Abstract
(1) Background: Only a few studies on histoplasmosis in immunocompetent patients have been reported in French Guiana. Therefore, we conducted a detailed clinical description of hospitalized patients suffering with histoplasmosis among non-HIV patients. (2) Methods: This is a single-center, retrospective study conducted at Cayenne Hospital Center between 2008 and 2022. (3) Results: Our cohort was composed of 31 (91%) adults (>18 years of age) and 3 (9%) children, with a sex ratio, M:F, of 1:2. The median age was higher among the women than among the men (70 versus 54 years). The collection of respiratory samples constituted the majority of the performed examinations (38%). Fever (>37 °C) was found in 56% of patients. Surprisingly, the histoplasmosis was disseminated in 82% of patients with an overall case fatality rate of 14.7%. However, immunosuppressive conditions were found in 52% (16/31) of the adult patients, including lymphoid hemopathies, diabetes and immunosuppressive drugs. Conclusions: This disease, though rare and usually considered a mostly benign disease in non-HIV patients, presented a relatively high mortality rate in our cohort. Thus, histoplasmosis should be suspected, screened and investigated as a first line of defense in highly endemic areas, even in immunocompetent and non-HIV patients, especially those with fever or chronic respiratory symptoms.
1. Introduction
Histoplasmosis, described for the first time by an American pathologist in 1905, is actually one of the most frequent endemic fungal infections in Latin America, notably on the Guiana Shield [1,2,3]. The pathogenic agent is a thermally dimorphic fungus, Histoplasma capsulatum. It can persist for years as mold in the soil after its colonization, notably through bird feces and bat guano [4]. Thus, the long survival in the environment offers prolonged opportunities for spores’ inhalation during visits to contaminated areas, causing a primary pulmonary infestation [5].
Pulmonary histoplasmosis is a primary disease with a wide spectrum of clinical manifestations: acute pulmonary histoplasmosis, histoplasmoma and chronic pulmonary histoplasmosis; beyond the lungs, it becomes disseminated and can take acute or chronic forms [1], involving every organ in systemic infections, or it can be the only manifestation when it is focalized in a single organ [1]. Dissemination occurs frequently in immunocompromised patients and is often fatal if not treated [6,7,8,9], but it sometimes also occurs in apparently immunocompetent persons [10].
In French Guiana, an overseas French territory located on the northeast coast of South America, histoplasmosis has been studied since the 1950s. Several studies have described histoplasmosis as the most frequent opportunistic infection in people living with the Human Immunodeficiency Virus (PLHIV) since the use of highly active antiretroviral therapy (HAART) [11,12,13,14]. In French Guiana, it is considered the first AIDS-defining condition, and it used to be the first cause of AIDS-related death [14,15,16,17].
A few clinical cases of histoplasmosis in apparently immunocompetent patients have been reported [18,19,20,21]. Given the high prevalence of HIV in French Guiana, much of the research on histoplasmosis has focused on patients with advanced HIV disease. However, this focus does not imply that the burden of histoplasmosis does not also affect HIV-negative patients. Therefore, given the scarcity of studies on this topic in French Guiana, we conducted a detailed clinical description of hospitalized patients suffering from histoplasmosis among HIV-negative individuals.
2. Patients and Methods
This was a single-center, retrospective, observational study conducted at Cayenne Hospital Center from 1 January 2008 to 31 March 2022. Cayenne Hospital is the reference center in French Guiana for the management of tropical infectious diseases. The Parasitology and Mycology laboratory has performed fungal culture since 1997. The Department of Pathology has specialized in tropical infectious disease since 2004, and it systematically performs special stains for cytologic and histologic specimens suspected of fungal infections (May Grunwald Giemsa, Gömöri–Grocott and Periodic Acid Schiff stains).
Files were reviewed based on the Pathology Department records of histoplasmosis, and they were identified by searching for the histoplasmosis code (“B39” within the International Statistical Classification of Diseases and Related Health Problems, “ICD-10”).
The samples collected and studied in the Pathology and Mycology laboratories included fluid samples (respiratory samples with broncho-alveolar lavage (BAL), tracheobronchial aspiration and sputum, ascites, urine, blood, joint fluid and bone marrow aspirate), and tissue biopsies (lung, lymph node, skin, larynx, colon and spinal cord).
The inclusion criteria were as follows: hospitalized patients of any age with an episode of histoplasmosis confirmed according to the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MGS) criteria (Histopathology or direct microscopy and/or direct mycological examination or mycological culture of specimens obtained from an affected site showing the distinctive form of the fungus) [22]. First episodes and relapses of histoplasmosis infection were all included.
Isolated positive PCR or serology and unproven histoplasmosis (improvement after empirical antifungal therapy) were excluded. A positive serology for HIV was an exclusion criterion.
Socio-demographic, clinical, biological (standard biological examinations, mycology and pathology on any type of samples), radiologic and therapeutic data were collected from medical records on a standardized file.
2.1. Statistical Analysis
The collected data were then processed using Microsoft Excel® 2019—version number 1808, for statistical analysis. Frequencies and proportions were calculated for categorical variables. For quantitative variables, means, medians and interquartile ranges [Q1; Q3] were reported.
2.2. Ethical Statement
The project consisted of reutilizing available personal health data; thus, it was classified as research not involving human persons and conducted according to reference methodology n°004 of the Commission Nationale Informatique et Libertés (CNIL) in compliance with the General Data Protection Regulation (EU 2016/679). A letter of information was sent to all participants to inform them of the collection of their data and the purpose of the study.
3. Results
3.1. Epidemiological Data
A total of 34 hospitalized patients from the Medicine, Intensive Care Unit (ICU), Infectious Tropical Diseases Unit (ITDU), Dermatology and Paediatrics Departments were included in our cohort. The cohort was composed of 91% (31/34) adults (≥18 years of age) and 9% (3/34) children (Table 1), with a sex ratio, M:F, of 2. The median age was higher among the women than among the men (70 versus 54 years). The patients were originally from French Guiana (47%), Mainland France (21%) and Brazil (18%). Patients from Surinam and Haiti were less represented (6 and 3% respectively). Nearly half of the patients (48%) lived in Cayenne and its surroundings, including Cayenne, Rémire-Montjoly and Matoury, the most inhabited areas of French Guiana.

Table 1.
Epidemiological and clinical characteristics of non-HIV patients diagnosed with histoplasmosis.
3.2. Clinical Presentation
All cases were first episodes Histoplasma capsulatum infection. General condition was impaired in 62% of patients with a WHO performance score >2. Fever (>37 °C) was found in 56% of patients.
In 82% of the cases, the histoplasmosis was disseminated: acute disseminated histoplasmosis accounted for 8 cases (23%), and chronic disseminated histoplasmosis accounted for 20 cases (59%), in contrast with 18% of cases in with pulmonary localization: acute pulmonary histoplasmosis in one case (3%) and chronic pulmonary histoplasmosis in five cases (15%).
The pulmonary features found in thoracic computerized tomography (CT) scans varied. They presented as diffuse miliary micronodules (four cases) (Figure 1) or non-diffuse micronodules (one case). Bilateral pulmonary nodules with regular contours (one case), localized ground glass nodules (one case), a calcified nodule (one case), alveolar pulmonary condensations (four cases) and excavated condensations (two cases) were also found via CT scan. In one case, the pulmonary histoplasmosis coexisted with a non-small-cell lung carcinoma in the same lung lobe.
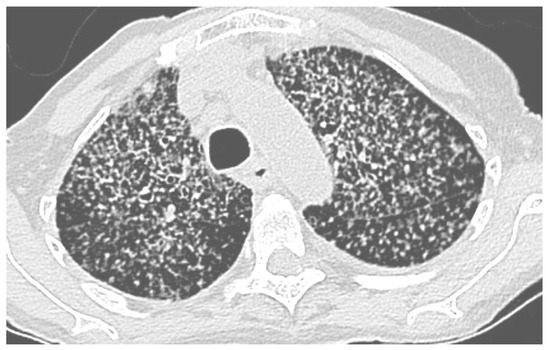
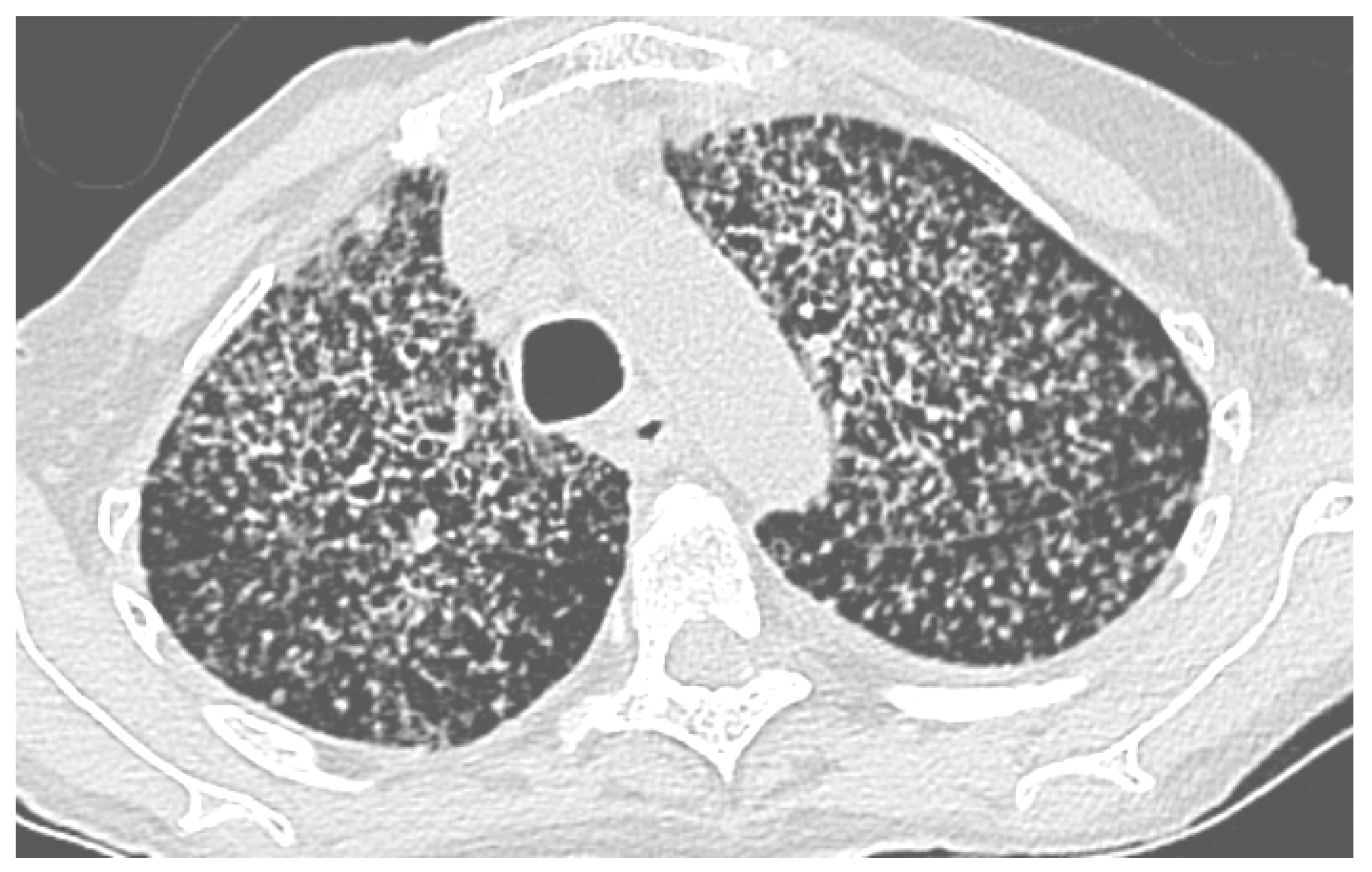
Figure 1.
Chest CT scan, miliary histoplasmosis in 91-year-old woman occurring after cleaning droppings, particularly those of bats, in a confined place without protection. She unfortunately died despite treatment. Scale bar: 25%.
Regarding digestive involvement, focal colonic involvement (without evidence of other localization) was the most frequent. The four cases of colonic histoplasmosis presented as ulcerations upon endoscopy (unique ulceration localized in the coecum in one case and multiple ulcerations spread throughout the colon in three cases).
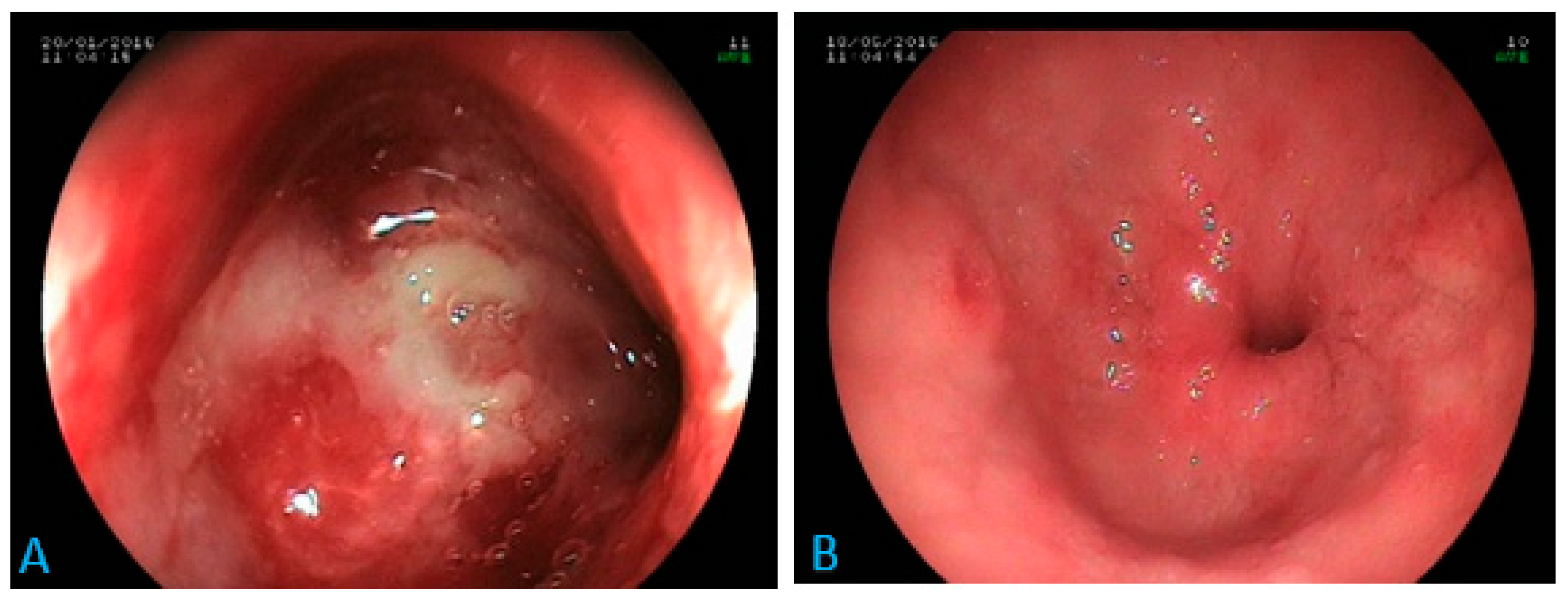
Ascites were found in both focal and disseminated forms. Gastric or hepatic involvements were found only in disseminated forms. One case of isolated duodenal involvement, as a tight stenosis of the duodenal bulb (Figure 2), was found.
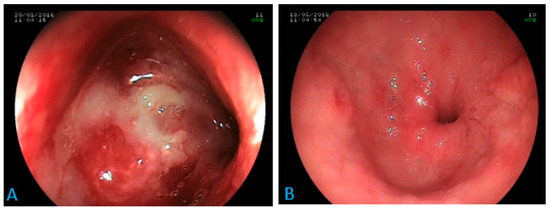
Figure 2.
Digestive endoscopy: inflammatory stenosis at the tip of the duodenal bulb in a 70-year-old patient (A) suffering from epigastric pain, anorexia and weight loss. Diagnosis of histoplasmosis via biopsy with favorable evolution under antifungal treatment (B). Scale bar: 100%.
Only one case of cerebral involvement was found in our cohort in a severely malnourished 10-year-old child suffering from disseminated histoplasmosis. Similarly, one oral mucosa histoplasmosis was found in a 79-year-old man in the context of disseminated histoplasmosis after anticancer chemotherapy.
Immunosuppressive conditions were found in 52% (16/31) of adult patients: immunosuppressive or biologic agents (long-term corticosteroid therapy, anticancer chemotherapy, methotrexate and anti-TNF alpha), malignant lymphoid hemopathies and solid organ cancer, kidney transplant, autoimmune disease, diabetes, hepatitis cirrhosis, alcoholism and HTLV-1 infection (Table 2). In our cohort, four patients had between two and three immunosuppressive pathologies. The three pediatric cases were all chronic disseminated forms. In immunosuppressed, non-HIV histoplasmosis patients, all forms were disseminated.

Table 2.
This table shows the number of immunosuppressive pathologies in non-HIV patients when histoplasmosis had been diagnosed.
We found five cases of histoplasmosis in patients with lymphoid malignancies. In four of these cases, Histoplasma capsulatum fungemia was diagnosed via blood cultures in the presence of fever during the course of chemotherapy.
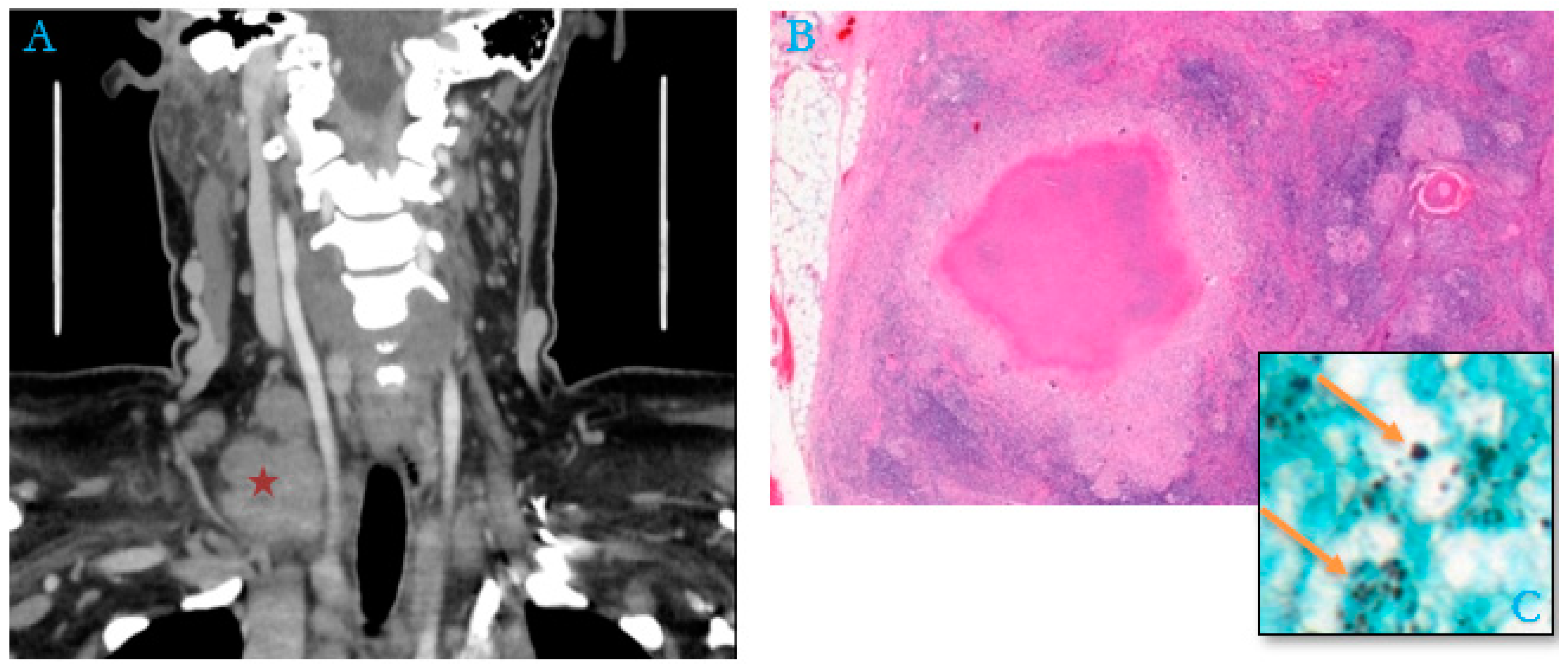
By contrast, in 48% of the adult cases (15/31), no cause of immunosuppression or associated conditions was mentioned in the patient’s medical record (an example in Figure 3).
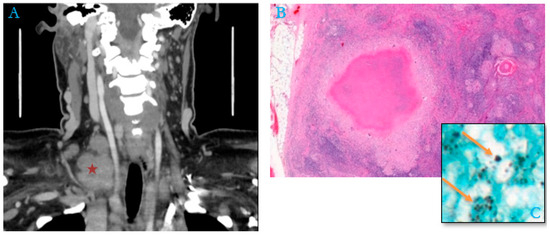
Figure 3.
Package of right supraclavicular adenopathy (red asterisk) in a 51-year-old woman, a housemaid with no previous history (A). (B) Histological section of lymph node shows numerous granulomas with epithelioid cells associated with multinucleated giant cells, centered by caseous necrosis mimicking tuberculoid granuloma (Hematoxylin–Eosin–Safran, “HES,” Stain, ×200). (C) Numerous extracellular and intra-histiocytic yeasts of Histoplasma capsulatum (Gömöri–Grocott stain, orange arrows, ×100).
3.3. Biological Data
An inflammatory syndrome with elevated CRP was found in 91% of cases with a median of 45 mg/L. Anemia (hemoglobin level < 11.5 g/dL), thrombocytopenia (platelets < 150 G/L) and neutropenia (neutrophils < 2 G/L) were found in 51.5%, 31% and 9% of cases, respectively. Aspartate amino transaminase (AST) was increased (>35 Ui/L) in 42% of cases and alanine amino transaminase (ALT) in 19.5% of cases. The greatest disturbances were found in GGT in 55% of cases (up to 19 times the upper normal level). LDH levels were elevated in 37% of cases (up to eight times the upper normal level).
3.4. Diagnostic Methods
Respiratory samples accounted for the majority of the examined specimens (38%), including 69% of BAL. In addition to being the main examination performed in the respiratory tract, BAL was, in fact, the most frequently performed examination in general (26.5% of all specimens). Fungal blood cultures came in second place (20.5%), followed by digestive biopsies (15%). The puncture of ascites fluid and lymph node biopsies represented 9% each.
Concerning the analyses requested by the physicians, mycological examination was the most frequently requested (88% of cases) with 82% of positive Histoplasma capsulatum cultures. Cytological analysis was less frequently requested (20.5%), but it was nevertheless frequently helpful. For example, a cytological examination was requested for 85% of the performed BALs, allowing the rapid detection of Histoplasma capsulatum in 54% of cases (Table 3).

Table 3.
Diagnostic methods of histoplasmosis in non-HIV patients.
3.5. Treatment Data
Antifungal therapy was initiated in 24/34 patients (71%), with Itraconazole monotherapy upfront (17/24) and Ambisome relayed with Itraconazole (7/24). Treatment was completed for only 18/24 patients. The duration of treatment varied from one patient to another: 2 months for two patients, 3 months for five patients, 4 months for one patient, 6 months for seven patients, 12 months for two patients and 20 months for one patient. Among them, the prescribed dose of Itraconazole varied from 400 mg for nine patients (50%) to 200 mg for six patients (33.3%), 100 mg for two patients (11.2%) and 300 mg for one patient (5.5%).
Five patients out of 34 died from histoplasmosis, including four patients who died in the ICU, leading to an overall case fatality rate of 14.7%. All five patients who died of histoplasmosis in our cohort, aged 36, 45, 60, 65 and 93, were diagnosed early and treated for disseminated histoplasmosis.
Four patients who died in the ICU presented an acute form of histoplasmosis with multi-visceral failure. One of these cases (a patient aged 36 years) had septic shock with progressive, disseminated histoplasmosis.
Two of these four patients had immunosuppressive pathologies (chronic alcoholism with HTLV1 infection and kidney transplantation with immunosuppressive drugs).
4. Discussion
This 14-year study identified 34 cases of histoplasmosis in non-HIV patients requiring hospitalization and treatment. This small sample is similar to that presented in studies carried out in neighboring countries in the same geographical area of the Amazon with an endemic presence of histoplasmosis, such as Brazil [2] and Peru [17]. On the other hand, although the exact incidence of mild or non-symptomatic histoplasmosis in endemic areas in French Guiana or in other neighboring countries of South America is unknown, histoplasmin skin test prevalence used in the early of 1950s allowed for the revelation of the extent of Histoplasma capsulatum infection in many areas [23]. A previous study supposed that 90% of the tested population in some parts of the USA (endemic arears of Mississippi and the Ohio River valleys of Missouri) had been exposed to Histoplasma by the age of ≥15 years [24]; hundreds of thousands of individuals in these endemic areas are infected each year and remain asymptomatic [1]. In French Guiana, 32.5% of the general population in the early 1950s was infected or had contact with Histoplasma capsulatum [25]. However, these skin tests are no longer marketed today because of cross-reactions with other fungi [1].
In persons with HIV, histoplasmosis takes a progressive, disseminated form in more than 95% of patients [26]. Although much research has been performed in the context of HIV, the problem of histoplasmosis extends beyond the realm of HIV, albeit with a much lower incidence.
Histoplasma capsulatum infection is usually due to inhalation of spores contained in enriched soils, which constitute the only reservoir of this mold [1]. Therefore, any domestic or professional activity in endemic regions can lead to the aerosolization of these spores, favoring inhalation and infection [1]. The occurrence of infection after the inhalation of spores depends on the inhaled inoculum and the immune response of the host [22].
The endogenous reactivation of previously inhaled spores that have remained quiescent yeasts in the host organism, similar to what is known for tuberculosis, has been suggested [7,8,9,11,12,13,14,15,16,17,18,19,20,21,22,24,25,26,27] and could explain certain forms of histoplasmosis occurring in immunocompetent patients several months after their return from a trip to an endemic area. However, in French Guiana, data from HIV cohorts have shown a substantial seasonal component, suggesting de novo infections [28].
In comparison with previous data in HIV patients infected with histoplasmosis [16], the sex ratio, M:F, appears identical to our study at 2, but the median age of onset in males and females was greater in the non-HIV population than in PLHIV (54–70 for non-HIV versus 41.4–37.5 for HIV patients). Patients from French Guiana were the most represented in both groups, but patients from Haiti were the second most represented among PLHIV, probably due to the high prevalence of HIV infection in Haiti. In our HIV-negative cohort, patients from mainland France represented the second most frequent group among non-HIV patients, followed by patients from Brazil.
In nearly 48% of cases, the patients were apparently immunocompetent. However, it is important to mention that no specific investigation of underlying immunosuppression was performed in these patients (serum protein electrophoresis, determination of immunoglobulin class, lymphocyte immunophenotyping, etc.), as the patients did not show patent signs of immunosuppression, which could be considered a limitation in this study.
Thus, in French Guiana, HIV infection remains the main immunosuppressive condition that favors histoplasmosis, especially since it is one of the most prevalent forms of immunosuppression, and it is mostly discovered at a late stage in nearly one-third of cases [29], with heterogeneous clinical presentations [30] most often fatal in the absence of appropriate treatment [31].
In our study, the immunosuppression-associated conditions—in particular lymphoid malignancies or treatment with long-term corticosteroids, chemotherapies or immunosuppressant drugs—are causes of cellular immunity impairment similar to the HIV-mediated immune impairment, highlighting the role of cell-mediated immunity against the dissemination of histoplasmosis beyond the pulmonary barrier. [1].
The analysis of cases of Histoplasma capsulatum fungemia should pay particular attention to the occurrence of acute febrile peaks during chemotherapy treatment, especially in hematologic lymphoid malignancies, and should lead to a search for histoplasmosis in the initial investigation.
Adrenal involvement is the most frequently described in the literature in immunocompetent patients [12], especially in Asia [32,33,34], as a bilateral adrenal hypertrophy or adrenal calcifications on CT scan [32]. This localization can sometimes lead to adrenal insufficiency suggestive of Addison’s disease or tuberculosis [34]. In our cohort of non-HIV patients, no morphological abnormalities of the adrenals in imaging were reported, and no clinico-biological signs of adrenal insufficiency were found. In HIV-infected patients in French Guiana, a low rate of 0.85% of adrenal damage via histoplasmosis was previously described [12]. Perhaps there again, the immunosuppression circumstances explain why it is rare, by contrast with localized adrenal presentations, where the host is immunocompetent but the local immunosuppression due to the adrenal hormonal environment creates a niche for infection.
Biology findings in non-HIV patients with histoplasmosis were non-specific (Table 4). In PLHIV with histoplasmosis, a platelet count of less than 100,000/mm3 and an LDH level greater than twice the normal level were defined as severity factors [13]. In our study, we calculated the mean LDH level for the five severe cases of patients who died from histoplasmosis, and we found an increase in this level to four times the normal value threshold. The median platelet count was normal at 227 G/L. However, cytolytic impairment predominantly via AST and cholestasis predominantly via GGT were found in a comparable manner to HIV [35].

Table 4.
Biological data found in non-HIV patients with histoplasmosis.
Regarding the analyzed specimens (Table 3), respiratory samples constituted the majority of the performed examinations (38% of samples). BAL accounted for 69% of these respiratory specimens with a good sensitivity compared to other examinations (71% positive direct examination in mycology, 71% in culture and 54% in cytology).
Cultures dominated all other diagnostic methods, confirming their place as the gold standard in the diagnosis of histoplasmosis. Few cytological examinations were performed, despite their potential contribution, in line with the same results found in disseminated histoplasmosis in PLHIV [5].
Concerning the therapeutic data, we found a lot of missing information concerning the follow-ups and outcomes of patients’ treatments, in contrast to the cohort of PLHIV. In French Guiana, specialized care structures dedicated to PLHIV have allowed a better organization of their treatment and follow-up, particularly the establishment of an HIV cohort and an HIV histoplasmosis database created in 1992 [5,8,12].
Itraconazole was the main antifungal agent used in the treatment of histoplasmosis, but its dosage and duration of treatment varied considerably from one patient to another, without any conformity with the guidelines [9,14]. Thus, it appears that the management of histoplasmosis in PLHIV is better codified and more consistent. All these data showed that, while histoplasmosis is considered a neglected infection in Latin America [15], it seems even more neglected in non-HIV and immunocompromised patients. While there is a growing recognition that febrile HIV-infected persons should be suspected of potentially having histoplasmosis, there is no such heuristic for patients without HIV, and clinicians should be vigilant.
5. Conclusions
In endemic areas such as French Guiana, symptomatic histoplasmosis in non-HIV patients appears to be a relatively rare disease, affecting both apparently immunocompetent individuals and those with comorbidities and immunosuppressive pathologies. In our study, the majority of hospitalized non-HIV patients with comorbidities had mainly lymphoid malignancies or diabetes, or had been treated with immunosuppressive drugs. This disease, though rare and usually considered a mostly benign disease in non-HIV patients, presented a relatively high mortality estimated at 14.7% in our cohort. Thus, histoplasmosis should be suspected, screened and investigated as a first line of defense in highly endemic areas, even in immunocompetent patients, especially those with fever or chronic respiratory symptoms.
Author Contributions
Conceptualization, H.A. and K.D.A.; formal analysis, H.A. and M.B.-W.; investigation, H.A., B.L., G.G., A.A. (Alolia Aboikoni), P.C., D.G., G.H.-A., R.M., M.O., C.S., M.D., L.E., H.K., M.N., K.D.A. and D.L.; methodology, H.A., M.B.-W., A.A. (Antoine Adenis) and K.D.A.; validation, M.N. and K.D.A.; writing—original draft, H.A. and M.B.-W.; writing—review and editing, H.A., A.A. (Antoine Adenis), M.N. and K.D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This project was approved by the Institutional Review Board of the Department of Research and Innovation in Public Health. The project consisted of reutilizing available personal health data; thus, it was classified as research not involving human persons according to reference methodology n°004 of the Commission Nationale Informatique et Libertés (CNIL) in compliance with the General Data Protection Regulation (EU 2016/679).
Informed Consent Statement
A letter of information was sent to all participants to inform them of the collection of their data and the purpose of the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carol, A. Kauffman Histoplasmosis: A Clinical and Laboratory Update. Clin. Microbiol. Rev. 2007, 20, 115–132. [Google Scholar] [CrossRef]
- Leimann, B.C.; Pizzini, C.V.; Muniz, M.M.; Albuquerque, P.C.; Monteiro, P.C.; Reis, R.S.; Almeida-Paes, R.; Lazera, M.S.; Wanke, B.; Pérez, M.A.; et al. Histoplasmosis in a Brazilian center: Clinical forms and laboratory tests. Rev. Iberoam. Micol. 2005, 22, 141–146. [Google Scholar] [CrossRef]
- Darling, S.T. A protozoon general infection producing pseudotubercles in the lungs and focal necroses in the liver, spleen and lymphnodes. JAMA 1906, 17, 1283. [Google Scholar] [CrossRef]
- Emmons, C.W. Isolation of Histoplasma capsulatum from soil. Public Health Rep. 1949, 64, 892–896. [Google Scholar] [CrossRef]
- Nacher, M.; Valdes, A.; Adenis, A.; Blaizot, R.; Ugo, F.; Abboud, P.; Demar, M.; Djossou, F.; Epelboin, L.; Misslin, C.; et al. Review of diagnostic methods and results for HIV-associated disseminated histoplasmosis: Pathologists are not sufficiently involved. Trop. Med. Int. Health 2021, 26, 1462–1469. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy; World Health Organization: Geneva, Switzerland, 2017. Available online: http://www.who.int/hiv/pub/toolkits/advanced-HIV-disease-policy/en/ (accessed on 2 April 2024).
- Woods, J.P. Revisiting old friends: Developments in understanding Histoplasma capsulatum pathogenesis. J. Microbiol. 2016, 54, 265–276. [Google Scholar] [CrossRef]
- Nacher, M.; Valdes, A.; Adenis, A.; Blaizot, R.; Abboud, P.; Demar, M.; Djossou, F.; Epelboin, L.; Misslin, C.; Ntab, B.; et al. Gastrointestinal disseminated histoplasmosis in HIV-infected patients: A descriptive and comparative study. PLoS Negl. Trop. Dis. 2021, 15, e0009050. [Google Scholar] [CrossRef]
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.E.; Kauffman, C.A.; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825. [Google Scholar] [CrossRef]
- Benedict, K.; Derado, G.; Mody, R.K. Histoplasmosis-Associated Hospitalizations in the United States, 2001–2012. Open Forum. Infect. Dis. 2016, 3, ofv219. [Google Scholar] [CrossRef]
- Nacher, M.; Adenis, A.; Adriouch, L.; Dufour, J.; Papot, E.; Hanf, M.; Vantilcke, V.; Calvez, M.; Aznar, C.; Carme, B.; et al. What is AIDS in the Amazon and the Guianas? Establishing the burden of disseminated histoplasmosis. Am. J. Trop. Med. Hyg. 2011, 84, 239–240. [Google Scholar] [CrossRef]
- Nacher, M.; Alsibai, K.D.; Valdes, A.; Abboud, P.; Adenis, A.; Blaizot, R.; Blanchet, D.; Demar, M.; Djossou, F.; Epelboin, L.; et al. HIV-Associated Disseminated Histoplasmosis and Rare Adrenal Involvement: Evidence of Absence or Absence of Evidence. Front. Cell Infect. Microbiol. 2021, 11, 619459. [Google Scholar] [CrossRef]
- Couppié, P.; Sobesky, M.; Aznar, C.; Bichat, S.; Clyti, E.; Bissuel, F.; El Guedj, M.; Alvarez, F.; Demar, M.; Louvel, D.; et al. Histoplasmosis and acquired immunodeficiency syndrome: A study of prognostic factors. Clin. Infect. Dis. 2004, 38, 134–138. [Google Scholar] [CrossRef]
- Perez, F.; Caceres, D.H.; Ford, N.; Ravasi, G.; Gomez, B.L.; Pasqualotto, A.C.; Hine, P.; Adenis, A.A.; Nacher, M.; Chiller, T.; et al. For The Guideline Development Group for Diagnosing and Managing Disseminated Histoplasmosis among People Living with HIV. Summary of Guidelines for Managing Histoplasmosis among People Living with HIV. J. Fungi 2021, 7, 134. [Google Scholar] [CrossRef]
- Nacher, M.; Couppié, P.; Epelboin, L.; Djossou, F.; Demar, M.; Adenis, A. Disseminated Histoplasmosis: Fighting a neglected killer of patients with advanced HIV disease in Latin America. PLoS Pathog. 2020, 16, e1008449. [Google Scholar] [CrossRef]
- Huber, F.; Nacher, M.; Aznar, C.; Pierre-Demar, M.; El Guedj, M.; Vaz, T.; Vantilcke, V.; Mahamat, A.; Magnien, C.; Chauvet, E.; et al. AIDS-related Histoplasma capsulatum var. capsulatum infection: 25 years experience of French Guiana. AIDS 2008, 22, 1047–1053. [Google Scholar] [CrossRef]
- Chincha, O.; Bustamante, B. Disseminated histoplasmosis in Peruvian non-HIV patients: An under recognized endemic disease. J. Mycol. Med. 2021, 31, 101177. [Google Scholar] [CrossRef]
- Bonnet, D.; Balandraud, P.; Lonjon, T.; Rey, P.; Van De Walle, J.P.; Cador, L.; Jancovici, R.; Martet, G.; Branquet, D.; De Muizon, H. Round pulmonary lesions after returning from French Guyana. Six cases of American pulmonary histoplasmosis. Med. Trop. (Mars) 1995, 55, 55–60. [Google Scholar]
- Cuguillière, A.; N’Guyen, G.; Maslin, J.; Lonjon, T.; Miltgen, J.; Bonnet, D. Tumorform of histoplasmosis in subjects returning from French Guiana. Treatment of an immunocompetentpatient. Presse Medicale 1997, 26, 1724. [Google Scholar]
- Cormier, H.; Perez, N.; Blanchet, D.; Couppié, P.; Carme, B.; Aznar, C. Fatal histoplasmosis in a non-HIV patient in French Guiana. J. Mycol. Médicale/J. Med. Mycol. 2012, 22, 189–191. [Google Scholar] [CrossRef]
- Barthes, N.; Morin, F.; Pommierd, S.V.; Briolant, S. Lung nodule in French Guiana in an immunocompetent patient. Med. Sante Trop. 2017, 27, 26. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Mochi, A.; Edwards, P.Q. Geographical distribution of histoplasmosis and histoplasmin sensitivity. Bull. World Health Organ. 1952, 5, 259–291. [Google Scholar]
- Edwards, P.Q.; Billings, E.L. Worldwide pattern of skin sensitivity to histoplasmin. Am. J. Trop. Med. Hyg. 1971, 20, 288–319. [Google Scholar] [CrossRef]
- Floch, H. Revue critique des investigations et de la littérature mycologiques pour les annés 1946–1956 en Guyane Française. Mycopathol. Mycol. 1957, 8, 194–205. [Google Scholar] [CrossRef]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Bourgeois, N.; Douard-Enault, C.; Reynes, J.; Lechiche, C.; Basset, D.; Rispail, P.; Lachaud, L. Seven imported histoplasmosis cases due to Histoplasma capsulatum var. capsulatum: From few weeks to more than three decades asymptomatic period. J. Mycol. Med. 2011, 21, 19–23. [Google Scholar] [CrossRef]
- Hanf, M.; Adenis, A.; Couppie, P.; Carme, B.; Nacher, M. HIV-associated histoplasmosis in French Guiana: Recent. infection or reactivation? AIDS 2010, 24, 1777–1778. [Google Scholar] [CrossRef]
- Nacher, M.; Ouedraogo, E.; Succo, T.; Guarmit, B.; Djossou, F.; Stettler, L.; Alvarez, F.; Elenga, N.; Adenis, A.; Couppié, P. L’infection VIH en Guyane, revue historique et tendances actuelles. Bull. Epidémiol. Hebd. 2020, 9, 43–51. [Google Scholar]
- Nacher, M.; Valdes, A.; Adenis, A.; Blaizot, R.; Abboud, P.; Demar, M.; Djossou, F.; Epelboin, L.; Drak Alsibai, K.; Misslin, C.; et al. Heterogeneity of Clinical Presentations and Paraclinical Explorations to Diagnose Disseminated Histoplasmosis in Patients with Advanced HIV: 34 Years of Experience in French Guiana. J. Fungi 2020, 6, 165. [Google Scholar] [CrossRef]
- Nacher, M.; Valdes, A.; Adenis, A.; Blaizot, R.; Abboud, P.; Demar, M.; Djossou, F.; Epelboin, L.; Misslin, C.; Ntab, B.; et al. Disseminated Histoplasmosis in HIV-Infected Patients: A Description of 34 Years of Clinical and Therapeutic Practice. J. Fungi 2020, 6, 164. [Google Scholar] [CrossRef]
- Samaddar, A.; Sharma, A.; Kumar Ph, A.; Srivastava, S.; Shrimali, T.; Gopalakrishnan, M.; Bohra, G.K. Disseminated histoplasmosis in immunocompetent patients from an arid zone in Western India: A case series. Med. Mycol. Case Rep. 2019, 25, 49–52. [Google Scholar] [CrossRef]
- Rahim, M.A.; Zaman, S.; Amin, M.R.; Nazim Uddin, K.; Chowdhury, M.J. Adrenal histoplasmosis among immunocompetent Bangladeshi patients. Trop. Doct. 2019, 49, 132–133. [Google Scholar] [CrossRef]
- Kathuria, S.; Capoor, M.R.; Yadav, S.; Singh, A.; Ramesh, V. Disseminated histoplasmosis in an apparently immunocompetent individual from north India: A case report and review. Med. Mycol. 2013, 51, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Couppié, P.; Herceg, K.; Bourne-Watrin, M.; Thomas, V.; Blanchet, D.; Alsibai, K.D.; Louvel, D.; Djossou, F.; Demar, M.; Blaizot, R.; et al. The Broad Clinical Spectrum of Disseminated Histoplasmosis in HIV-Infected Patients: A 30 Years’ Experience in French Guiana. J. Fungi 2019, 5, 115. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).