Colonization and Biodegradation Potential of Fungal Communities on Immersed Polystyrene vs. Biodegradable Plastics: A Time Series Study in a Marina Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Immersion Experiments
2.2. Culture-Independent Approach
2.2.1. DNA Extraction, Amplification, and Sequencing
2.2.2. Bioinformatics and Statistical Analyses
2.3. Culture-Dependent Approach
2.4. Screening for Use of Plastics as a Source of Carbon
2.4.1. Stratified and Weighted Random Sampling
2.4.2. Solid-Based Screening
2.4.3. Liquid-Based Screening
2.5. Sanger Sequencing
3. Results
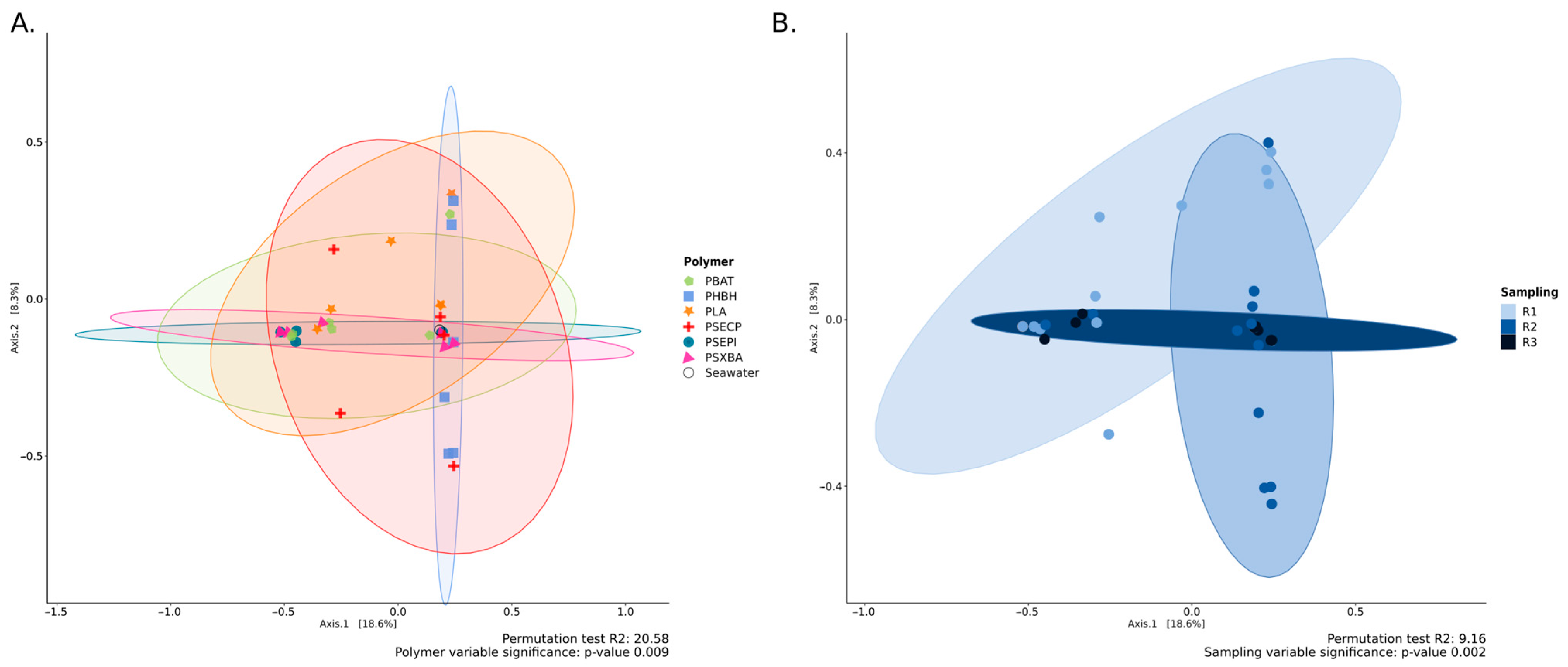
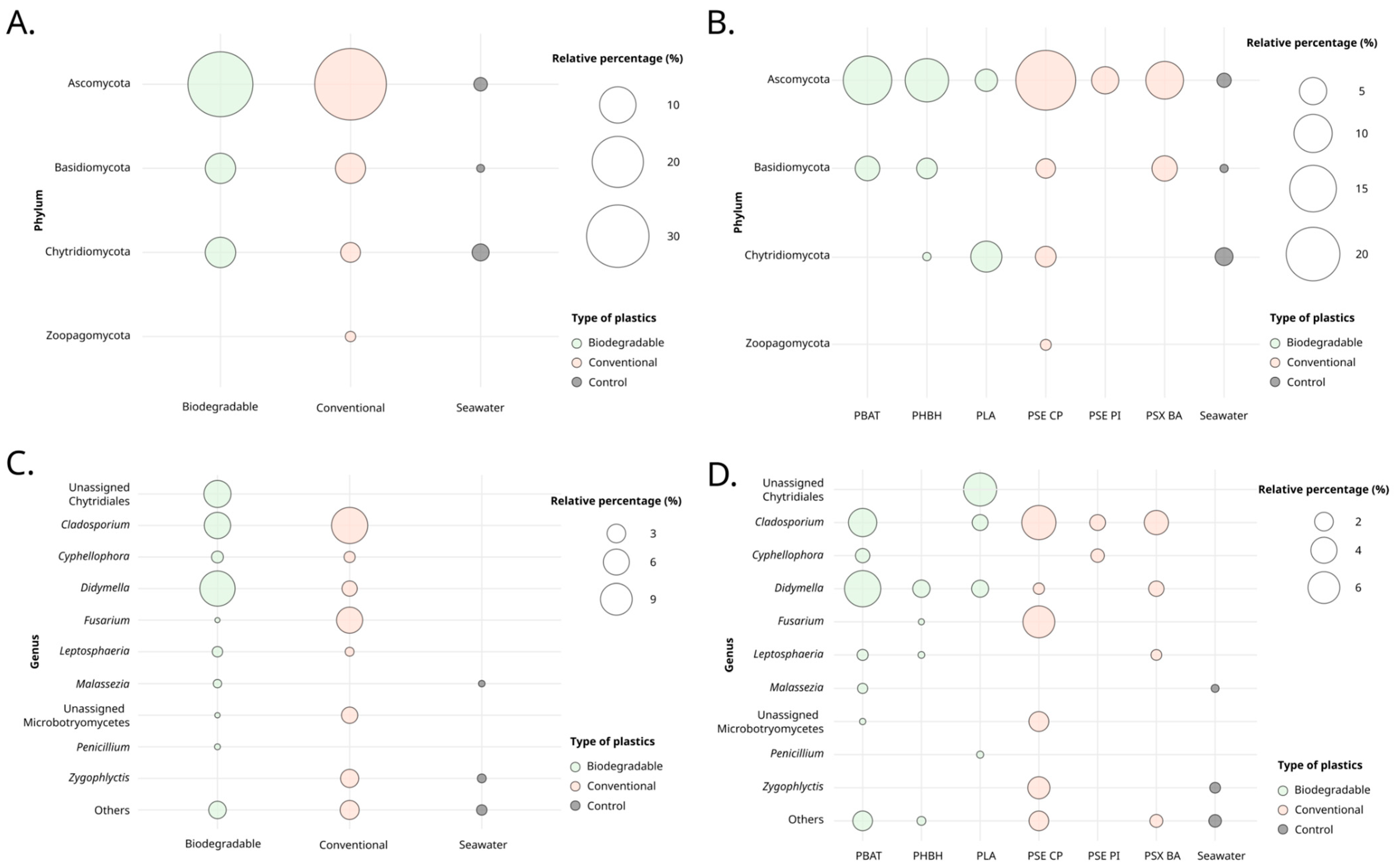
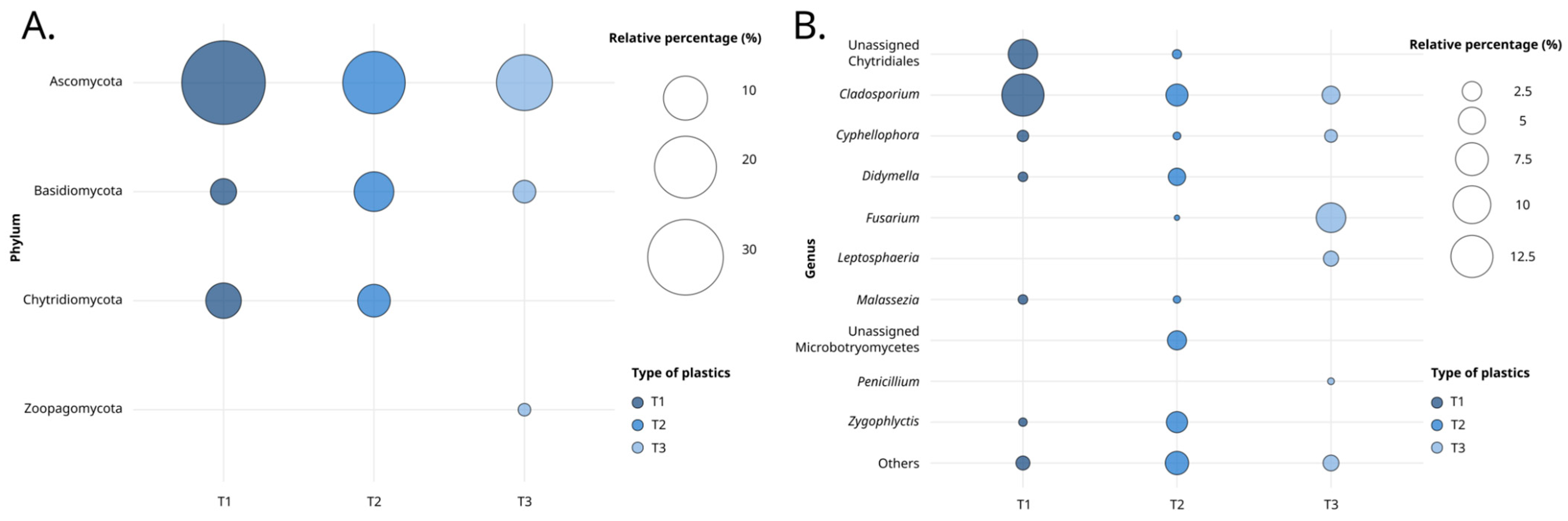
3.1. Fungal Diversity Detected by Metabarcoding Analysis
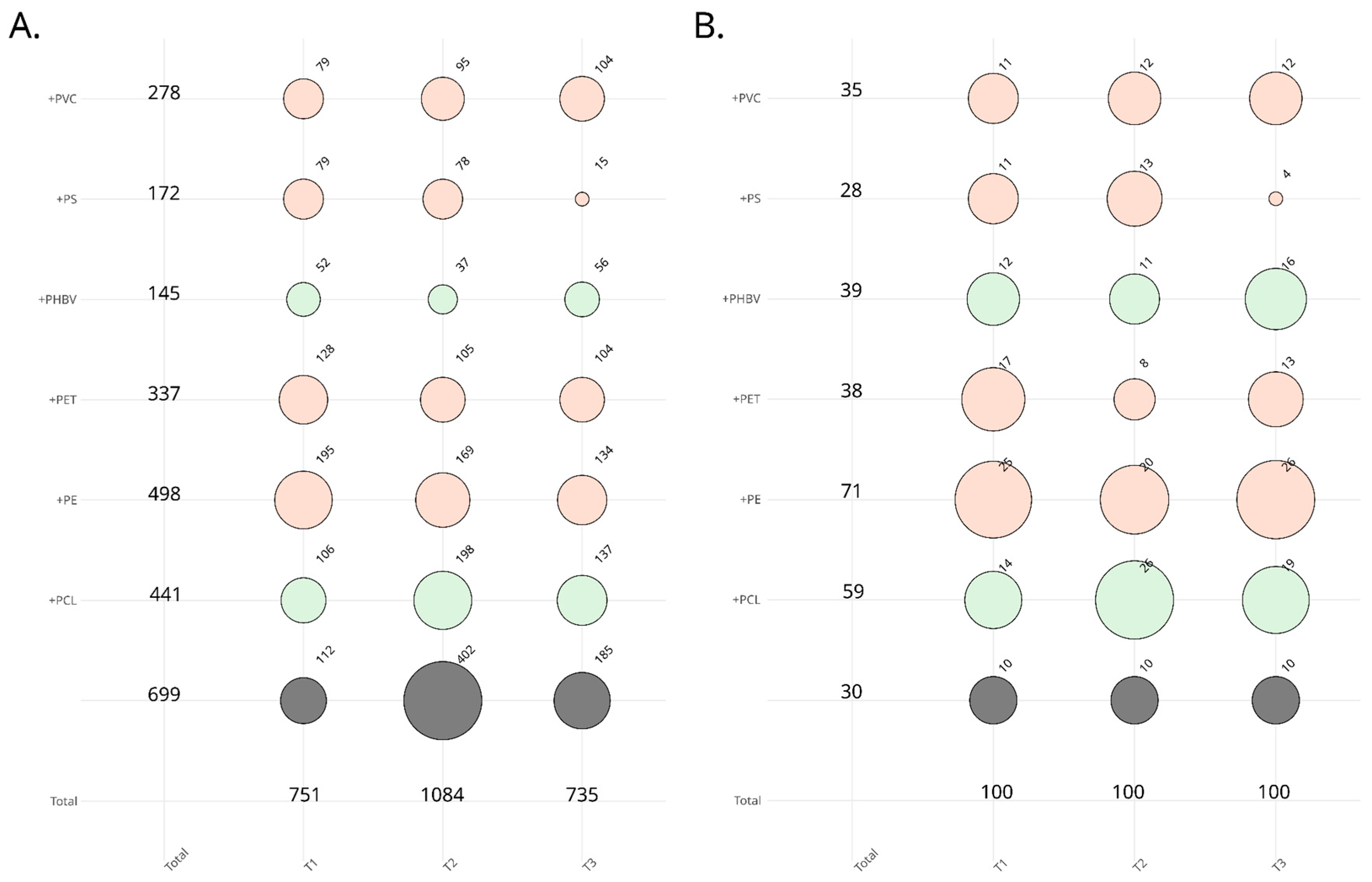
3.2. Assessing Fungal Colonization Potential through Cultivation
3.3. Screening for the Ability to Utilize Polymers as Carbon Sources
4. Discussion
4.1. The Fungal Plastisphere
4.2. Ability to Utilize Polymers as Carbon Sources
4.3. Taxonomic Identification of Putative Fungal Degraders
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2022; Plastics Europe: Brussels, Belgium, 2022. [Google Scholar]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating Scenarios toward Zero Plastic Pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Porta, R. Anthropocene, the Plastic Age and Future Perspectives. FEBS Open Bio 2021, 11, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of Plastic Polymer Types in the Marine Environment; A Meta-Analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef]
- Turner, A. Foamed Polystyrene in the Marine Environment: Sources, Additives, Transport, Behavior, and Impacts. Environ. Sci. Technol. 2020, 54, 10411–10420. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; André, S.; Loon, W.M.G.M. Abundance, Composition and Trends of Beach Litter. In OSPAR, 2023: The 2023 Quality Status Report for the North-East Atlantic; OSPAR Commission: London, UK, 2022. [Google Scholar]
- Mincer, T.; Zettler, E.; Amaral-Zettler, L. Biofilms on Plastic Debris and Their Influence on Marine Nutrient Cycling, Productivity, and Hazardous Chemical Mobility. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-95566-7. [Google Scholar]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Azuma, T.; Cordova, M.R.; Cózar, A.; Galgani, F.; Hagita, R.; Kanhai, L.D.; Imai, K.; Iwasaki, S.; Kako, S.; et al. A Multilevel Dataset of Microplastic Abundance in the World’s Upper Ocean and the Laurentian Great Lakes. Microplast. Nanoplast. 2021, 1, 16. [Google Scholar] [CrossRef]
- Agostini, L.; Moreira, J.C.F.; Bendia, A.G.; Kmit, M.C.P.; Waters, L.G.; Santana, M.F.M.; Sumida, P.Y.G.; Turra, A.; Pellizari, V.H. Deep-Sea Plastisphere: Long-Term Colonization by Plastic-Associated Bacterial and Archaeal Communities in the Southwest Atlantic Ocean. Sci. Total Environ. 2021, 793, 148335. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.P.; Schratzberger, M.; Sapp, M.; Osborn, A.M. Rapid Bacterial Colonization of Low-Density Polyethylene Microplastics in Coastal Sediment Microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef]
- Latva, M.; Dedman, C.J.; Wright, R.J.; Polin, M.; Christie-Oleza, J.A. Microbial Pioneers of Plastic Colonisation in Coastal Seawaters. Mar. Pollut. Bull. 2022, 179, 113701. [Google Scholar] [CrossRef]
- Woodall, L.C.; Jungblut, A.D.; Hopkins, K.; Hall, A.; Robinson, L.F.; Gwinnett, C.; Paterson, G.L.J. Deep-sea anthropogenic macrodebris harbours rich and diverse communities of bacteria and archaea. PLoS ONE 2018, 13, e0206220. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.J.; Erni-Cassola, G.; Zadjelovic, V.; Latva, M.; Christie-Oleza, J.A. Marine Plastic Debris: A New Surface for Microbial Colonization. Environ. Sci. Technol. 2020, 54, 11657–11672. [Google Scholar] [CrossRef] [PubMed]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal Dynamics of Bacterial and Fungal Colonization on Plastic Debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef]
- Debroas, D.; Mone, A.; Ter Halle, A. Plastics in the North Atlantic Garbage Patch: A Boat-Microbe for Hitchhikers and Plastic Degraders. Sci. Total Environ. 2017, 599–600, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Florio Furno, M.; Poli, A.; Ferrero, D.; Tardelli, F.; Manzini, C.; Oliva, M.; Pretti, C.; Campani, T.; Casini, S.; Fossi, M.C.; et al. The Culturable Mycobiota of Sediments and Associated Microplastics: From a Harbor to a Marine Protected Area, a Comparative Study. J. Fungi 2022, 8, 927. [Google Scholar] [CrossRef]
- Kettner, M.T.; Rojas-Jimenez, K.; Oberbeckmann, S.; Labrenz, M.; Grossart, H.-P. Microplastics Alter Composition of Fungal Communities in Aquatic Ecosystems: Fungal Communities on Microplastics. Environ. Microbiol. 2017, 19, 4447–4459. [Google Scholar] [CrossRef]
- Kettner, M.T.; Oberbeckmann, S.; Labrenz, M.; Grossart, H.-P. The Eukaryotic Life on Microplastics in Brackish Ecosystems. Front. Microbiol. 2019, 10, 538. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Wichels, A.; Krohne, G.; Gerdts, G. Mature Biofilm Communities on Synthetic Polymers in Seawater—Specific or General? Mar. Environ. Res. 2018, 142, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, A.L.d.F.; Proietti, M.C.; Secchi, E.R.; Taylor, J.D. Diverse Groups of Fungi Are Associated with Plastics in the Surface Waters of the Western South Atlantic and the Antarctic Peninsula. Mol. Ecol. 2020, 29, 1903–1918. [Google Scholar] [CrossRef]
- Lacerda, A.L.d.F.; Taylor, J.D.; Rodrigues, L.d.S.; Kessler, F.; Secchi, E.; Proietti, M.C. Floating Plastics and Their Associated Biota in the Western South Atlantic. Sci. Total Environ. 2022, 805, 150186. [Google Scholar] [CrossRef] [PubMed]
- Oberbeckmann, S.; Osborn, A.M.; Duhaime, M.B. Microbes on a Bottle: Substrate, Season and Geography Influence Community Composition of Microbes Colonizing Marine Plastic Debris. PLoS ONE 2016, 11, e0159289. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Noël, C.; Eyheraguibel, B.; Briand, J.-F.; Paul-Pont, I.; Ghiglione, J.-F.; Coton, E.; Burgaud, G. Fungal Diversity and Dynamics during Long-Term Immersion of Conventional and Biodegradable Plastics in the Marine Environment. Diversity 2023, 15, 579. [Google Scholar] [CrossRef]
- Sérvulo, T.; Taylor, J.D.; Proietti, M.C.; Rodrigues, L.d.S.; Puertas, I.P.; Barutot, R.A.; Lacerda, A.L.d.F. Plastisphere Composition in a Subtropical Estuary: Influence of Season, Incubation Time and Polymer Type on Plastic Biofouling. Environ. Pollut. 2023, 332, 121873. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, W.; Zhang, K.; Hu, J.; Gao, Y.; Cui, G.; Grossart, H.-P.; Luo, Z. Fungal Communities Differ with Microplastic Types in Deep Sea Sediment Enrichments of the Eastern Pacific. Int. Biodeterior. Biodegrad. 2022, 173, 105461. [Google Scholar] [CrossRef]
- Yang, Y.; Suyamud, B.; Liang, S.; Liang, X.; Wan, W.; Zhang, W. Distinct Spatiotemporal Succession of Bacterial Generalists and Specialists in the Lacustrine Plastisphere. Environ. Microbiol. 2023, 25, 2746–2760. [Google Scholar] [CrossRef] [PubMed]
- Zeghal, E.; Vaksmaa, A.; Vielfaure, H.; Boekhout, T.; Niemann, H. The Potential Role of Marine Fungi in Plastic Degradation—A Review. Front. Mar. Sci. 2021, 8, 738877. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Pang, K.-L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J.; et al. An Online Resource for Marine Fungi. Fungal Divers. 2019, 96, 347–433. [Google Scholar] [CrossRef]
- Lear, G.; Kingsbury, J.M.; Franchini, S.; Gambarini, V.; Maday, S.D.M.; Wallbank, J.A.; Weaver, L.; Pantos, O. Plastics and the Microbiome: Impacts and Solutions. Environ. Microbiome 2021, 16, 2. [Google Scholar] [CrossRef]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. Phylogenetic Distribution of Plastic-Degrading Microorganisms. mSystems 2021, 6, e01112-20. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Zeng, Y.-H.; Zhu, J.-M.; Cai, Z.-H.; Zhou, J. The Structure and Assembly Mechanisms of Plastisphere Microbial Community in Natural Marine Environment. J. Hazard. Mater. 2022, 421, 126780. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Guo, Z.; Yang, X.; Dai, Y. Comprehensive Understanding of the Aging and Biodegradation of Polystyrene-Based Plastics. Environ. Pollut. 2024, 342, 123034. [Google Scholar] [CrossRef] [PubMed]
- Karimah, S.; Yanto, D.; Rahayu, G.; Dwi Nurhayat, O. The Potential of White Rot Fungi from Indonesia for Biodegradation of Expanded Polystyrene. Bioremediation J. 2024, 1–14. [Google Scholar] [CrossRef]
- Cedre Assessment and Comparison of Potential Impacts of Expanded and Extruded Polystyrenes (EPS/XPS) and Their Alternatives on the Marine Environment. Report of Intereg OCEANWISE Projet. Available online: https://doc.cedre.fr/index.php?lvl=author_see&id=5959 (accessed on 15 April 2024).
- Noel, C.; Cormier, A.; Leroi, L.; Durand, P. SAMBA: Standardized and Automated MetaBarcoding Analyses Workflow. WorkflowHub 2021. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Sasada, R.; Weinstein, M.; Prem, A.; Jin, M.; Bhasin, J. FIGARO: An Efficient and Objective Tool for Optimizing Microbiome rRNA Gene Trimming Parameters. J. Biomol. Tech. JBT 2020, 31, S2. [Google Scholar]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Olesen, S.W.; Duvallet, C.; Alm, E.J. dbOTU3: A New Implementation of Distribution-Based OTU Calling. PLoS ONE 2017, 12, e0176335. [Google Scholar] [CrossRef]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference Database (PR2): A Catalog of Unicellular Eukaryote Small Sub-Unit rRNA Sequences with Curated Taxonomy. Nucleic Acids Res. 2013, 41, D597–D604. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef] [PubMed]
- Odobel, C.; Dussud, C.; Philip, L.; Derippe, G.; Lauters, M.; Eyheraguibel, B.; Burgaud, G.; Ter Halle, A.; Meistertzheim, A.-L.; Bruzaud, S.; et al. Bacterial Abundance, Diversity and Activity During Long-Term Colonization of Non-Biodegradable and Biodegradable Plastics in Seawater. Front. Microbiol. 2021, 12, 734782. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.A.; Clemente, T.M.; Viviani, D.A.; Fong, A.A.; Thomas, K.A.; Kemp, P.; Karl, D.M.; White, A.E.; DeLong, E.F. Diversity and Activity of Communities Inhabiting Plastic Debris in the North Pacific Gyre. mSystems 2016, 1, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Dussud, C.; Meistertzheim, A.L.; Conan, P.; Pujo-Pay, M.; George, M.; Fabre, P.; Coudane, J.; Higgs, P.; Elineau, A.; Pedrotti, M.L.; et al. Evidence of Niche Partitioning among Bacteria Living on Plastics, Organic Particles and Surrounding Seawaters. Environ. Pollut. Barking Essex 1987 2018, 236, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Debroas, D.; Domaizon, I.; Humbert, J.-F.; Jardillier, L.; Lepère, C.; Oudart, A.; Taïb, N. Overview of Freshwater Microbial Eukaryotes Diversity: A First Analysis of Publicly Available Metabarcoding Data. FEMS Microbiol. Ecol. 2017, 93, fix023. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Maimone, G.; Rappazzo, A.C.; Dell’Acqua, O.; Laganà, P.; Azzaro, M. Microbial Biofilm Colonizing Plastic Substrates in the Ross Sea (Antarctica): First Overview of Community-Level Physiological Profiles. J. Mar. Sci. Eng. 2023, 11, 1317. [Google Scholar] [CrossRef]
- Cheng, J.; Jacquin, J.; Conan, P.; Pujo-Pay, M.; Barbe, V.; George, M.; Fabre, P.; Bruzaud, S.; Ter Halle, A.; Meistertzheim, A.-L.; et al. Relative Influence of Plastic Debris Size and Shape, Chemical Composition and Phytoplankton-Bacteria Interactions in Driving Seawater Plastisphere Abundance, Diversity and Activity. Front. Microbiol. 2021, 11, 610231. [Google Scholar] [CrossRef]
- Dodhia, M.S.; Rogers, K.L.; Fernández-Juárez, V.; Carreres-Calabuig, J.A.; Löscher, C.R.; Tisserand, A.A.; Keulen, N.; Riemann, L.; Shashoua, Y.; Posth, N.R. Microbe-Mineral Interactions in the Plastisphere: Coastal Biogeochemistry and Consequences for Degradation of Plastics. Front. Mar. Sci. 2023, 10, 1134815. [Google Scholar] [CrossRef]
- Kesy, K.; Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Spatial Environmental Heterogeneity Determines Young Biofilm Assemblages on Microplastics in Baltic Sea Mesocosms. Front. Microbiol. 2019, 10, 1665. [Google Scholar] [CrossRef]
- Ogonowski, M.; Motiei, A.; Ininbergs, K.; Hell, E.; Gerdes, Z.; Udekwu, K.I.; Bacsik, Z.; Gorokhova, E. Evidence for Selective Bacterial Community Structuring on Microplastics. Environ. Microbiol. 2018, 20, 2796–2808. [Google Scholar] [CrossRef]
- Rajeev, M.; Sushmitha, T.J.; Toleti, S.R.; Pandian, S.K. Culture Dependent and Independent Analysis and Appraisal of Early Stage Biofilm-Forming Bacterial Community Composition in the Southern Coastal Seawater of India. Sci. Total Environ. 2019, 666, 308–320. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Shyu, D.J.H.; Domínguez, R.; Lorenzo, J.M.; Pereira, J.A.M.; Câmara, J.S. Polystyrene Microplastic Particles in the Food Chain: Characteristics and Toxicity—A Review. Sci. Total Environ. 2023, 892, 164531. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Vijayakumar, R.P. Studies on Biological Degradation of Polystyrene by Pure Fungal Cultures. Environ. Dev. Sustain. 2020, 22, 4495–4508. [Google Scholar] [CrossRef]
- Oviedo-Anchundia, R.; del Castillo, D.S.; Naranjo-MorÃn, J.; Francois, N.; Ãlvarez-Barreto, J.; AlarcÃn, A.; Villafuerte, J.S.; Barcos-Arias, M. Analysis of the Degradation of Polyethylene, Polystyrene and Polyurethane Mediated by Three Filamentous Fungi Isolated from the Antarctica. Afr. J. Biotechnol. 2021, 20, 66–76. [Google Scholar] [CrossRef]
- Wu, F.; Guo, Z.; Cui, K.; Dong, D.; Yang, X.; Li, J.; Wu, Z.; Li, L.; Dai, Y.; Pan, T. Insights into Characteristics of White Rot Fungus during Environmental Plastics Adhesion and Degradation Mechanism of Plastics. J. Hazard. Mater. 2023, 448, 130878. [Google Scholar] [CrossRef]
- Sánchez, C. Fungal Potential for the Degradation of Petroleum-Based Polymers: An Overview of Macro- and Microplastics Biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.E.S.; Chaudhary, A.; Kelly, J.J.; Hoellein, T.J. Biofilm Assemblage and Activity on Plastic in Urban Streams at a Continental Scale: Site Characteristics Are More Important than Substrate Type. Sci. Total Environ. 2022, 835, 155398. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of Plastic Polymers by Fungi: A Brief Review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.W.; Kim, J.S.; Lee, W.; Park, M.S.; Lim, Y.W. Plastic-Inhabiting Fungi in Marine Environments and PCL Degradation Activity. Antonie Van Leeuwenhoek 2022, 115, 1379–1392. [Google Scholar] [CrossRef]
- Gao, R.; Liu, R.; Sun, C. A Marine Fungus Alternaria Alternata FB1 Efficiently Degrades Polyethylene. J. Hazard. Mater. 2022, 431, 128617. [Google Scholar] [CrossRef] [PubMed]
- Paço, A.; Duarte, K.; da Costa, J.P.; Santos, P.S.M.; Pereira, R.; Pereira, M.E.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Biodegradation of Polyethylene Microplastics by the Marine Fungus Zalerion Maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Vaksmaa, A.; Polerecky, L.; Dombrowski, N.; Kienhuis, M.V.M.; Posthuma, I.; Gerritse, J.; Boekhout, T.; Niemann, H. Polyethylene Degradation and Assimilation by the Marine Yeast Rhodotorula Mucilaginosa. ISME Commun. 2023, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Leitão, A.L. Potential of Penicillium species in the bioremediation field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Prabhu, G.; Prasad, M.; Mishra, M.; Chaudhary, M.; Srivastava, R. Penicillium. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Mohamed, H.; Shah, A.M.; Nazir, Y.; Naz, T.; Nosheen, S.; Song, Y. Biodegradation of Poly (Vinyl Alcohol) by an Orychophragmus Rhizosphere-Associated Fungus Penicillium Brevicompactum OVR-5, and Its Proposed PVA Biodegradation Pathway. World J. Microbiol. Biotechnol. 2021, 38, 10. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Pantelic, B.; Ponjavic, M.; Jankovic, V.; Aleksic, I.; Stevanovic, S.; Murray, J.; Fournet, M.B.; Nikodinovic-Runic, J. Upcycling Biodegradable PVA/Starch Film to a Bacterial Biopigment and Biopolymer. Polymers 2021, 13, 3692. [Google Scholar] [CrossRef] [PubMed]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef] [PubMed]
- Rämä, T.; Nordén, J.; Davey, M.L.; Mathiassen, G.H.; Spatafora, J.W.; Kauserud, H. Fungi Ahoy! Diversity on Marine Wooden Substrata in the High North. Fungal Ecol. 2014, 8, 46–58. [Google Scholar] [CrossRef]
- Anbalagan, S.; Venkatakrishnan, H.R.R.; Ravindran, J.; Sathyamoorthy, J.; Rangabashyam, K.A.; Ragini, Y.P.; Sathasivam, J.; Sureshbabu, K. Hydrolytic Degradation of Polyethylene Terephthalate by Cutinase Enzyme Derived from Fungal Biomass–Molecular Characterization. Biointerface Res. Appl. Chem. 2022, 12, 653–667. [Google Scholar] [CrossRef]
- Pangging, M.; Nguyen, T.T.T.; Lee, H.B. Seven New Records of Penicillium Species Belonging to Section Lanata-Divaricata in Korea. Mycobiology 2021, 49, 363–375. [Google Scholar] [CrossRef]
- Santacruz-Juárez, E.; Buendia-Corona, R.E.; Ramírez, R.E.; Sánchez, C. Fungal Enzymes for the Degradation of Polyethylene: Molecular Docking Simulation and Biodegradation Pathway Proposal. J. Hazard. Mater. 2021, 411, 125118. [Google Scholar] [CrossRef]
- Romero-Soto, I.C.; Martínez-Pérez, R.B.; Rodríguez, J.A.; Camacho-Ruiz, R.M.; Barbachano-Torres, A.; Martín del Campo, M.; Napoles-Armenta, J.; Pliego-Sandoval, J.E.; Concha-Guzmán, M.O.; Angeles Camacho-Ruiz, M. Galactomannans for Entrapment of Gliomastix Murorum Laccase and Their Use in Reactive Blue 2 Decolorization. Sustainability 2021, 13, 9019. [Google Scholar] [CrossRef]
- Nasrabadi, A.E.; Ramavandi, B.; Bonyadi, Z. Recent Progress in Biodegradation of Microplastics by Aspergillus Sp. in Aquatic Environments. Colloid Interface Sci. Commun. 2023, 57, 100754. [Google Scholar] [CrossRef]
- Ekanayaka, A.H.; Tibpromma, S.; Dai, D.; Xu, R.; Suwannarach, N.; Stephenson, S.L.; Dao, C.; Karunarathna, S.C. A Review of the Fungi That Degrade Plastic. J. Fungi 2022, 8, 772. [Google Scholar] [CrossRef] [PubMed]
- El-Dash, H.A.; Yousef, N.E.; Aboelazm, A.A.; Awan, Z.A.; Yahya, G.; El-Ganiny, A.M. Optimizing Eco-Friendly Degradation of Polyvinyl Chloride (PVC) Plastic Using Environmental Strains of Malassezia Species and Aspergillus Fumigatus. Int. J. Mol. Sci. 2023, 24, 15452. [Google Scholar] [CrossRef] [PubMed]
- Muhonja, C.N.; Makonde, H.; Magoma, G.; Imbuga, M. Biodegradability of Polyethylene by Bacteria and Fungi from Dandora Dumpsite Nairobi-Kenya. PLoS ONE 2018, 13, e0198446. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; Alcazar-Fuoli, L. Diseases Caused by Aspergillus Fumigatus. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-809633-8. [Google Scholar]
- Morelli, K.A.; Kerkaert, J.D.; Cramer, R.A. Aspergillus Fumigatus Biofilms: Toward Understanding How Growth as a Multicellular Network Increases Antifungal Resistance and Disease Progression. PLoS Pathog. 2021, 17, e1009794. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus Fumigatus—What Makes the Species a Ubiquitous Human Fungal Pathogen? PLoS Pathog. 2013, 9, e1003743. [Google Scholar] [CrossRef]
- Alshehrei, F. Biodegradation of Low Density Polyethylene by Fungi Isolated from Red Sea Water. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1703–1709. [Google Scholar] [CrossRef]
- Gkoutselis, G.; Rohrbach, S.; Harjes, J.; Brachmann, A.; Horn, M.A.; Rambold, G. Plastiphily Is Linked to Generic Virulence Traits of Important Human Pathogenic Fungi. Commun. Earth Environ. 2024, 5, 51. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippe, A.; Salaun, M.; Quemener, M.; Noël, C.; Tallec, K.; Lacroix, C.; Coton, E.; Burgaud, G. Colonization and Biodegradation Potential of Fungal Communities on Immersed Polystyrene vs. Biodegradable Plastics: A Time Series Study in a Marina Environment. J. Fungi 2024, 10, 428. https://doi.org/10.3390/jof10060428
Philippe A, Salaun M, Quemener M, Noël C, Tallec K, Lacroix C, Coton E, Burgaud G. Colonization and Biodegradation Potential of Fungal Communities on Immersed Polystyrene vs. Biodegradable Plastics: A Time Series Study in a Marina Environment. Journal of Fungi. 2024; 10(6):428. https://doi.org/10.3390/jof10060428
Chicago/Turabian StylePhilippe, Aurélie, Marie Salaun, Maxence Quemener, Cyril Noël, Kévin Tallec, Camille Lacroix, Emmanuel Coton, and Gaëtan Burgaud. 2024. "Colonization and Biodegradation Potential of Fungal Communities on Immersed Polystyrene vs. Biodegradable Plastics: A Time Series Study in a Marina Environment" Journal of Fungi 10, no. 6: 428. https://doi.org/10.3390/jof10060428
APA StylePhilippe, A., Salaun, M., Quemener, M., Noël, C., Tallec, K., Lacroix, C., Coton, E., & Burgaud, G. (2024). Colonization and Biodegradation Potential of Fungal Communities on Immersed Polystyrene vs. Biodegradable Plastics: A Time Series Study in a Marina Environment. Journal of Fungi, 10(6), 428. https://doi.org/10.3390/jof10060428








