Allochthonous Trichoderma Isolates Boost Atractylodes lancea Herb Quality at the Cost of Rhizome Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Fungal Materials
2.2. Isolation, Identification and Culturing of Fungi
2.3. Dual-Culture Experiments
2.4. Inoculation of A. lancea Seedlings
2.5. Measurement of A. lancea Biomass and Content of Medicinal Compounds
2.6. Studies on the Optimal Culturing and Sporulation Conditions for the Trichoderma Isolates
2.7. Statistical Analyses
3. Results
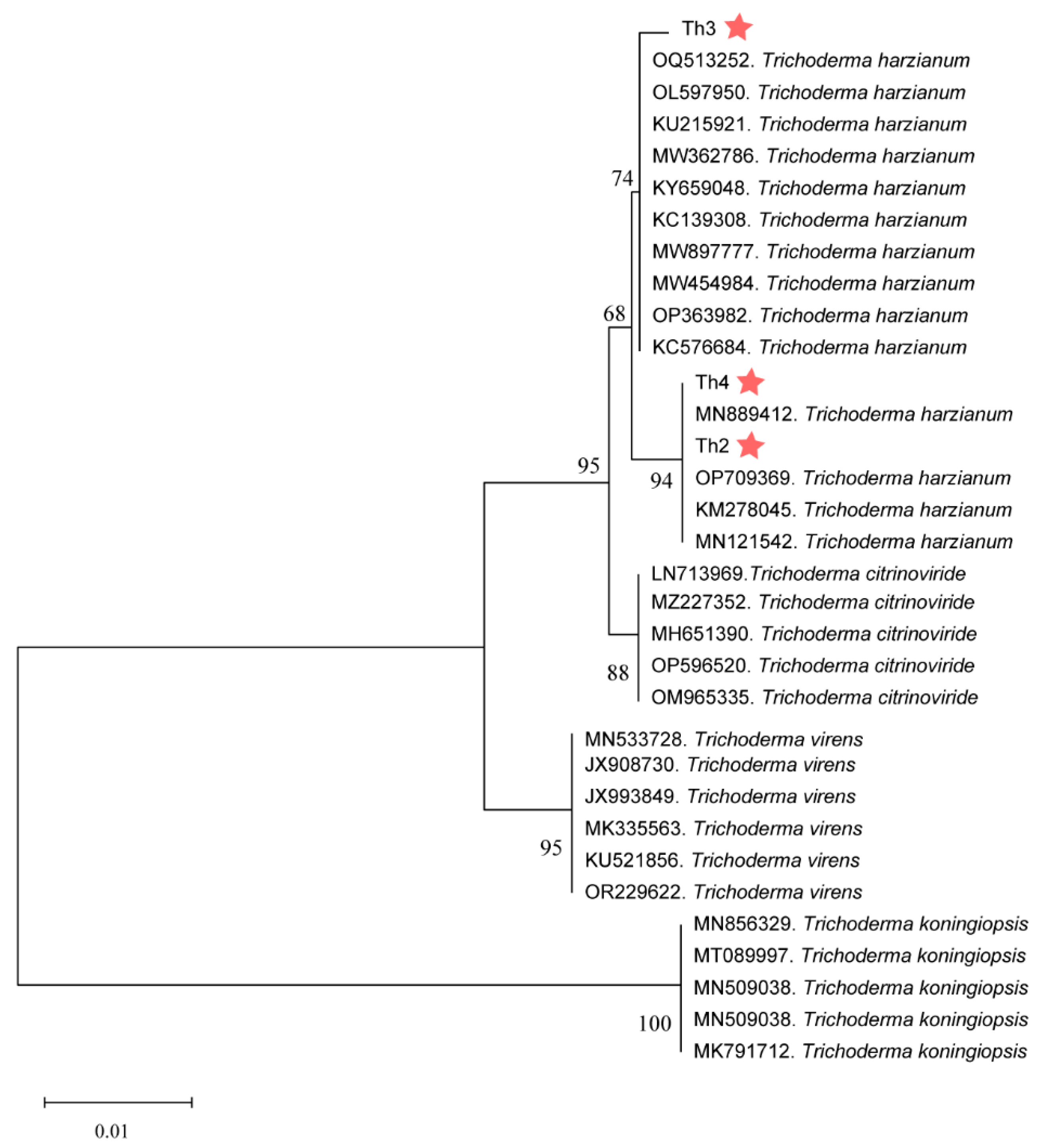
3.1. Trichoderma Strains Were Isolated and Identified
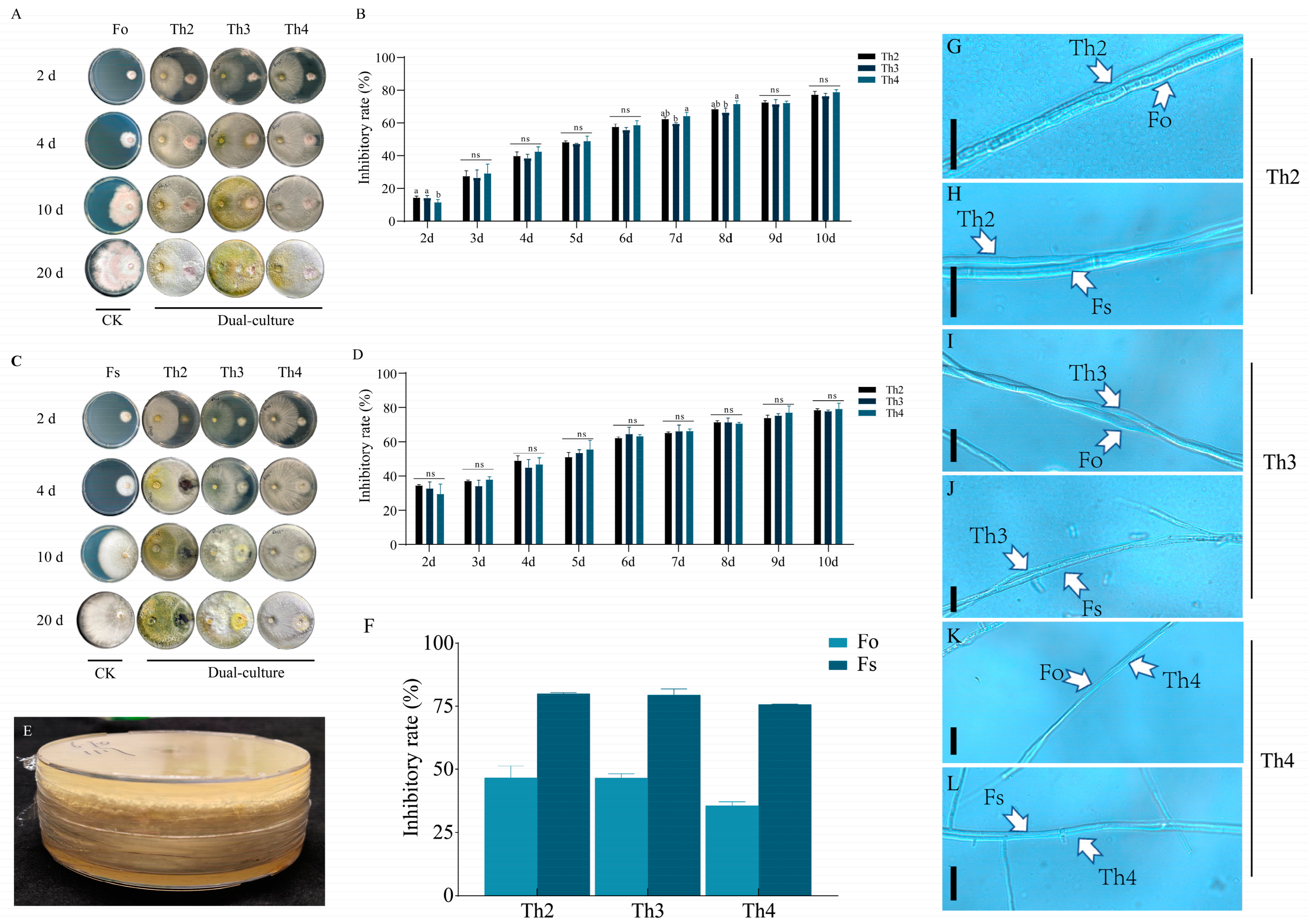
3.2. Screening of Strong Biocontrol Trichoderma Candidates
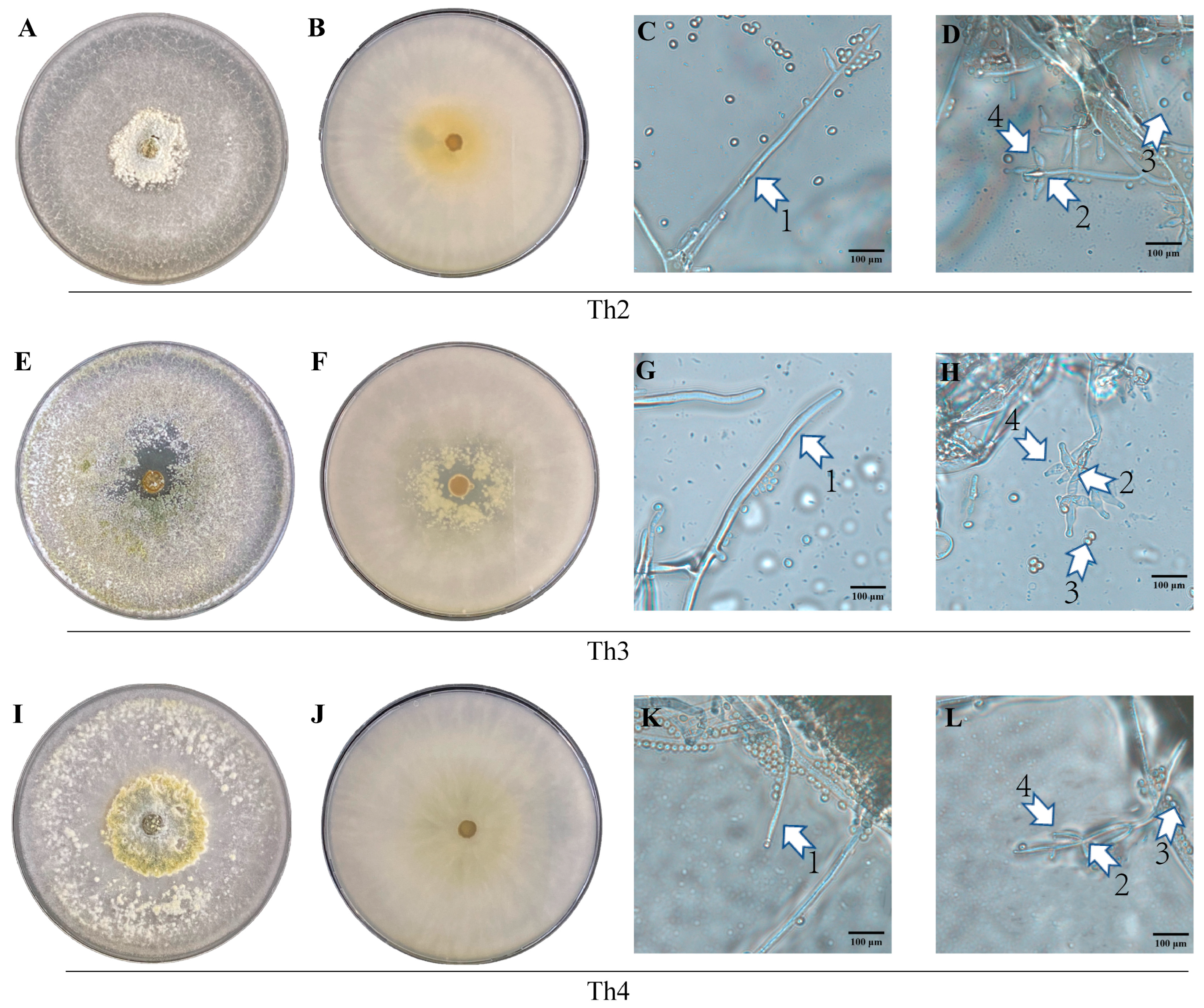
3.3. Morphological and Phylogenetic Characterization of Th2, Th3 and Th4
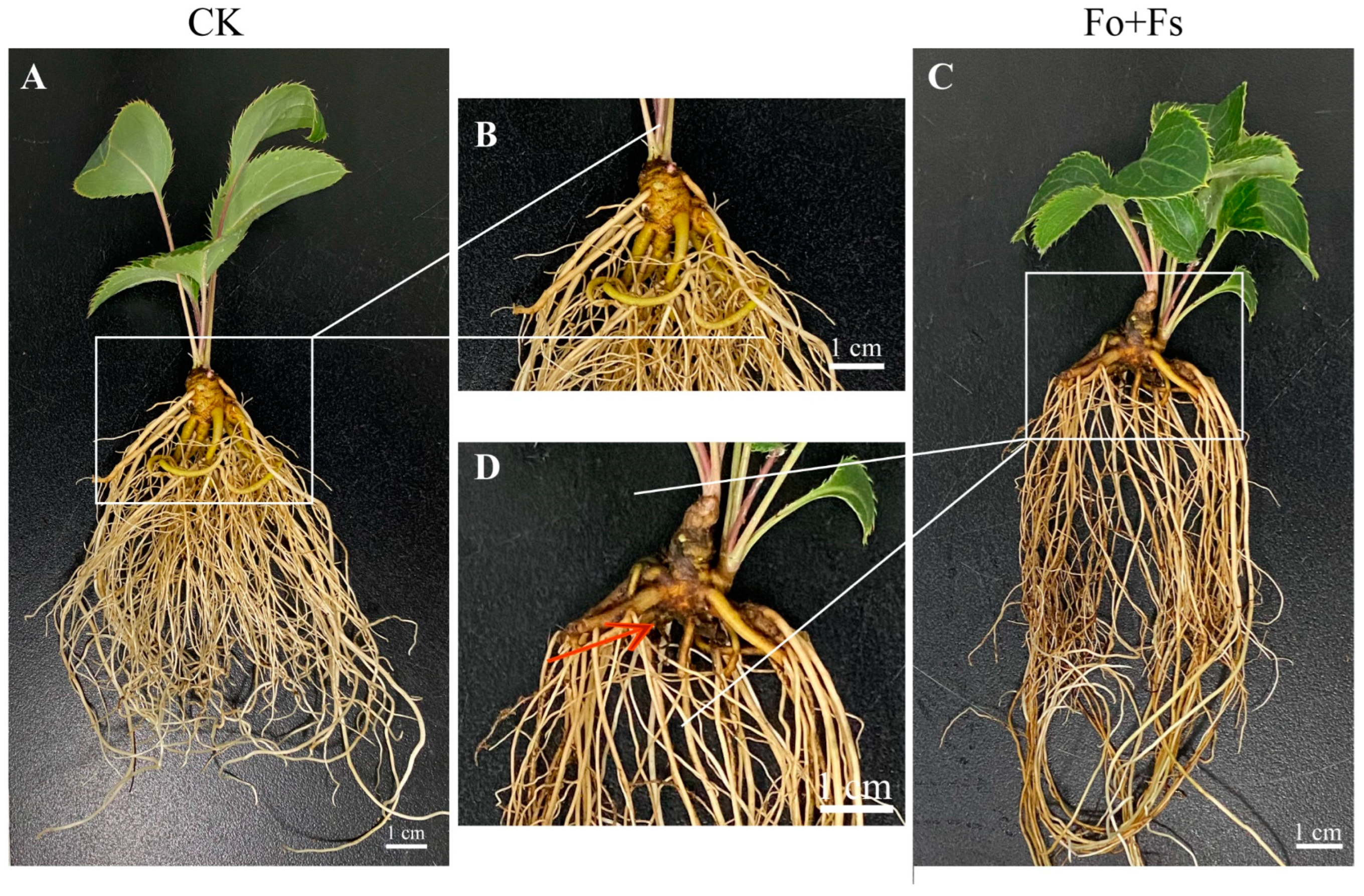
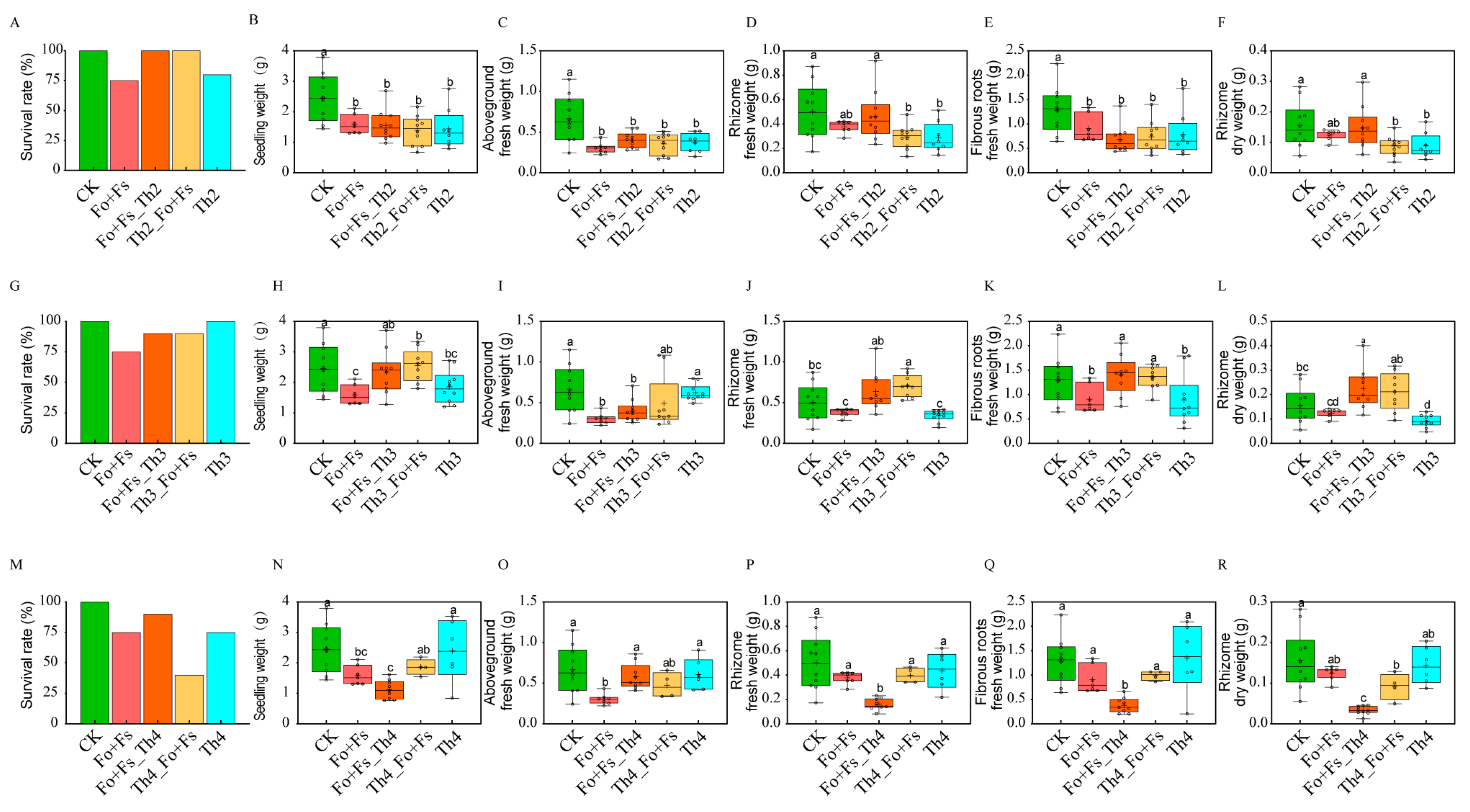
3.4. Th2, Th3 and Th4 Promoted the Medicinal Compound Accumulation of A. lancea Seedlings at the Cost of Plant Growth
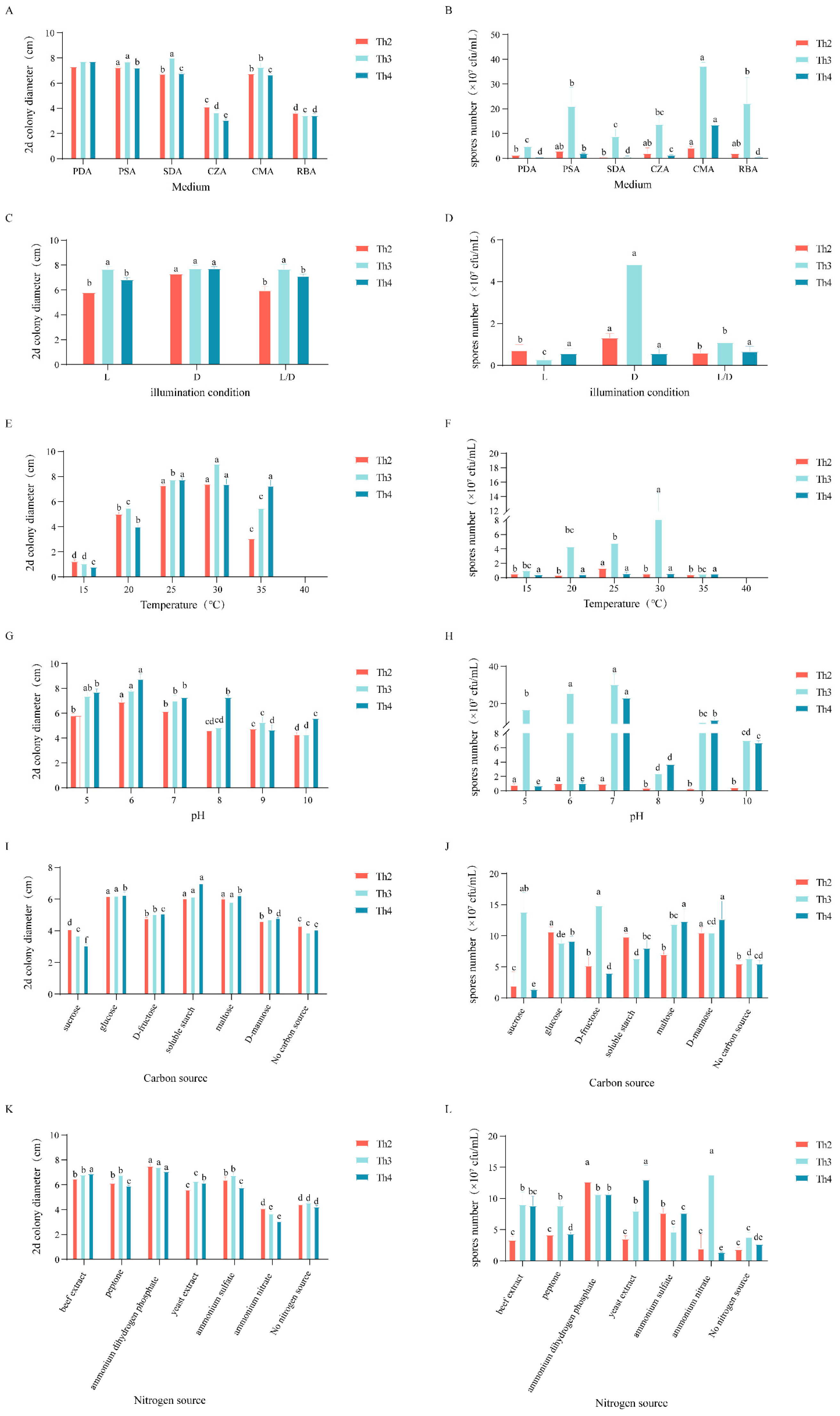
3.5. Establishment of the Optimal Culturing and Sporulation Conditions of Th2, Th3 and Th4
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia (Part I); China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Yang, L.P.; Gai, C.; Zhou, M.Q.; Ma, H.J.; Zhang, Y.X.; Zhang, J.K.; Sun, H.M. Network Pharmacology Study of Ephedrae Herba-Atractylodis Rhizoma in the Treatment of Novel Coronavirus Pneumonia. J. Med. Chem. 2020, 43, 1779–1785. [Google Scholar]
- Hu, W.S.; Wu, Q.F. Review of Historical Evaluation and Modern Application of Chinese Materia Medica Fumigation in Prevention of Epidemic Disease. Chin. Herb. Med. 2020, 51, 895–901. [Google Scholar]
- Wang, H.Y.; Kang, C.Z.; Zhang, W.J.; Zhou, L.Y.; Wan, X.F.; Lyu, C.G.; Huang, L.Q.; Guo, L.P. Land Use Strategy of Ecological Agriculture of Chinese Materia Medica in Future Development. Chin. Med. J. 2020, 45, 1990–1995. [Google Scholar]
- Gao, X.Y.; Bai, R.Y.; Wei, J.Y.; Zhao, C.Y. Research Progress on Germplasm Resources and Cultivation Techniques of Atractylodes Lancea. J. Med. Chem. 2022, 39, 237–241. [Google Scholar]
- Kang, C.Z.; Zhang, Y.; Wang, S.; Wan, X.F.; Jiang, J.Y.; Zeng, Y.; He, Y.C.; Wang, R.S.; Guo, L.P. Analysis of Economic Benefits of Chinese Medicine Eco-Agriculture Based on Multiple Stakeholders. Chin. Med. J. 2021, 46, 1858–1863. [Google Scholar]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal Volatile Organic Compounds: A Review with Emphasis on Their Biotechnological Potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Viterbo, A.; Landau, U.; Kim, S.; Chernin, L.; Chet, I. Characterization of ACC Deaminase from the Biocontrol and Plant Growth-Promoting Agent Trichoderma Asperellum T203. FEMS Microbiol. Lett. 2010, 305, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Buensanteai, N.; Moran-Diez, M.E.; Druzhinina, I.S.; Kenerley, C.M. Functional Analysis of Non-Ribosomal Peptide Synthetases (NRPSs) in Trichoderma Virens Reveals a Polyketide Synthase (PKS)/NRPS Hybrid Enzyme Involved in the Induced Systemic Resistance Response in Maize. Microbiology 2012, 158, 155–165. [Google Scholar] [CrossRef]
- Sharma, V.; Salwan, R.; Sharma, P. The Comparative Mechanistic Aspects of Trichoderma and Probiotics: Scope for Future Research. Physiol. Mol. Plant Pathol. 2017, 100, 84–96. [Google Scholar] [CrossRef]
- Gualtieri, L.; Monti, M.M.; Mele, F.; Russo, A.; Pedata, P.A.; Ruocco, M. Volatile Organic Compound (VOC) Profiles of Different Trichoderma Species and Their Potential Application. J. Fungi 2022, 8, 989. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Herrmann-Andrade, A.M.; Calabrese, C.D.; Bello, F.; Vázquez, D.; Musumeci, M.A. Effectiveness of Trichoderma Strains Isolated from the Rhizosphere of Citrus Tree to Control Alternaria alternata, Colletotrichum gloeosporioides and Penicillium digitatum A21 Resistant to Pyrimethanil in Post-harvest Oranges (Citrus sinensis L. (Osbeck)). J. Appl. Microbiol. 2020, 129, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Rojo, F.G.; Reynoso, M.M.; Ferez, M.; Chulze, S.N.; Torres, A.M. Biological Control by Trichoderma Species of Fusarium solani Causing Peanut Brown Root Rot under Field Conditions. Crop Prot. 2007, 26, 549–555. [Google Scholar] [CrossRef]
- Sivan, A.; Chet, I. Integrated Control of Fusarium Crown and Root Rot of Tomato with Trichoderma Harzianum in Combination with Methyl Bromide or Soil Solarization. Crop Prot. 1993, 12, 380–386. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Nemec, S.; Pernezny, K. Biological Control of Fusarium Crown and Root Rot of Tomato in Florida Using Trichoderma Harzianum and Glomus Intraradices. Biol. Control. 1995, 5, 427–431. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Eke, P.; Zabalgogeazcoa, Í.; de Aldana, B.R.V.; Nana, L.W.; Boyom, F.F. Biocontrol and Growth Enhancement Potential of Two Endophytic Trichoderma Spp. from Terminalia catappa against the Causative Agent of Common Bean Root Rot (Fusarium solani). Biol. Control. 2016, 96, 8–20. [Google Scholar] [CrossRef]
- Erazo, J.G.; Palacios, S.A.; Pastor, N.; Giordano, F.D.; Rovera, M.; Reynoso, M.M.; Venisse, J.S.; Torres, A.M. Biocontrol Mechanisms of Trichoderma Harzianum ITEM 3636 against Peanut Brown Root Rot Caused by Fusarium solani RC 386. Biol. Control. 2021, 164, 104774. [Google Scholar] [CrossRef]
- Wang, H.; Chang, K.F.; Hwang, S.F.; Turnbull, G.D.; Howard, R.J.; Blade, S.F.; Callan, N.W. Fusarium Root Rot of Coneflower Seedlings and Integrated Control Using Trichoderma and Fungicides. Biocontrol 2005, 50, 317–329. [Google Scholar] [CrossRef]
- Chen, S.C.; Ren, J.J.; Zhao, H.J.; Wang, X.L.; Jin, S.D.; Li, C.Y.; Liu, A.R.; Lin, X.M.; Ahammed, G.J. Trichoderma Harzianum Improves Defense against Fusarium oxysporum by Regulating ROS and RNS Metabolism, Redox Balance, and Energy Flow in Cucumber Roots. Phytopathology 2019, 109, 972–982. [Google Scholar] [CrossRef]
- Gupta, K.J.; Brotman, Y.; Mur, L.A.J. Trichoderma Asperelloides Suppresses Nitric Oxide Generation Elicited by Fusarium oxysporum in Arabidopsis Roots. Mol. Plant Microbe Interact. 2014, 27, 307–314. [Google Scholar] [CrossRef]
- Ding, W.L.; Cheng, H.Z.; Chen, J. Presearch on Preventing the Medicinal Plant Diseases with Trichoderma Harzianum Preparation. Chin. Med. J. 2003, 28, 28. [Google Scholar]
- Li, Y.B.; Liu, Y.X.; Zhang, Z.P.; Li, J.Q.; Zhu, S.S.; Yang, M.; Luo, L.X. Application of Plant Survival-Promoting and Pathogen-Suppressing Trichoderma Species for Crop Biofertilization and Biocontrol of Root Rot in Panax Notoginseng. J. Plant Pathol. 2022, 104, 1361–1369. [Google Scholar] [CrossRef]
- Hu, R.P.; Gong, G.S.; Ye, H.L.; Jiang, X.; Ma, Y.F.; He, Z.Q.; Lin, Y.; Zhou, Y. Preliminary Study on Control Effect to Root Rot Disease of Ligusticum Chuanxiong. Southwest China J. Agric. Sci. 2013, 26, 1873–1877. [Google Scholar]
- Qiang, W.; Guo, S.; Zhang, A.H.; Lei, F.J.; Chi, K.; Wang, Z.; Wang, E.H.; Zhang, L.X. Screening Antagonistic Microorganisms against Ginseng Diseases from Panax Ginseng Rhizosphere Soil. J. South China Agric. Univ. 2014, 35, 76–81. [Google Scholar]
- Sun, G.L.; Chen, Y.W.; Xia, G.X.; Wu, S.H.; Zhang, Q.; Li, Z.Y.; Li, S.L.; Yang, L.Y.; Huang, R. Isolation of Endophytic Fungi in Paris Polyphylla Smith Var.Yunnanensis and The Screening of Its Antibiotic Activity. J. Microbiol. 2005, 25, 59–62. [Google Scholar]
- Hussein, K.A.; Lee, Y.D.; Joo, J.H. Effect of Rosemary Essential Oil and Trichoderma Koningiopsis VOCs on Pathogenic Fungi Responsible for Ginseng Root-Rot Disease. J. Microbiol. Biotechnol. 2020, 30, 1018–1026. [Google Scholar] [CrossRef]
- Huang, Y.J.; Dong, L.L.; Wei, G.F.; Zhang, X.H. Development and Evaluation of Biocontrol Agents against Panax Ginseng Root Rot. J. Tradit. Chin. Med. 2022, 28, 182. [Google Scholar]
- Wang, H.Y.; Wang, Y.F.; Jiang, D.Q.; Xiang, Z.X.; Wang, S.; Kang, C.Z.; Zhang, W.J.; Ge, Y.; Wang, T.L.; Huang, L.Q.; et al. Soil Microbe Inoculation Alters the Bacteria Communities and Promotes Root Growth of Atractylodes lancea under Heat Stress. Plant Soil 2022, 478, 371–389. [Google Scholar] [CrossRef]
- Wang, T.L.; Huang, L.Q.; Guo, L.P. China Medicinal Plant Disease Atlas; China Agriculture Press Co., Ltd.: Beijing, China, 2024. [Google Scholar]
- Wang, Y.F.; Hou, X.Y.; Jiang, C.Y.; Zhai, T.T.; Miao, R.; Deng, J.J.; Yao, Z.H.; Zhang, R.S. A Native Trichoderma Harzianum Strain Th62 Displays Antagonistic Activities against Phytopathogenic Fungi and Promotes the Growth of Celosia cristata. Hortic. Environ. Biotechnol. 2021, 62, 169–179. [Google Scholar]
- Tian, M.; Peng, Y.F.; Lyu, H.; Qin, N.; Ren, L.; Yin, H.; Zhao, X.J. Trichoderma Afroharzianum LMNS-M9: Identification, Biological Characteristics, and Growth-Promoting Effect on Quinoa. Microbiol. China 2023, 50, 3848–3865. [Google Scholar]
- Dou, K.; Lu, Z.; Wu, Q.; Ni, M.; Yu, C.; Wang, M.; Li, Y.; Wang, X.; Xie, H.; Chen, J.; et al. MIST: A Multilocus Identification System for Trichoderma. Appl. Environ. Microbiol. 2020, 86, e01532-20. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Yang, S.; Li, Y.; Zhu, Z. Biocontrol Potential of Trichoderma Asperellum Strain 576 against Exserohilum turcicum in Zea mays. J. Fungi 2023, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a Mechanism of Trichoderma-Mediated Suppression of Plant Diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A Multipurpose, Plant-Beneficial Microorganism for Eco-Sustainable Agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Kang, C.; Wang, S.; Zhang, Y.; Yang, G.; Zhou, L.; Xiang, Z.; Huang, L.; Liu, D.; et al. Drought Stress Modifies the Community Structure of Root-Associated Microbes That Improve Atractylodes Lancea Growth and Medicinal Compound Accumulation. Front. Plant Sci. 2022, 13, 1032480. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Webster, S.; He, S.Y. Growth-Defense Trade-Offs in Plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef]
- Adedeji, A.A.; Babalola, O.O. Secondary Metabolites as Plant Defensive Strategy: A Large Role for Small Molecules in the near Root Region. Planta 2020, 252, 61. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly)Phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Baiyee, B.; Ito, S.; Sunpapao, A. Trichoderma Asperellum T1 Mediated Antifungal Activity and Induced Defense Response against Leaf Spot Fungi in Lettuce (Lactuca sativa L.). Physiol. Mol. Plant Pathol. 2019, 106, 96–101. [Google Scholar] [CrossRef]
- Wang, C.; El-Shetehy, M.; Shine, M.B.; Yu, K.; Navarre, D.; Wendehenne, D.; Kachroo, A.; Kachroo, P. Free Radicals Mediate Systemic Acquired Resistance. Cell Rep. 2014, 7, 348–355. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Lim, G.-H.; De Lorenzo, L.; Yu, K.; Zhang, K.; Hunt, A.G.; Kachroo, A.; Kachroo, P. Pipecolic Acid Confers Systemic Immunity by Regulating Free Radicals. Sci. Adv. 2018, 4, eaar4509. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Kaimoyo, E.; Kumar, D.; Mosher, S.; Klessig, D.F. Methyl Salicylate Is a Critical Mobile Signal for Plant Systemic Acquired Resistance. Science 2007, 318, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Mohnike, L.; Rekhter, D.; Huang, W.; Feussner, K.; Tian, H.; Herrfurth, C.; Zhang, Y.; Feussner, I. The Glycosyltransferase UGT76B1 Modulates N-Hydroxy-Pipecolic Acid Homeostasis and Plant Immunity. Plant Cell 2021, 33, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of Defense Responses in Cucumber Plants (Cucumis sativus L.) By the Biocontrol Agent Trichoderma Harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant Induction of Systemic Resistance to Pseudomonas Syringae Pv. Lachrymans in Cucumber by Trichoderma Asperellum (T-203) and Accumulation of Phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef]


| Species Name | Total Isolate Count | Isolate Count by Geographic Origin | Isolate Count by Association to A. lancea Compartment | ||
|---|---|---|---|---|---|
| Liaoning Province | Inner Mongolia Autonomous Region | Rhizome Rhizosphere | Fibrous Root Rhizosphere | ||
| Trichoderma atroviride | 1 | 1 | 0 | 0 | 1 |
| Trichoderma lixii | 1 | 1 | 0 | 0 | 1 |
| Trichoderma chlamydosporicum | 3 | 3 | 0 | 1 | 2 |
| Trichoderma harzianum | 7 | 2 | 5 | 5 | 2 |
| Trichoderma velutinum | 3 | 1 | 2 | 3 | 0 |
| Trichoderma koningiopsis | 1 | 1 | 0 | 0 | 0 |
| Trichoderma brevicompactum | 4 | 0 | 4 | 3 | 1 |
| Trichoderma citrinoviride | 1 | 0 | 1 | 0 | 1 |
| Sum | 21 | 9 | 12 | 12 | 8 |
| Name | Microscopic Morphology of Conidiophores |
|---|---|
| Th2 | Gliocladium type, containing 2–4 branches (1–2 cells long), attached to 3–5 phialides or a combination of secondary branches and phialides. Phialides were short with a thin base and an enlarged middle part. |
| Th3 | Pachybasium type, with a main axis, short secondary branches forming a nearly right angle with the axis, with a terminal whorl of 1–3 and occasionally 4 phialides. Phialides were short, barrel-shaped, forming large angles between each other. |
| Th4 | Verticillium type. Conidiophore had septate base and was dark in color, with 1–3 (occasionally up to 5) slim phialides per whorl at the terminal. |
| T (°C) | 40 | 45 | 50 | 55 | 60 | 65 | |
|---|---|---|---|---|---|---|---|
| Trichoderma | |||||||
| Th2 | + | + | + | − | − | − | |
| Th3 | + | + | + | − | − | − | |
| Th4 | + | + | + | − | − | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Lin, H.; Guo, X.; Wang, S.; Wang, H.; Wang, T.; Peng, Z.; Wang, Y.; Guo, L. Allochthonous Trichoderma Isolates Boost Atractylodes lancea Herb Quality at the Cost of Rhizome Growth. J. Fungi 2024, 10, 351. https://doi.org/10.3390/jof10050351
Li K, Lin H, Guo X, Wang S, Wang H, Wang T, Peng Z, Wang Y, Guo L. Allochthonous Trichoderma Isolates Boost Atractylodes lancea Herb Quality at the Cost of Rhizome Growth. Journal of Fungi. 2024; 10(5):351. https://doi.org/10.3390/jof10050351
Chicago/Turabian StyleLi, Kuo, Huaibin Lin, Xiuzhi Guo, Sheng Wang, Hongyang Wang, Tielin Wang, Zheng Peng, Yuefeng Wang, and Lanping Guo. 2024. "Allochthonous Trichoderma Isolates Boost Atractylodes lancea Herb Quality at the Cost of Rhizome Growth" Journal of Fungi 10, no. 5: 351. https://doi.org/10.3390/jof10050351
APA StyleLi, K., Lin, H., Guo, X., Wang, S., Wang, H., Wang, T., Peng, Z., Wang, Y., & Guo, L. (2024). Allochthonous Trichoderma Isolates Boost Atractylodes lancea Herb Quality at the Cost of Rhizome Growth. Journal of Fungi, 10(5), 351. https://doi.org/10.3390/jof10050351






