In Vitro Activitiy of Rezafungin in Comparison with Anidulafungin and Caspofungin against Invasive Fungal Isolates (2017 to 2022) in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Antifungal Agents
2.2. Strains
2.3. Identification of Isolates
2.4. Antifungal Susceptibility Testing
2.5. FKS Mutation Sequencing
2.6. Data Analysis
3. Results
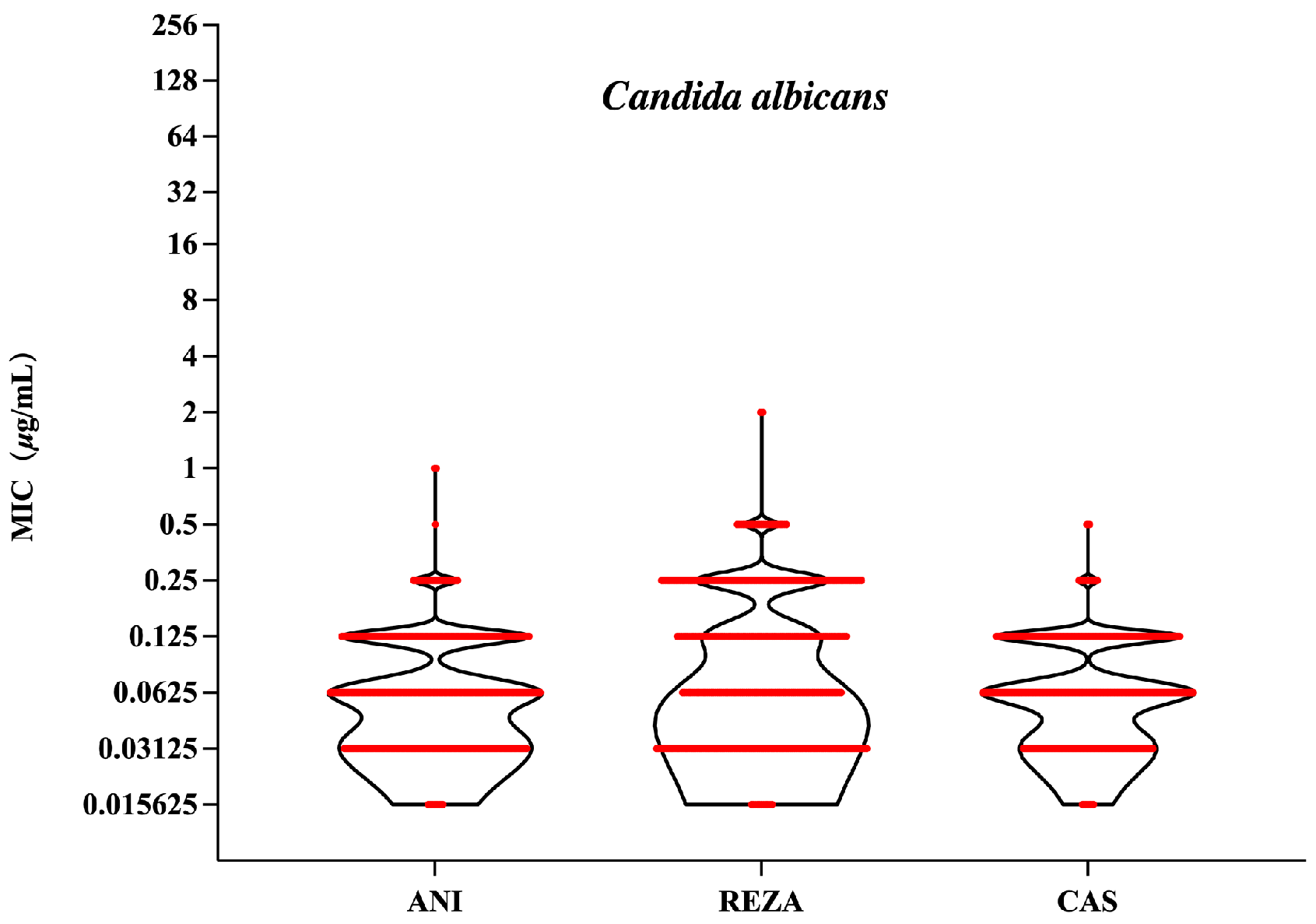
3.1. Candida albicans
3.2. Non-Albicans Candida
3.3. Activity of Fluconazole against Candida Species
3.4. Aspergillus Species
3.5. Resistance Mutations in Echinocandin-Resistant C. tropicalis Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Lara, M.F.; Ostrosky-Zeichner, L. Invasive Candidiasis. Semin. Respir. Crit. Care Med. 2020, 41, 3–12. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.J.; Tullio, V.; Rodloff, A.C.; Fu, W.; Ling, T.A.; Global Antifungal Surveillance Group. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef]
- Xiao, M.; Sun, Z.-Y.; Kang, M.; Guo, D.-W.; Liao, K.; Chen, S.C.-A.; Kong, F.; Fan, X.; Cheng, J.-W.; Hou, X.; et al. Five-Year National Surveillance of Invasive Candidiasis: Species Distribution and Azole Susceptibility from the China Hospital Invasive Fungal Surveillance Net (CHIF-NET) Study. J. Clin. Microbiol. 2018, 56, e00577-18. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Johnson, C.; Berman, J.; Coste, A.T.; Cuomo, C.A.; Perlin, D.S.; Bicanic, T.; Harrison, T.S.; Wiederhold, N.; Bromley, M.; et al. The importance of antimicrobial resistance in medical mycology. Nat. Commun. 2022, 13, 5352. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Kontoyiannis, D.P. Resistance to Antifungal Drugs. Infect. Dis. Clin. N. Am. 2021, 35, 279–311. [Google Scholar] [CrossRef]
- Morgan, E.T.; Coon, M.J. Effects of cytochrome b5 on cytochrome P-450-catalyzed reactions. Studies with manganese-substituted cytochrome b5. Drug Metab. Dispos. 1984, 12, 358–364. [Google Scholar]
- Ham, Y.Y.; Lewis, J.S., 2nd; Thompson, G.R., 3rd. Rezafungin: A novel antifungal for the treatment of invasive candidiasis. Future Microbiol. 2021, 16, 27–36. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. S1), e1–e38. [Google Scholar] [CrossRef]
- Banche, G.; Mandras, N.; Giacchino, F.; Scalas, D.; Allizond, V.; Roana, J.; Tullio, V.; Garneri, G.; Castagno, F.; Merlino, C.; et al. Caspofungin benefit on phagocytes from patients with renal dysfunction infected with multidrug-resistant Candida glabrata. Future Microbiol. 2013, 8, 1091–1096. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Castanheira, M.; Messer, S.A.; Rhomberg, P.R.; Pfaller, M.A. Antifungal susceptibility patterns of a global collection of fungal isolates: Results of the SENTRY Antifungal Surveillance Program (2013). Diagn. Microbiol. Infect. Dis. 2016, 85, 200–204. [Google Scholar] [CrossRef]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732, Erratum in Clin. Infect. Dis. 2014, 58, 754. [Google Scholar] [CrossRef]
- Zhao, Y.; Perlin, D.S. Review of the Novel Echinocandin Antifungal Rezafungin: Animal Studies and Clinical Data. J. Fungi 2020, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R., 3rd; Soriano, A.; Cornely, O.A.; Kullberg, B.J.; Kollef, M.; Vazquez, J.; Honore, P.M.; Bassetti, M.; Pullman, J.; Chayakulkeeree, M.; et al. Rezafungin versus caspofungin for treatment of Candidaemia and invasive candidiasis (ReSTORE): A multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet 2023, 401, 49–59. [Google Scholar] [CrossRef]
- The U.S. Food and Drug Administration. Drugs@FDA: FDA-Approved Drug [EB/OL]. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021948 (accessed on 17 February 2006).
- US Food and Drug Administration. Rezzayo Prescribing Information. 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217417s000lbl.pdf (accessed on 3 May 2024).
- CLSI Standard M27; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 4th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- CLSI Standard M38; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 3rd ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- CLSI Document M27M44S; Performance Standards for Antifungal Susceptibility Testing of Yeasts. 3rd ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022.
- Carvalhaes, C.G.; Klauer, A.L.; Rhomberg, P.R.; Pfaller, M.A.; Castanheira, M. Evaluation of Rezafungin Provisional CLSI Clinical Breakpoints and Epidemiological Cutoff Values Tested against a Worldwide Collection of Contemporaneous Invasive Fungal Isolates (2019 to 2020). J. Clin. Microbiol. 2022, 60, e0244921. [Google Scholar] [CrossRef]
- CLSI Document M57S; Epidemiological Cutoff Values for Antifungal Susceptibility Testing. 4th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022.
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.B.; Pillar, C.M.; Castanheira, M.; Carvalhaes, C.G.; Andes, D.; Aram, J.A.; Andrzejewski, C.; Bartizal, K.; Das, A.F.; Sandison, T.; et al. Outcomes by Candida spp. in the ReSTORE Phase 3 trial of rezafungin versus caspofungin for candidemia and/or invasive candidiasis. Antimicrob. Agents Chemother. 2024, 68, e0158423. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Z.; Forgács, L.; Locke, J.B.; Kardos, G.; Nagy, F.; Kovács, R.; Szekely, A.; Borman, A.M.; Majoros, L. In vitro activity of rezafungin against common and rare Candida species and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2019, 74, 3505–3510. [Google Scholar] [CrossRef] [PubMed]
- Sandison, T.; Ong, V.; Lee, J.; Thye, D. Safety and Pharmacokinetics of CD101 IV, a Novel Echinocandin, in Healthy Adults. Antimicrob. Agents Chemother. 2017, 61, e01627-16. [Google Scholar] [CrossRef]
- Ong, V.; Hough, G.; Schlosser, M.; Bartizal, K.; Balkovec, J.M.; James, K.D.; Krishnan, B.R. Preclinical Evaluation of the Stability, Safety, and Efficacy of CD101, a Novel Echinocandin. Antimicrob. Agents Chemother. 2016, 60, 6872–6879. [Google Scholar] [CrossRef]
- Helleberg, M.; Jorgensen, K.M.; Hare, R.K.; Datcu, R.; Chowdhary, A.; Arendrup, M.C. Rezafungin In Vitro Activity against Contemporary Nordic Clinical Candida Isolates and Candida auris Determined by the EUCAST Reference Method. Antimicrob. Agents Chemother. 2020, 64, e02438-19. [Google Scholar] [CrossRef]
- CLSI Document M23; Development of In Vitro Susceptibility Testing Criteria and Quality Control Parameters. 5th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019.
- Espinel-Ingroff, A.; Arendrup, M.C.; Pfaller, M.A.; Bonfietti, L.X.; Bustamante, B.; Canton, E.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Fothergill, A.; et al. Multicentre determination of rezafungin (CD101) susceptibility of Candida species by the EUCAST method. Clin. Microbiol. Infect. 2018, 24, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Arendrup, M.C.; Pfaller, M.A.; Bonfietti, L.X.; Bustamante, B.; Canton, E.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Fothergill, A.; et al. Interlaboratory variability of Caspofungin MICs for Candida spp. Using CLSI and EUCAST methods: Should the clinical laboratory be testing this agent? Antimicrob. Agents Chemother. 2013, 57, 5836–5842. [Google Scholar] [CrossRef]
- Fothergill, A.W.; McCarthy, D.I.; Albataineh, M.T.; Sanders, C.; McElmeel, M.; Wiederhold, N.P. Effects of Treated versus Untreated Polystyrene on Caspofungin In Vitro Activity against Candida Species. J. Clin. Microbiol. 2016, 54, 734–738. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Wang, H.; Kudinha, T.; Mei, Y.-N.; Ni, F.; Pan, Y.-H.; Gao, L.-M.; Xu, H.; Kong, H.-S.; et al. Continual Decline in Azole Susceptibility Rates in Candida tropicalis over a 9-Year Period in China. Front. Microbiol. 2021, 12, 702839. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Watanabe, A.; Kamei, K. Azole and echinocandin resistance mechanisms and genotyping of Candida tropicalis in Japan: Cross-boundary dissemination and animal-human transmission of C. tropicalis infection. Clin. Microbiol. Infect. 2022, 28, 302.e5–302.e8. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Badiee, P.; Abastabar, M.; Morovati, H.; Haghani, I.; Noorbakhsh, M.; Mohammadi, R. A 3-year study of Candida infections among patients with malignancy: Etiologic agents and antifungal susceptibility profile. Front. Cell. Infect. Microbiol. 2023, 13, 1152552. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.; Chmielewska, S.; Czyzewska, U.; Malinowska, M.; Tylicki, A. Echinocandins–structure, mechanism of action and use in antifungal therapy. J. Enzyme Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Locke, J.B.; Daruwala, P.; Bartizal, K. Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole-resistant Aspergillus fumigatus isolates and cryptic species. J. Antimicrob. Chemother. 2018, 73, 3063–3067. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.S.; Mohsin, J.; Al-Huraizi, A.; Khamis, F. COVID-19 associated invasive candidiasis. J. Infect. 2021, 82, e45–e46. [Google Scholar] [CrossRef] [PubMed]
- Floridia, M.; Giuliano, M.; Monaco, M.; Palmieri, L.; Noce, C.L.; Palamara, A.T.; Pantosti, A.; Brusaferro, S.; Onder, G.; Agazio, E.; et al. Microbiologically confirmed infections and antibiotic-resistance in a national surveillance study of hospitalised patients who died with COVID-19, Italy 2020–2021. Antimicrob. Resist. Infect. Control 2022, 11, 74. [Google Scholar] [CrossRef]
- Logan, C.; Martin-Loeches, I.; Bicanic, T. Invasive candidiasis in critical care: Challenges and future directions. Intensive Care Med. 2020, 46, 2001–2014. [Google Scholar] [CrossRef]
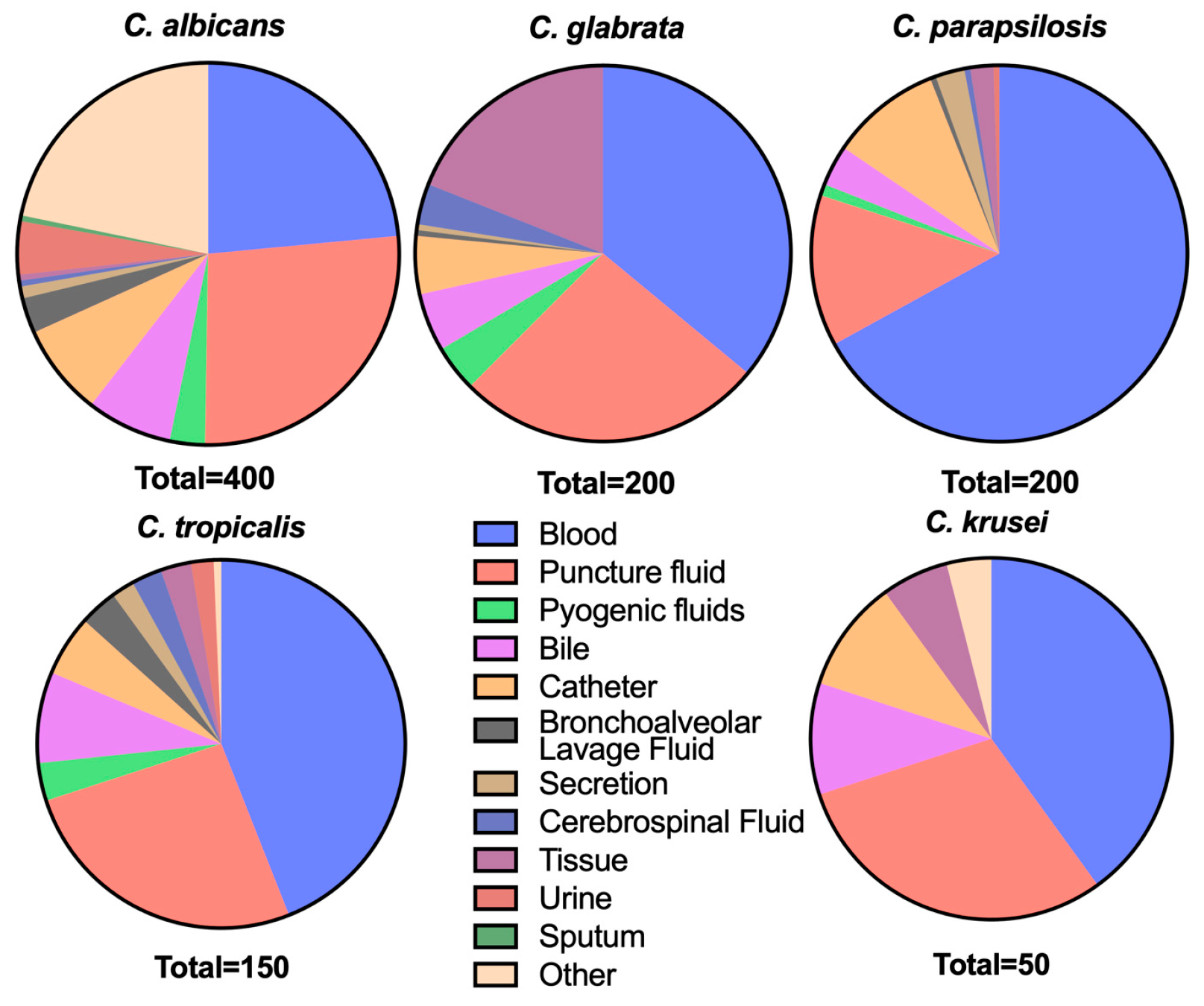


| Species | Antifungal Agents | MIC Range (μg/mL) | MIC50 (μg/mL) | MIC90 (μg/mL) | GM (μg/mL) | Susceptibility (%) | ||
|---|---|---|---|---|---|---|---|---|
| S | I/SDD | R | ||||||
| C. albicans (n = 400) | ANI | ≤0.016–1 | 0.06 | 0.12 | 0.068 | 99.25 | 0.25 | 0.5 |
| REZA | ≤0.016–2 | 0.12 | 0.25 | 0.095 | 93.3 | |||
| CAS | ≤0.016–0.5 | 0.06 | 0.12 | 0.069 | 100 | |||
| FLC | 0.5–32 | 2 | 4 | 1.591 | 73.25 | 20 | 6.75 | |
| C. glabrata (n = 200) | ANI | 0.03–1 | 0.125 | 0.25 | 0.140 | 84.5 | 13.5 | 2 |
| REZA | 0.03–1 | 0.125 | 0.25 | 0.174 | 99 | |||
| CAS | 0.03–1 | 0.125 | 0.25 | 0.134 | 87.5 | 11 | 1.5 | |
| FLC | 0.5–128 | 2 | 4 | 1.613 | 98 | 2 | ||
| C. parapsilosis (n = 200) | ANI | 0.5–4 | 2 | 2 | 1.647 | 97.5 | 2.5 | |
| REZA | 0.5–4 | 2 | 2 | 1.564 | 97.5 | |||
| CAS | 0.25–1 | 0.5 | 1 | 0.551 | 100 | |||
| FLC | 0.25–16 | 0.5 | 1 | 0.502 | 98.5 | 1 | 0.5 | |
| C. tropicalis (n = 150) | ANI | 0.03–4 | 0.12 | 0.25 | 0.120 | 91.33 | 8.67 | |
| REZA | 0.03–4 | 0.12 | 0.25 | 0.163 | 90.67 | |||
| CAS | 0.03–8 | 0.06 | 0.25 | 0.110 | 90.67 | 0.67 | 8.67 | |
| FLC | 0.5–256 | 1 | 128 | 4.093 | 55.33 | 4 | 40.67 | |
| C. krusei (n = 50) | ANI | 0.06–0.25 | 0.12 | 0.12 | 0.127 | 100 | ||
| REZA | 0.12–0.5 | 0.25 | 0.25 | 0.240 | 92 | |||
| CAS | 0.12–0.5 | 0.25 | 0.25 | 0.253 | 96 | 4 | ||
| FLC | 8–64 | 32 | 32 | 25.992 | ||||
| Fluconazole-Sensitive C. albicans | Fluconazole-Intermediate/Resistant C. albicans | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (μg/mL) | MIC90 (μg/mL) | GM (μg/mL) | MIC Range (μg/mL) | Susceptibility (%) | MIC50 (μg/mL) | MIC90 (μg/mL) | GM (μg/mL) | MIC Range (μg/mL) | Susceptibility (%) | |
| ANI | 0.06 | 0.12 | 0.076 | 0.016 −0.25 | 100 | 0.06 | 0.25 | 0.067 | 0.016 −1 | 97.20 |
| REZA | 0.12 | 0.25 | 0.102 | 0.016 −0.5 | 94.54 | 0.06 | 0.35 | 0.09 | 0.016 −2 | 89.72 |
| CAS | 0.06 | 0.12 | 0.079 | 0.016 −0.25 | 100 | 0.06 | 0.12 | 0.072 | 0.016 −0.5 | 97.20 |
| Aspergillus Species | Antifungal Drugs | MEC Range (μg/mL) | MEC50 (μg/mL) | MEC90 (μg/mL) | GM MEC (μg/mL) | ||
|---|---|---|---|---|---|---|---|
| WT | NWT | ||||||
| A. fumigatus (n = 100) | ANI | 0.03–0.25 | 0.06 | 0.12 | 0.064 | ||
| REZA | 0.03–0.25 | 0.12 | 0.25 | 0.116 | |||
| CAS | 0.06–0.25 | 0.12 | 0.25 | 0.122 | 100 | ||
| A. flavus (n = 50) | ANI | 0.03–0.12 | 0.06 | 0.12 | 0.059 | ||
| REZA | 0.03–0.25 | 0.12 | 0.12 | 0.110 | |||
| CAS | 0.03–0.25 | 0.12 | 0.25 | 0.142 | 50 | ||
| No. | MIC (μg/mL) | FKS1 Mutation | |||
|---|---|---|---|---|---|
| ANI | REZA | CAS | FLC | ||
| CTR3 | 1 | 2 | 4 | 64 | S654P |
| CTR8 | 2 | 2 | 4 | 128 | S654P |
| CTR12 | 4 | 4 | 4 | 128 | S654P |
| CTR16 | 2 | 2 | 4 | 128 | S654P, R1220T |
| CTR17 | 2 | 2 | 4 | 128 | S654P |
| CTR18 | 1 | 2 | 2 | 128 | S654P, G324R |
| CTR20 | 1 | 2 | 2 | 64 | S654P |
| CTR21 | 1 | 2 | 2 | 128 | S654P |
| CTR22 | 1 | 2 | 2 | 128 | S654P |
| CTR24 | 1 | 0.5 | 2 | 64 | S654P |
| CTR25 | 1 | 1 | 2 | 64 | S654P |
| CTR30 | 4 | 2 | 4 | 128 | S654P |
| CTR36 | 1 | 2 | 4 | 128 | S654P |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Wan, F.; Zhang, M.; Lin, H.; Hu, L.; Zhou, Z.; Wang, D.; Zhou, A.; Ni, L.; Guo, J.; et al. In Vitro Activitiy of Rezafungin in Comparison with Anidulafungin and Caspofungin against Invasive Fungal Isolates (2017 to 2022) in China. J. Fungi 2024, 10, 397. https://doi.org/10.3390/jof10060397
Yang S, Wan F, Zhang M, Lin H, Hu L, Zhou Z, Wang D, Zhou A, Ni L, Guo J, et al. In Vitro Activitiy of Rezafungin in Comparison with Anidulafungin and Caspofungin against Invasive Fungal Isolates (2017 to 2022) in China. Journal of Fungi. 2024; 10(6):397. https://doi.org/10.3390/jof10060397
Chicago/Turabian StyleYang, Simin, Feifei Wan, Min Zhang, Huiping Lin, Liang Hu, Ziyi Zhou, Dongjiang Wang, Aiping Zhou, Lijun Ni, Jian Guo, and et al. 2024. "In Vitro Activitiy of Rezafungin in Comparison with Anidulafungin and Caspofungin against Invasive Fungal Isolates (2017 to 2022) in China" Journal of Fungi 10, no. 6: 397. https://doi.org/10.3390/jof10060397
APA StyleYang, S., Wan, F., Zhang, M., Lin, H., Hu, L., Zhou, Z., Wang, D., Zhou, A., Ni, L., Guo, J., & Wu, W. (2024). In Vitro Activitiy of Rezafungin in Comparison with Anidulafungin and Caspofungin against Invasive Fungal Isolates (2017 to 2022) in China. Journal of Fungi, 10(6), 397. https://doi.org/10.3390/jof10060397







