Effects of MrwetA on Sexual Reproduction and Secondary Metabolism of Monascus ruber M7 Based on Transcriptome Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Construction of the MrwetA Complemented Strain
2.3. Observations of Sexual Reproduction
2.4. Detection of Monascus Pigments and Citrinin
2.5. RNA Extraction and RNA Sequencing
2.6. Statistical Analysis
3. Results
3.1. Construction of the MrwetA Complemented Strain
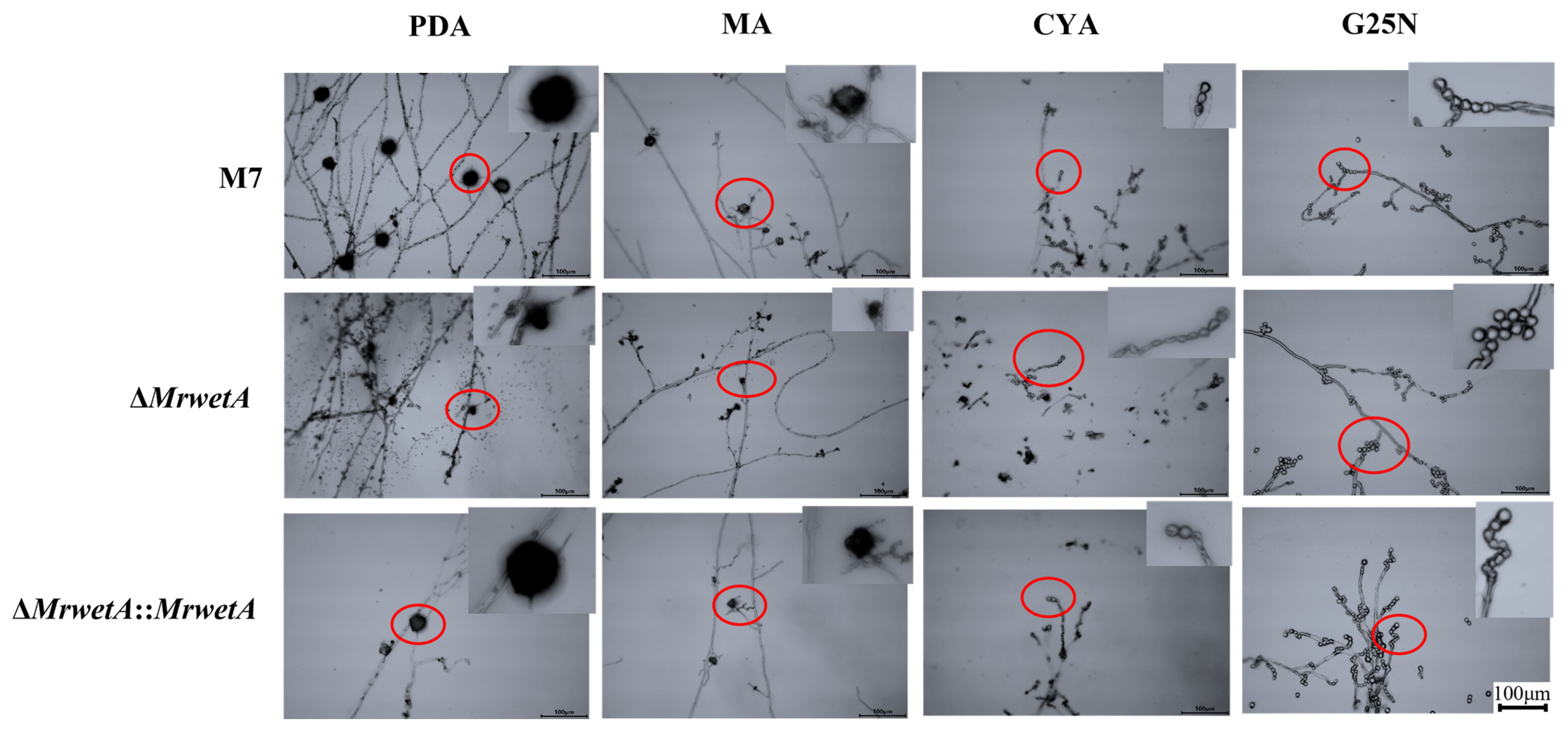
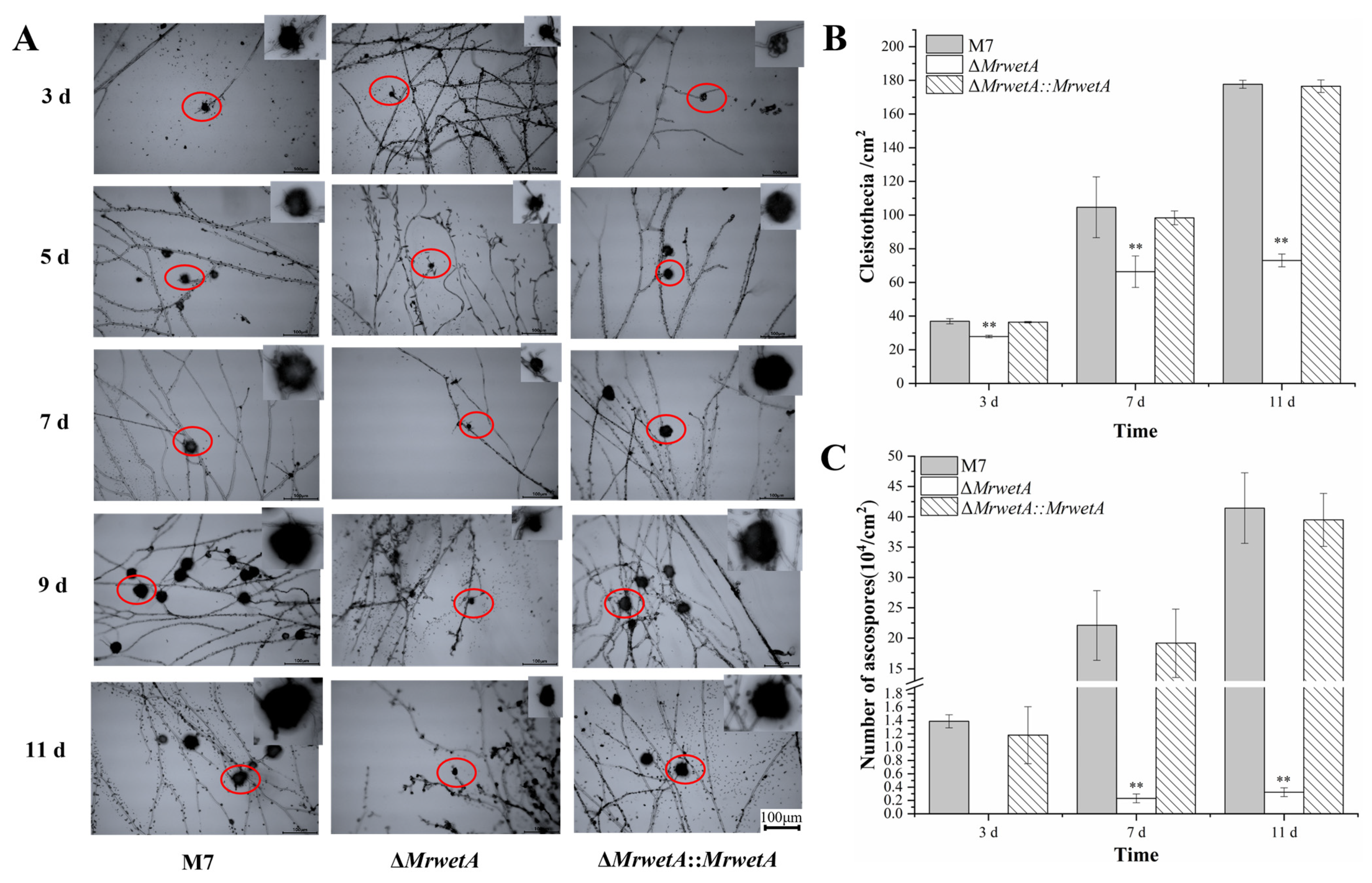
3.2. Reproduction Observations of M. ruber M 7 and Its MrwetA Modified Strains
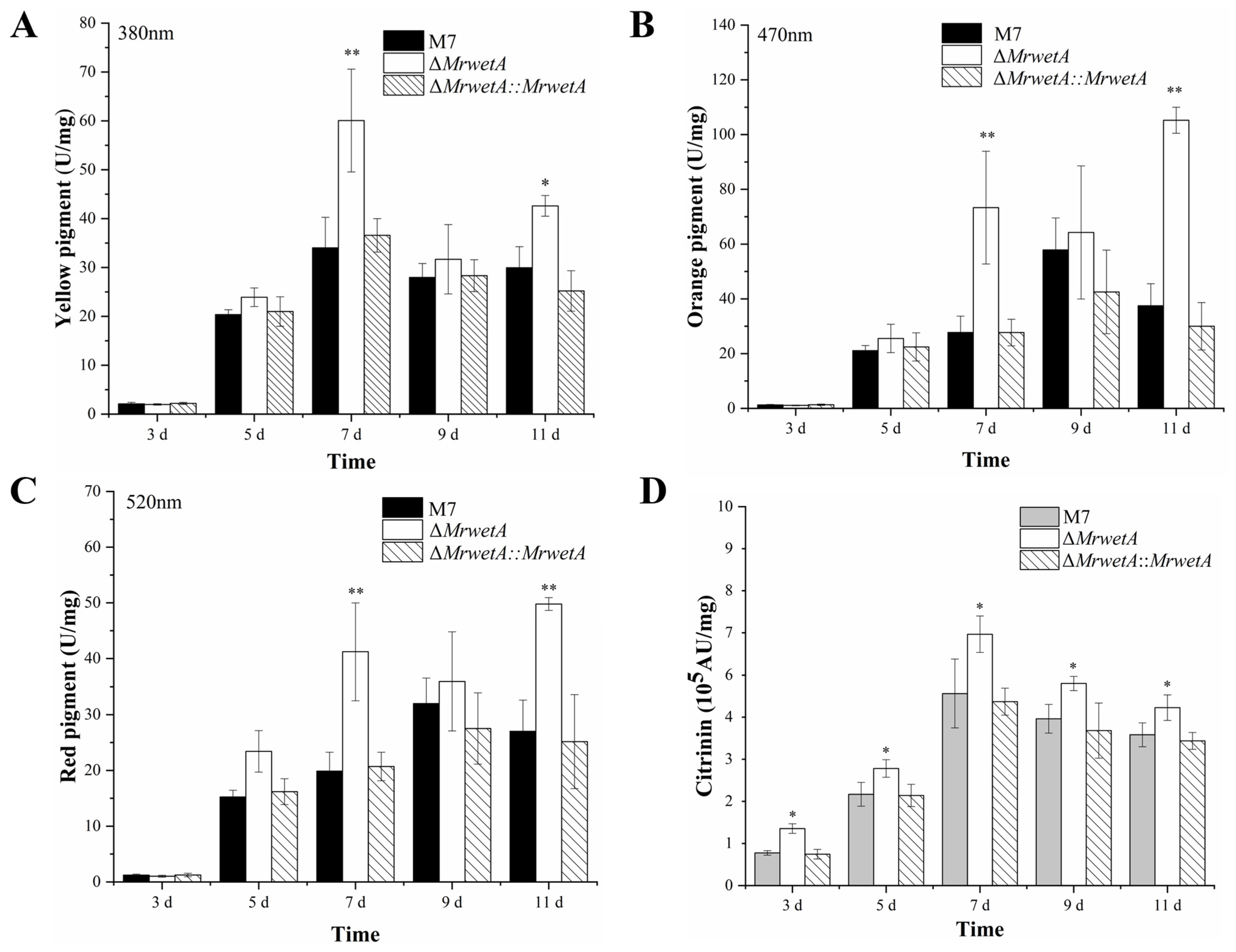
3.3. MPs and CIT Analysis of WT and Its MrwetA Modified Strains
3.4. The Transcriptomic Analysis of WT and ΔMrwetA
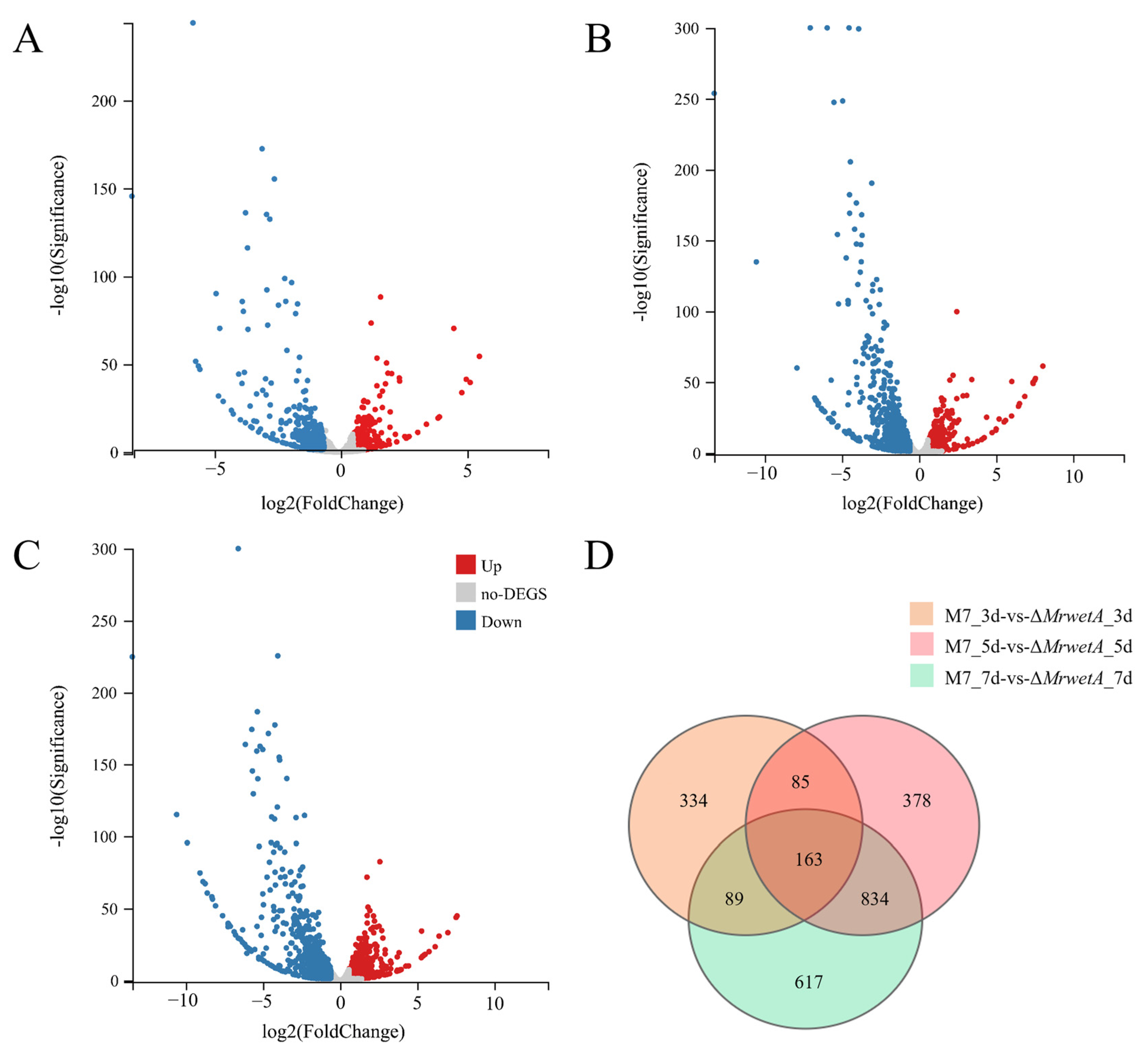
3.4.1. Analysis of Differentially Expressed Genes
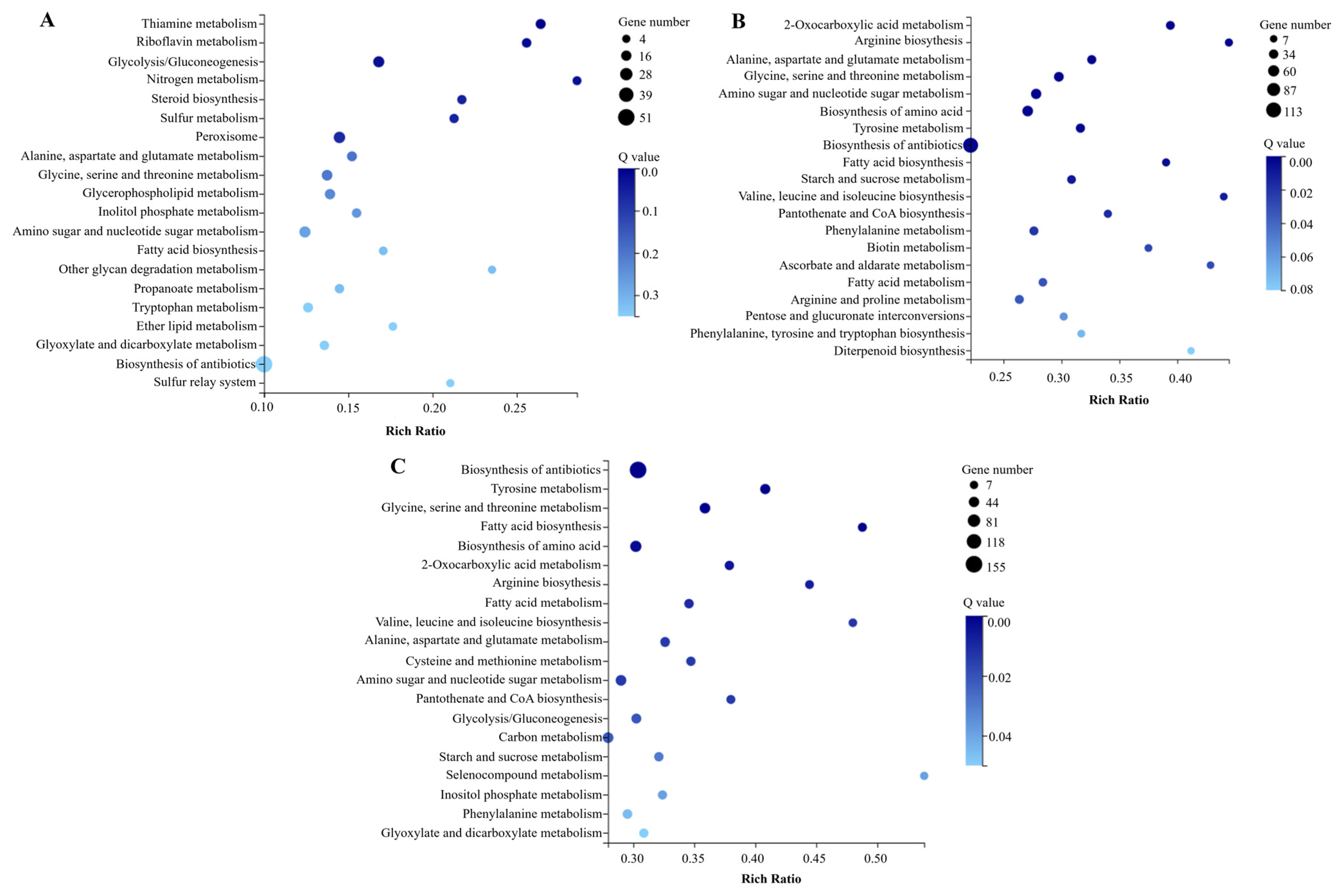
3.4.2. KEGG Pathway Enrichment Analysis of DEGs
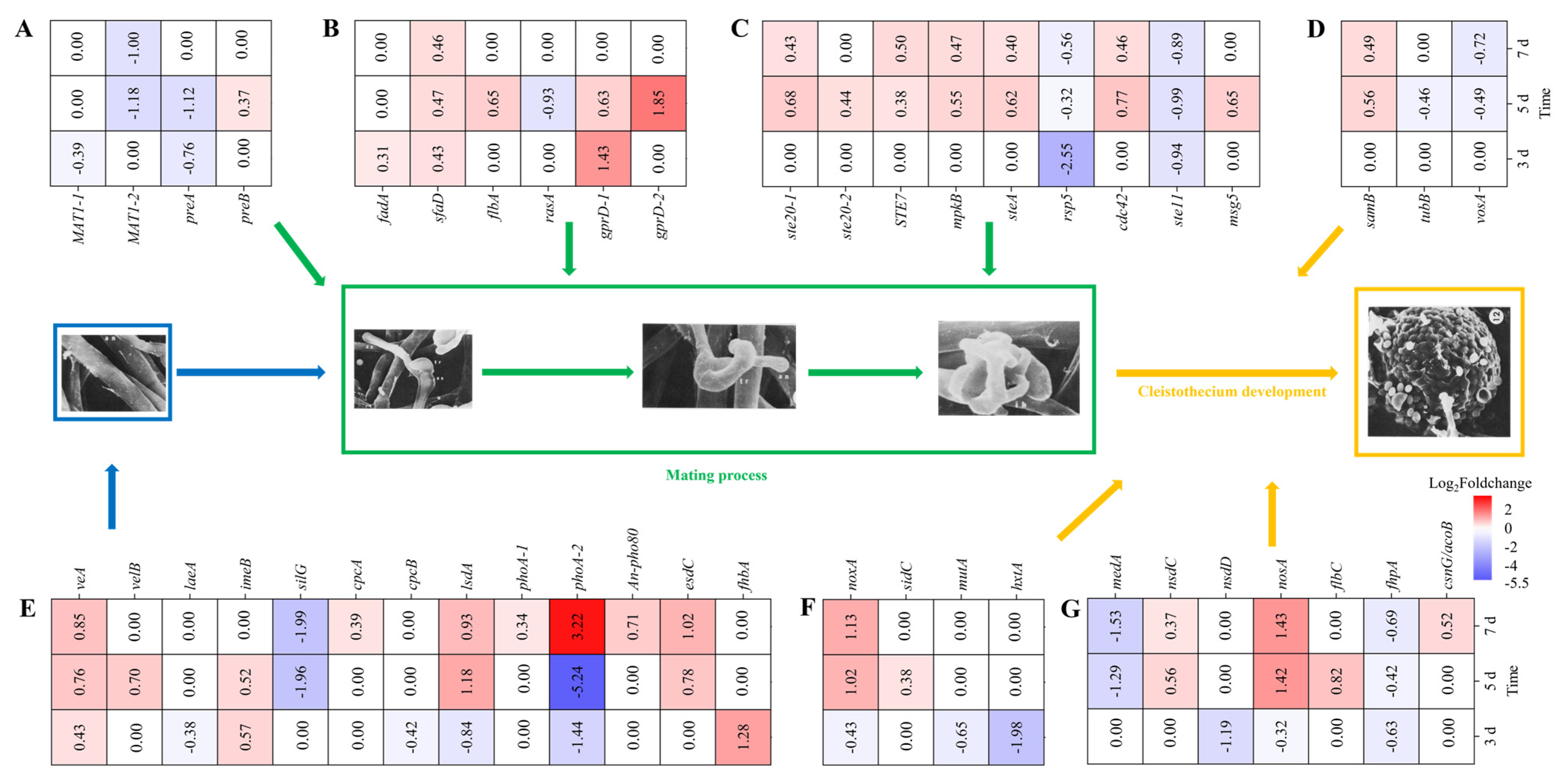
3.4.3. Effects of MrwetA on Sexual Reproduction
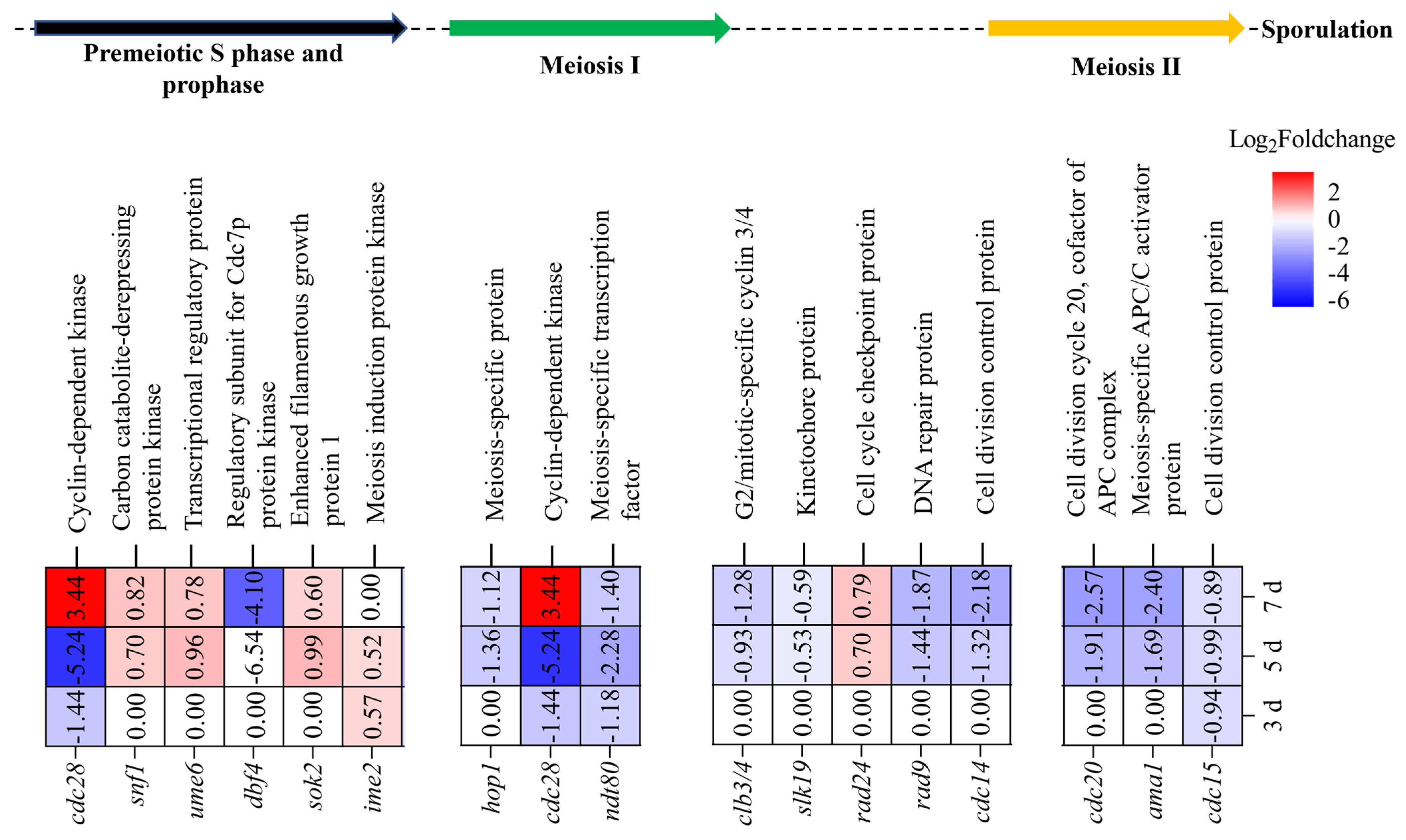
3.4.4. Effects of MrwetA on Meiosis
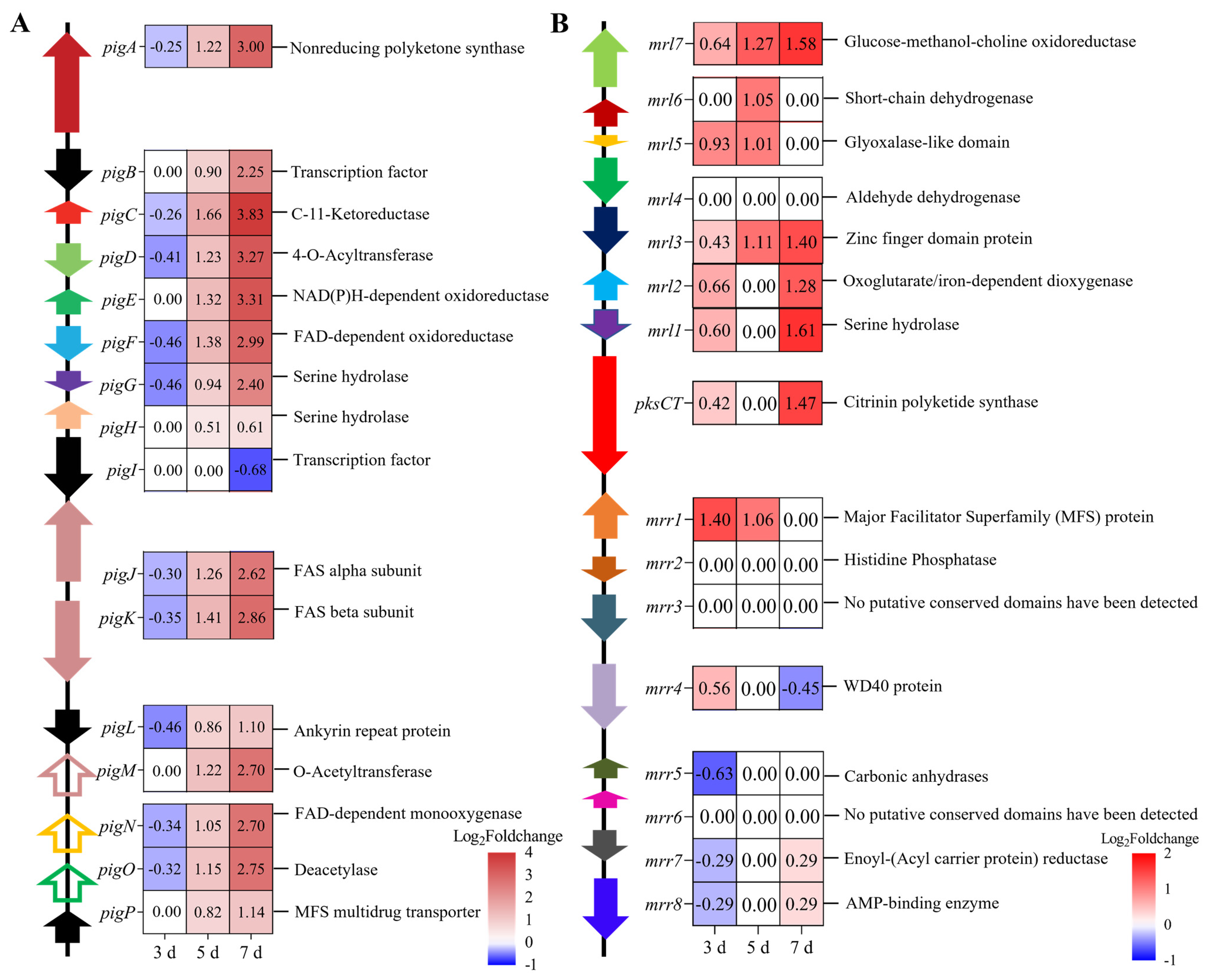
3.4.5. Effects of MrwetA on Secondary Metabolites
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; He, Y.; Zhou, Y.; Shao, Y.; Feng, Y.; Li, M.; Chen, F. Edible filamentous fungi from the species Monascus: Early traditional fermentations, modern molecular biology, and future genomics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 555–567. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Feng, Y.; Chen, W.; He, Y.; Liu, J.; Lei, M.; Liu, Q.; Shao, Y.; Chen, F. Historic and charming Monascus spp. Chin. Sci.Bull. 2023, 68, 479–494. [Google Scholar] [CrossRef]
- Patakova, P. Monascus secondary metabolites: Production and biological activity. J. Ind. Microbiol. Biotechnol. 2013, 40, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Feng, Y.; Molnar, I.; Chen, F. Nature and nurture: Confluence of pathway determinism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Nat. Prod. Rep. 2019, 36, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Compactin (ML-236B) and related compounds as potential cholesterol-lowering agents that inhibit HMG-CoA reductase. J. Med. Chem. 1985, 8, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.; Laussac, J.P.; Le Bars, J.; Le Bars, P.; Loret, M.O.; Pareilleux, A.; Prome, D.; Prome, J.C.; Santerre, A.L.; Goma, G. Characterization of monascidin A from Monascus as citrinin. Int. J. Food Microbiol. 1995, 27, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shao, Y.; Zhou, Y.; Chen, F. Production and optimization of monacolin K by citrinin-free Monascus pilosus MS-1 in solid-state fermentation using non-glutinous rice and soybean flours as substrate. Eur. Food Res. Technol. 2014, 239, 629–636. [Google Scholar] [CrossRef]
- Kang, B.; Zhang, X.; Wu, Z.; Wang, Z.; Park, S. Production of citrinin-free Monascus pigments by submerged culture at low pH. Enzym. Microb. Technol. 2014, 55, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qin, X.; Zhao, Y.; Sun, X.; Yu, X.; Feng, Y. Strategies to enhance the production efficiency of Monascus pigments and control citrinin contamination. Process Biochem. 2022, 117, 19–29. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Dong, X.; Li, A.; Xu, S.; Wang, L.; Shao, Y. Metabolic regulation of two pksCT gene transcripts in Monascus ruber impacts citrinin biosynthesis. J. Fungi 2023, 9, 1174. [Google Scholar] [CrossRef]
- He, Y.; Cox, R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016, 7, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kang, L.; Ding, X.; Liu, J.; Liu, Q.; Shao, Y.; Molnár, I.; Chen, F. Monasone naphthoquinone biosynthesis and resistance in Monascus fungi. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Srianta, I.; Ristiarini, S.; Nugerahani, I.; Sen, S.K.; Zhang, B.B.; Xu, G.R.; Blanc, P.J. Recent research and development of Monascus fermentation products. Int. Food Res. J. 2014, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Yang, J.G.; Ma, Y.Y.; Lin, Y.; Wu, H.C. Research article optimization of process conditions of Monascus seed Koji by orthogonal test design. Adv. J. Food Sci. Technol. 2015, 7, 250–254. [Google Scholar] [CrossRef]
- Wong, H.C.; Chien, C.Y. Ultrastructure of sexual reproduction of Monascus Purpureos. Mycologia 1986, 78, 713–721. [Google Scholar] [CrossRef]
- Dyer, P.S.; O’Gorman, C.M. Sexual development and cryptic sexuality in fungi: Insights from Aspergillus species. FEMS Microbiol. Rev. 2012, 36, 165–192. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.N.; Leong, S.L.; Vinnere-Pettersson, O.; Chen, A.J.; Souza-Motta, C.M.; Frisvad, J.C.; Samson, R.A.; Oliveira, N.T.; Houbraken, J. Phylogenetic analysis of Monascus and new species from honey, pollen and nests of stingless bees. Stud. Mycol. 2017, 86, 29–51. [Google Scholar] [CrossRef]
- De Vries, R.P.; Riley, R.; Wiebenga, A.; Aguilar-Osorio, G.; Amillis, S.; Uchima, C.A.; Anderluh, G.; Asadollahi, M.; Askin, M.; Barry, K.; et al. Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus. Genome Biol. 2017, 18, 28. [Google Scholar] [CrossRef]
- Jia, L.; Yu, J.H.; Chen, F.; Chen, W. Characterization of the asexual developmental genes brlA and wetA in Monascus ruber M7. Fungal Genet. Biol. 2021, 151, 103564. [Google Scholar] [CrossRef]
- Mirabito, P.M.; Adams, T.H.; Timberlake, W.E. Interactions of three sequentially expressed genes control temporal and spatial specificity in Aspergillus development. Cell 1989, 57, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Sewall, T.C.; Mims, C.W.; Timberlake, W.E. abaA controls phialide differentiation in Aspergillus nidulans. Plant Cell 1990, 2, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.A.; Timberlake, W.E. Aspergillus nidulans wetA activates spore-specific gene expression. Mol. Cell. Biol. 1991, 11, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Tokuoka, M.; Jin, F.J.; Takahashi, T.; Koyama, Y. Genetic analysis of conidiation regulatory pathways in koji-mold Aspergillus oryzae. Fungal Genet. Biol. 2010, 47, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Yu, J.H. AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiology 2011, 157, 313–326. [Google Scholar] [CrossRef]
- Shin, K.S.; Kim, Y.H.; Yu, J.H. Proteomic analyses reveal the key roles of BrlA and AbaA in biogenesis of gliotoxin in Aspergillus fumigatus. Biochem. Biophys. Res. Commun. 2015, 463, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Yu, J.H. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012, 15, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-López, M.; Chen, W.; Eagle, C.E.; Gutiérrez, G.; Jia, W.L.; Swilaiman, S.S.; Huang, Z.; Park, H.S.; Yu, J.H.; Cánovas, D.; et al. Evolution of asexual and sexual reproduction in the Aspergilli. Stud. Mycol. 2018, 91, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Sigl, C.; Haas, H.; Specht, T.; Pfaller, K.; Kürnsteiner, H.; Zadra, I. Among developmental regulators, StuA but not BrlA is essential for penicillin V production in Penicillium chrysogenum. Appl. Environ. Microbiol. 2011, 77, 972–982. [Google Scholar] [CrossRef]
- Li, F.; Shi, H.Q.; Ying, S.H.; Feng, M.G. WetA and VosA are distinct regulators of conidiation capacity, conidial quality, and biological control potential of a fungal insect pathogen. Appl. Microbiol. Biotechnol. 2015, 99, 10069–10081. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Zhu, C.; Xu, Q.; Ruan, R.; Yu, D.; Li, H. PdbrlA, PdabaA and PdwetA control distinct stages of conidiogenesis in Penicillium digitatum. Res. Microbiol. 2015, 166, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Chen, X.; Zhang, X.; Zhang, Q.; Xu, C.; Mi, W.; Guo, N.; Zhao, H.; You, Y.; Dryburgh, F.J.; et al. Genome-wide identification of pathogenicity, conidiation and colony sectorization genes in Metarhizium robertsii. Environ. Microbiol. 2017, 19, 3896–3908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.X.; Mouhoumed, A.Z.; Tong, S.M.; Ying, S.H.; Feng, M.G. BrlA and AbaA govern virulence-required dimorphic switch, conidiation, and pathogenicity in a fungal insect pathogen. mSystems 2019, 4, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Mead, M.E.; Lee, M.K.; Ostrem Loss, E.M.; Kim, S.C.; Rokas, A.; Yu, J.H. Systematic dissection of the evolutionarily conserved WetA developmental regulator across a genus of filamentous fungi. mBio 2018, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hu, X. Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. Int. J. Food Microbiol. 2005, 103, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ding, Y.; Zhao, Y.; Yang, S.; Xie, B.; Chen, F. Characteristic analysis of transformants in T-DNA mutation library of Monascus ruber. World J. Microbiol. Biotechnol. 2009, 25, 989–995. [Google Scholar] [CrossRef]
- Li, L.; He, L.; Lai, Y.; Shao, Y.; Chen, F. Cloning and functional analysis of the Gβ gene Mgb1 and the Gγ gene Mgg1 in Monascus ruber. J. Microbiol. 2014, 52, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, F. A comprehensive analysis of the small GTPases Ypt7 involved in the regulation of fungal development and secondary metabolism in Monascus ruber M7. Front. Microbiol. 2019, 10, 436891. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yang, L.; Shao, Y.; Xiong, H. The preliminary study on autolytic process of Monaascus ruber M805. China Food Addit. 2009, 82–87. [Google Scholar]
- Kolotila, M.P.; Hollingsworth, P.J.; Volz, P.A. Suface features of Monascus ruber van Tieghem cleistothecia. Bot. Gaz. 1978, 139, 256–260. [Google Scholar] [CrossRef]
- Kwon, N.J.; Shin, K.S.; Yu, J.H. Characterization of the developmental regulator FlbE in Aspergillus fumigatus and Aspergillus nidulans. Fungal Genet. Biol. 2010, 47, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Li, Z.; Wu, R.; Qin, Y.; Liu, G.; Qu, Y. Penicillium oxalicum PoFlbC regulates fungal asexual development and is important for cellulase gene expression. Fungal Genet. Biol. 2016, 86, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Sopko, R.; Huang, D.; Preston, N.; Chua, G.; Papp, B.; Kafadar, K.; Snyder, M.; Oliver, S.G.; Cyert, M.; Hughes, T.R.; et al. Mapping pathways and phenotypes by systematic gene overexpression. Mol. Cell 2006, 21, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Sewall, T.C.; Mims, C.W.; Timberlake, W.E. Conidium differentiation in Aspergillus nidulans wild-type and wet-white (wetA) mutant strains. Dev. Biol. 1990, 138, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Kim, M.G.; Min, K.; Lim, J.Y.; Choi, G.J.; Kim, J.C.; Chae, S.K.; Lee, Y.W. WetA is required for conidiogenesis and conidium maturation in the ascomycete fungus Fusarium graminearum. Eukaryot. Cell 2014, 13, 87–98. [Google Scholar] [CrossRef]
- Bardwell, A.J.; Abdollahi, M.; Bardwell, L. Anthrax lethal factor-cleavage products of MAPK (mitogen-activated protein kinase) kinases exhibit reduced binding to their cognate MAPKs. Biochem. J. 2004, 378, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Ni, M.; Li, W.; Shertz, C.; Heitman, J. The evolution of sex: A perspective from the fungal kingdom. Microbiol. Mol. Biol. Rev. 2010, 74, 298–340. [Google Scholar] [CrossRef]
- Krijgsheld, P.; Bleichrodt, R.V.; Van Veluw, G.J.; Wang, F.; Müller, W.H.; Dijksterhuis, J.; Wösten, H.A.B. Development in Aspergillus. Stud. Mycol. 2013, 74, 1–29. [Google Scholar] [CrossRef]
- Chu, S.; DeRisi, J.; Eisen, M.; Mulholland, J.; Botstein, D.; Brown, P.O.; Herskowitz, I. The transcriptional program of sporulation in budding yeast. Science 1998, 282, 699–705. [Google Scholar] [CrossRef]
- Benjamin, K.R.; Zhang, C.; Shokat, K.M.; Herskowitz, I. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 2003, 17, 1524–1539. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Liu, Q.; Chen, F. Deletion of pigR gene in Monascus ruber leads to loss of pigment production. Biotechnol. Lett. 2013, 35, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Chen, C.C.; Pan, T.M.; Kwon, H.J. Mpp7 controls regioselective Knoevenagel condensation during the biosynthesis of Monascus azaphilone pigments. Tetrahedron Lett. 2014, 55, 1640–1643. [Google Scholar] [CrossRef]
- Bijinu, B.; Suh, J.W.; Park, S.H.; Kwon, H.J. Delineating Monascus azaphilone pigment biosynthesis: Oxidoreductive modifications determine the ring cyclization pattern in azaphilone biosynthesis. Rsc Adv. 2014, 4, 59405–59408. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, N.; He, Y.; Wang, L.; Shao, Y.; Zhao, H.; Chen, F. MpigE, a gene involved in pigment biosynthesis in Monascus ruber M7. Appl. Microbiol. Biotechnol. 2014, 98, 285–296. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Yi, T.; Zhao, M.; Xie, N.; Lei, M.; Liu, J.; Shao, Y.; Chen, F. Identification and role analysis of an intermediate produced by a polygenic mutant of Monascus pigments cluster in Monascus ruber M7. Appl. Microbiol. Biotechnol. 2016, 100, 7037–7049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Jia, L.; Chen, F. Effects of MrwetA on Sexual Reproduction and Secondary Metabolism of Monascus ruber M7 Based on Transcriptome Analysis. J. Fungi 2024, 10, 338. https://doi.org/10.3390/jof10050338
Huang Y, Jia L, Chen F. Effects of MrwetA on Sexual Reproduction and Secondary Metabolism of Monascus ruber M7 Based on Transcriptome Analysis. Journal of Fungi. 2024; 10(5):338. https://doi.org/10.3390/jof10050338
Chicago/Turabian StyleHuang, Yuyun, Lili Jia, and Fusheng Chen. 2024. "Effects of MrwetA on Sexual Reproduction and Secondary Metabolism of Monascus ruber M7 Based on Transcriptome Analysis" Journal of Fungi 10, no. 5: 338. https://doi.org/10.3390/jof10050338
APA StyleHuang, Y., Jia, L., & Chen, F. (2024). Effects of MrwetA on Sexual Reproduction and Secondary Metabolism of Monascus ruber M7 Based on Transcriptome Analysis. Journal of Fungi, 10(5), 338. https://doi.org/10.3390/jof10050338







