 2016; pp. 257–285. [Google Scholar]
- Wang, Y.; Xiao, B.; Wang, W.; Revillini, D.; Delgado-Baquerizo, M. Biocrust adaptations to microhabitat alter bacterial communities in a semiarid ecosystem. Plant Soil 2023, 492, 413–427. [Google Scholar] [CrossRef]
- Zhao, Y.D.; Hu, X.; Pan, P.Y. Positive feedback relationship between shrub encroachment and arbuscular mycorrhizal fungi in the Inner Mongolia grassland of northern China. Appl. Soil Ecol. 2022, 177, 104525. [Google Scholar] [CrossRef]
- Koorem, K.; Tulva, I.; Davison, J.; Jairus, T.; Öpik, M.; Vasar, M.; Zobel, M.; Moora, M. Arbuscular mycorrhizal fungal communities in forest plant roots are simultaneously shaped by host characteristics and canopy-mediated light availability. Plant Soil 2017, 410, 259–271. [Google Scholar] [CrossRef]
- Utaile, Y.U.; Van Geel, M.; Muys, B.; Cheche, S.S.; Helsen, K.; Honnay, O. Woody encroachment of an East-African savannah ecosystem alters its arbuscular mycorrhizal fungal communities. Plant Soil 2021, 464, 303–320. [Google Scholar] [CrossRef]
- Öpik, M.; Zobel, M.; Cantero, J.J.; Davison, J.; Facelli, J.M.; Hiiesalu, I.; Jairus, T.; Kalwij, J.M.; Koorem, K.; Leal, M.E.; et al. Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza 2013, 23, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Wang, X.; Liu, F.; Li, X.; Zhu, X. Soil properties and geography shape arbuscular mycorrhizal fungal communities in black land of China. Appl. Soil Ecol. 2021, 167, 104109. [Google Scholar] [CrossRef]
- Che, R.; Deng, Y.; Wang, F.; Wang, W.; Xu, Z.; Hao, Y.; Xue, K.; Zhang, B.; Tang, L.; Zhou, K.; et al. Autotrophic and symbiotic diazotrophs dominate nitrogen-fixing communities in Tibetan grassland soils. Sci. Total Environ. 2018, 639, 997–1006. [Google Scholar] [CrossRef]
- Dodds, W.K.; Gudder, D.A.; Mollenhauer, D. The ecology of nostoc. J. Phycol. 1995, 31, 2–18. [Google Scholar] [CrossRef]
- Li, J.; Xie, T.; Zhu, H.; Zhou, J.; Li, C.; Xiong, W.; Xu, L.; Wu, Y.; He, Z.; Li, X. Alkaline phosphatase activity mediates soil organic phosphorus mineralization in a subalpine forest ecosystem. Geoderma 2021, 404, 115376. [Google Scholar] [CrossRef]
- Di Martino, C.; Palumbo, G.; Vitullo, D.; Di Santo, P.; Fuggi, A. Regulation of mycorrhiza development in durum wheat by P fertilization: Effect on plant nitrogen metabolism. J. Plant Nutr. Soil Sci. 2018, 181, 429–440. [Google Scholar] [CrossRef]
- Peng, L.; Shan, X.; Yang, Y.; Wang, Y.; Druzhinina, I.S.; Pan, X.; Jin, W.; He, X.; Wang, X.; Zhang, X.; et al. Facultative symbiosis with a saprotrophic soil fungus promotes potassium uptake in American sweetgum trees. Plant Cell Environ. 2021, 44, 2793–2809. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; González-Guerrero, M. Unravelling potassium nutrition in ectomycorrhizal associations. New Phytol. 2014, 201, 707–709. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Xiao, D.; Zhang, W.; Wang, K. Impacts of Managed Vegetation Restoration on Arbuscular Mycorrhizal Fungi and Diazotrophs in Karst Ecosystems. J. Fungi 2024, 10, 280. https://doi.org/10.3390/jof10040280
Sun M, Xiao D, Zhang W, Wang K. Impacts of Managed Vegetation Restoration on Arbuscular Mycorrhizal Fungi and Diazotrophs in Karst Ecosystems. Journal of Fungi. 2024; 10(4):280. https://doi.org/10.3390/jof10040280
Chicago/Turabian StyleSun, Mingming, Dan Xiao, Wei Zhang, and Kelin Wang. 2024. "Impacts of Managed Vegetation Restoration on Arbuscular Mycorrhizal Fungi and Diazotrophs in Karst Ecosystems" Journal of Fungi 10, no. 4: 280. https://doi.org/10.3390/jof10040280
APA StyleSun, M., Xiao, D., Zhang, W., & Wang, K. (2024). Impacts of Managed Vegetation Restoration on Arbuscular Mycorrhizal Fungi and Diazotrophs in Karst Ecosystems. Journal of Fungi, 10(4), 280. https://doi.org/10.3390/jof10040280







