Molecular Characterization and Expression Analysis of a Gene Encoding 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGR) from Bipolaris eleusines, an Ophiobolin A-Producing Fungus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Inoculum
2.2. Transcriptome Sequencing
2.3. Cloning of the Full-Length cDNA of BeHMGR by RACE
2.3.1. Confirmation of EST Amplification from B. eleusines
2.3.2. 5′ End Amplification of BeHMGR Gene
2.3.3. 3′ End Amplification of BeHMGR Gene
2.3.4. Obtaining Full-Length cDNA of BeHMGR Gene
2.4. Bioinformatics Analysis
2.5. Expression Analysis of BeHMGR Gene under Methyl Jasmonate Treatment
2.5.1. Methyl Jasmonate Treatment Procedure for B. eleusines
2.5.2. Fluorescent Quantitative PCR Detection of BeHMGR Gene Expression Level
2.5.3. Data Analysis and Statistics
3. Results and Analysis
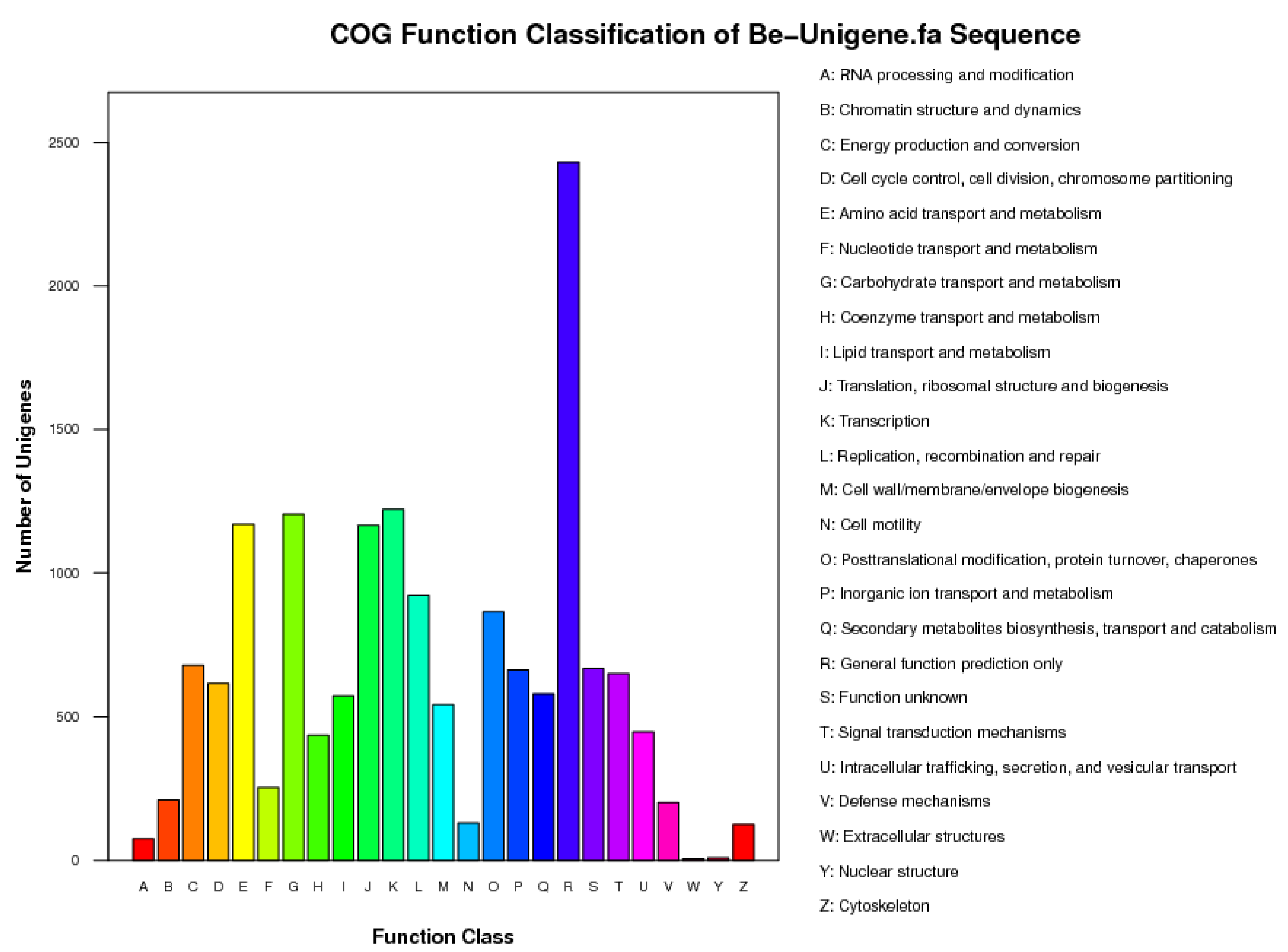
3.1. Results of Transcriptome Sequence Analysis of B. eleusines
3.2. Molecular Characterization of HMGR Gene of B. eleusines
3.3. Bioinformatics Analysis of BeHMGR Protein
3.3.1. Analysis of Physical and Chemical Properties of BeHMGR Protein
3.3.2. Conservation Analysis of BeHMGR Protein Sequence
3.3.3. Phylogenetic Analysis of BeHMGR Proteins
3.4. Effect of Methyl Jasmonate on BeHMGR Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Peng, G.; Duan, G.; Zhou, Y.; Yang, S.; Yu, L. Bipolaris eleusines, a potential mycoherbicide candidate for control of barnyardgrass (Echinochloa crus-galli). Biocontrol. Sci. Technol. 2014, 24, 839–846. [Google Scholar] [CrossRef]
- Duan, G.; Zhou, Y.; Yuan, Q.; Yu, L. Phytotoxic ophiobolins produced by Helminthosporium gramineum Rabenh, a potential bioherbicide for control of barnyard grass (Echinochloa crus-galli). Nat. Prod. Indian. J. 2007, 3, 11–17. [Google Scholar]
- Evidente, A.; Andolfi, A.; Cimmino, A.; Vurro, M.; Fracchiolla, M.; Charudattan, R. Herbicidal potential of ophiobolins produced by Drechslera gigantean. J. Agric. Food Chem. 2006, 54, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Zhang, Z.; Zhang, J.; Zhou, Y.; Yu, L.; Yuan, Q. Evaluation of crude toxin and metabolite produced by Helminthosporium gramineum Rabenh for the control of rice sheath blight in paddy field. Crop Prot. 2007, 26, 1036–1041. [Google Scholar] [CrossRef]
- Li, E.; Clark, A.M.; Rotella, D.P.; Hufford, C.D. Microbial metabolites of ophiobolin A and antimicrobial evaluation of ophiobolins. J. Nat. Prod. 1995, 58, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.; Gardiner, C.; Evidente, A.; Kiss, R.; Townley, H. Incorporation of ophiobolin A into novel chemoembolization particles for cancer cell treatment. Pharm. Res. 2014, 31, 2904–2917. [Google Scholar] [CrossRef] [PubMed]
- Chidley, C.; Trauger, S.A.; Birsoy, K.; O’Shea, E.K. The anticancer natural product ophiobolin A induces cytotoxicity by covalent modification of phosphatidylethanolamine. eLife 2016, 5, e14601. [Google Scholar] [CrossRef] [PubMed]
- Bury, M.; Novo-Uzal, E.; Andolfi, A.; Cimini, S.; Wauthoz, N.; Heffeter, P.; Lallemand, B.; Avolio, F.; Delporte, C.; Cimmino, A.; et al. Ophiobolin A, a sesterterpenoid fungal phytotoxin, displays higher in vitro growth-inhibitory effects in mammalian than in plant cells and displays in vivo antitumor activity. Int. J. Oncol. 2013, 43, 575–585. [Google Scholar] [CrossRef]
- Au, T.K.; Chick, W.S.; Leung, P.C. The biology of ophiobolins. Life Sci. 2000, 67, 733–742. [Google Scholar] [CrossRef]
- Sugawara, F.; Strobel, G.; Strange, R.N.; Siedow, J.N.; Vanduyne, G.D.; Clardyvi, J. Phytotoxins from the pathogenic fungi Drechslera maydis and Drechslera sorghicola. Proc. Natl. Acad. Sci. USA 1987, 84, 3081–3085. [Google Scholar] [CrossRef]
- Singh, S.B.; Smith, J.L.; Sabnis, G.S.; Dombrowski, A.W.; Schaeffer, J.M.; Goetz, M.A.; Bills, G.F. Structure and conformation of ophiobolinK and 6- epiophiobolinK from Aspergillus ustus as anematocidal agent. Tetrahedron 1991, 32, 6931–6938. [Google Scholar] [CrossRef]
- Sugawara, F.; Takahashi, N.; Strobel, G.; Yun, C.H.; Gray, G.; Fu, Y.; Clardy, J. Some new phytotoxic ophiobolins produced by Drechslera oryzae. J. Org. Chem. 1988, 53, 2170–2172. [Google Scholar] [CrossRef]
- Brill, Z.G.; Grover, H.K.; Maimone, T.J. Enantioselective synthesis of an ophiobolin sesterterpene via a programmed radical cascade. Science 2016, 352, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Tsuna, K.; Noguchi, N.; Nakada, M. Convergent total synthesis of (+)-ophiobolin A. Angew Chem Int Ed. 2011, 123, 9624–9627. [Google Scholar] [CrossRef]
- Thach, D.Q.; Brill, Z.G.; Grover, H.K.; Esguerra, K.V.; Thompson, J.K.; Maimone, T.J. Total synthesis of (+)-6-epi-ophiobolin A. Angew Chem. Int. Ed. 2020, 59, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, G.; Zhu, K.; Zhou, Y.; Lu, Y.; Yu, L. Screening and identification of insertion mutants from Bipolaris eleusines by mutagenesis based on restriction enzyme-mediated integration. FEMS Microbiol. Lett. 2012, 330, 90–97. [Google Scholar]
- Zhang, Z.; Burgos, N.; Zhang, J.; Yu, L. Biological control agent for rice weeds from protoplast fusion between Curvularia lunata and Helminthosporium gramineum. Weed Sci. 2007, 55, 603–609. [Google Scholar] [CrossRef]
- Shigeo, N.; Masuo, M.; Shigenobu, O.; Kyosuke, T. Biosynthesis of ophiobolins from the doubly labeled mevalonate. Tetrahedron Lett. 1968, 9, 2347–2349. [Google Scholar]
- Shigeo, N.; Masuo, M. Enzymic formation of a tricyclic sesterterpene alcohol from mevalonic acid and all-trans-geranylfarnesyl pyrophosphate. J. Chem. Soc. D. 1969, 22, 1319–1320. [Google Scholar]
- Istvan, E.S.; Deisenhofer, J. The structure of the catalytic portion of human HMG-CoA reductase. BBA-Mol. Cell. Biol. L 2000, 1529, 9–18. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Li, Z.; Kong, L.; Liu, G.; Fu, J.; Wang, A. Molecular cloning, tissue expression and protein structure prediction of the porcine 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) gene. Gene 2012, 495, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, Y.; Lou, Y.; Niu, J.; Yin, C.; Zhao, J.; Du, W.; Yue, A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase genes from Glycine max regulate plant growth and isoprenoid biosynthesis. Sci. Rep. 2023, 13, 1–14. [Google Scholar]
- Basson, M.E.; Thorsness, M.; Rine, J. Saccharomyces cerevisiae contains two functional genes encoding 3-hydroxy-3-methylglutaryl- coenzyme A reductase. Proc. Natl. Acad. Sci. USA 1986, 83, 5563–5567. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, N.; Zhong, J. Enhancement of Ganoderic Acid Accumulation by Overexpression of an N-Terminally Truncated 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Gene in the Basidiomycete Ganoderma lucidum. Appl. Environ. Microbiol. 2012, 78, 7968–7976. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Cui, G.; Zhou, S.; Zhang, X.; Huang, L. Cloning and characterization of a novel 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Salvia miltiorrhiza involved in diterpenoid tanshinone accumulation. J. Plant Physiol. 2011, 168, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Tan, Q.; Chai, Y.; Zuo, K.; Chen, M.; Gong, Y.; Wang, P.; Pi, Y.; Tan, F.; Sun, X.; et al. Cloning and characterisation of the gene encoding HMG-CoA reductase from Taxus media and its functional identification in yeast. Funct. Plant Biol. 2004, 31, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Meng, X.; Liao, Y.; Yu, T.; Cao, J.; Tan, J.; Xu, F.; Cheng, S. Characterization and functional analysis of two novel 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes (GbHMGR2 and GbHMGR3) from Ginkgo biloba. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- Condon, B.J.; Leng, Y.; Wu, D.; Bushley, K.E.; Ohm, R.A.; Otillar, R.; Martin, J.; Schackwitz, W.; Grimwood, J.; MohdZainudin, N.; et al. Comparative genome structure, secondary metabolite, and effector coding capacity across Cochliobolus pathogens. PLoS Genet. 2013, 9, e1003233. [Google Scholar] [CrossRef] [PubMed]
- Lum, P.Y.; Edwards, S.; Wright, R. Molecular, functional and evolutionary characterization of the gene encoding HMG-CoA reductase in the fission yeast, Schizosaccharomyces pombe. Yeast 1996, 12, 1107–1124. [Google Scholar] [CrossRef]
- Burmester, A.; Czempinski, K. Sequence comparison of a segment of the gene for 3-hydroxy-3-methylglutaryl-coenzyme A reductase in zygomycetes. Eur. J. Biochem. 1994, 220, 403–408. [Google Scholar] [CrossRef]
- Woitek, S.; Unkles, S.E.; Kinghorn, J.R.; Tudzynski, B. 3-Hydroxy-3-methylglutaryl-CoA reductase gene of Gibberella fujikuroi: Isolation and characterization. Curr. Genet. 1997, 31, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Croxen, R.; Goosey, M.W.; Keon, J.P.; Hargreaves, J.A. Isolation of a Ustilago maydis gene encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase and expression of a C-terminal-truncated form in Escherichia coli. Microbiology 1994, 140, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.; Li, L.; Hou, Z.; Tracy, M.; Kistler, H.C.; Xu, J. Cryptic promoter activity in the coding region of the HMG-CoA reductase gene in Fusarium graminearum. Fungal Genet. Biol. 2006, 43, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.H.; Zhu, F.; Li, N.; Ou-Yang, X.; Shi, L.; Zhao, M.W.; Li, Y.X. Cloning and characterization of a gene encoding HMG-CoA reductase from Ganoderma lucidum and its functional identification in yeast. Biosci. Biotechnol. Biochem. 2008, 72, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Dugan, R.E.; Katiyar, S.S. Evidence for catalytic site cysteine and histidine by chemical modification of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Biochem. Biophys. Res. Commun. 1986, 41, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Darnay, B.G.; Rodwell, V.W. His865 is the catalytically important histidyl residue of Syrian hamster 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 1993, 268, 8429–8435. [Google Scholar] [CrossRef] [PubMed]
- Darnay, B.G.; Wang, Y.; Rodwell, V.W. Identification of the catalytically important histidine of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 1992, 267, 15064–15070. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, K.; Rodwell, V.W. Catalysis by Syrian hamster 3-hydroxy-3-methylglutaryl-coenzyme A reductase: Proposed roles of histidine 865, glutamate 558 and aspartate 766. J. Biol. Chem. 1994, 259, 11478–11483. [Google Scholar] [CrossRef]
- Ren, A.; Qin, L.; Shi, L.; Dong, X.; Mu, D.S.; Li, Y.X.; Zhao, M.W. Methyl jasmonate induces ganoderic acid biosynthesis in the basidiomycetous fungus Ganoderma lucidum. Bioresour. Technol. 2010, 101, 6785–6790. [Google Scholar] [CrossRef]
- Zhao, M.W.; Zhong, J.Y.; Liang, W.Q.; Wang, N.; Chen, M.J.; Zhang, D.B.; Pan, Y.J.; Jong, S.C. Analysis of squalene synthase expression during the development of Ganoderma lucidum. J. Microbiol. Biotech. 2004, 14, 116–120. [Google Scholar]
- Nimsa, E.; Duboisb, C.P.; Robertsa, S.C.; Walker, E.L. Expression profiling of genes involved in paclitaxel biosynthesis for targeted metabolic engineering. Metab. Eng. 2006, 8, 385–394. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequences (5′-3′) |
|---|---|
| BeHMGR-ECT-F | CGTACCCCCGGCCCAGATGA |
| BeHMGR-ECT-R | CGCGCGAAGTTGAAGCGACG |
| BeHMGR3-1 | TCTACCTCTCGCTTCGCCAGGCTACAA |
| BeHMGR3-2 | TACCGACAAGAAGTCTGCCGCCATCAA |
| BeHMGR5-GSP1 | TGGCGTTGCTGCTCTT |
| BeHMGR5-GSP2 | AATCTCTGGCTGGGGTCTTGGC |
| BeHMGR5-GSP3 | TACGGGGCGGGGTAGGCATGTG |
| BeHMGR-FL-F | ACAATGCTAGGATCACTCGCCA |
| BeHMGR-FL-R | ACTTTCTCTATCGCTTGGGCAC |
| Actin177F | CATCAACCCCAAGTCCAACC |
| Actin177R | CCCTCGTAGATGGGGACAAC |
| HMGR158F | TGTCCCCGGAACCCCTCGCA |
| HMGR158R | GGCGTTGCTGCTCTTCCGTTG |
| Total Reads | Total Nucleotides * (nt) | Q20 Percent | N Percent | GC Percent |
|---|---|---|---|---|
| 26,555,560 | 2,390,000,400 | 92.91% | 0.00% | 50.77% |
| Nucleotide Length | 100–500 nt | 500–1000 nt | 1000–1500 nt | 1500–2000 nt | ≥2000 nt |
|---|---|---|---|---|---|
| Number | 20,075 | 7460 | 3021 | 1078 | 466 |
| Percent (%) | 62.54 | 23.24 | 9.41 | 3.36 | 1.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, K.; Tang, W.; Yang, Y.; Yu, X.; Lu, Y.; Yu, L. Molecular Characterization and Expression Analysis of a Gene Encoding 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGR) from Bipolaris eleusines, an Ophiobolin A-Producing Fungus. J. Fungi 2024, 10, 445. https://doi.org/10.3390/jof10070445
Zhang J, Yang K, Tang W, Yang Y, Yu X, Lu Y, Yu L. Molecular Characterization and Expression Analysis of a Gene Encoding 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGR) from Bipolaris eleusines, an Ophiobolin A-Producing Fungus. Journal of Fungi. 2024; 10(7):445. https://doi.org/10.3390/jof10070445
Chicago/Turabian StyleZhang, Jianping, Ke Yang, Wei Tang, Yongjie Yang, Xiaoyue Yu, Yongliang Lu, and Liuqing Yu. 2024. "Molecular Characterization and Expression Analysis of a Gene Encoding 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGR) from Bipolaris eleusines, an Ophiobolin A-Producing Fungus" Journal of Fungi 10, no. 7: 445. https://doi.org/10.3390/jof10070445






