MyC Factor Analogue CO5 Promotes the Growth of Lotus japonicus and Enhances Stress Resistance by Activating the Expression of Relevant Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Establishment of a Hydroponic System
2.2. Arbuscular Mycorrhizal Fungi and Mycorrhiza Factor Analogues
2.3. Plant Experiment Design
2.4. The Determination of Some Physiological and Biochemical Indexes
2.5. Trypan Blue Staining
2.6. RNA Sequencing and Differentially Expressed Gene Analysis
2.7. Statistical Analysis
3. Results
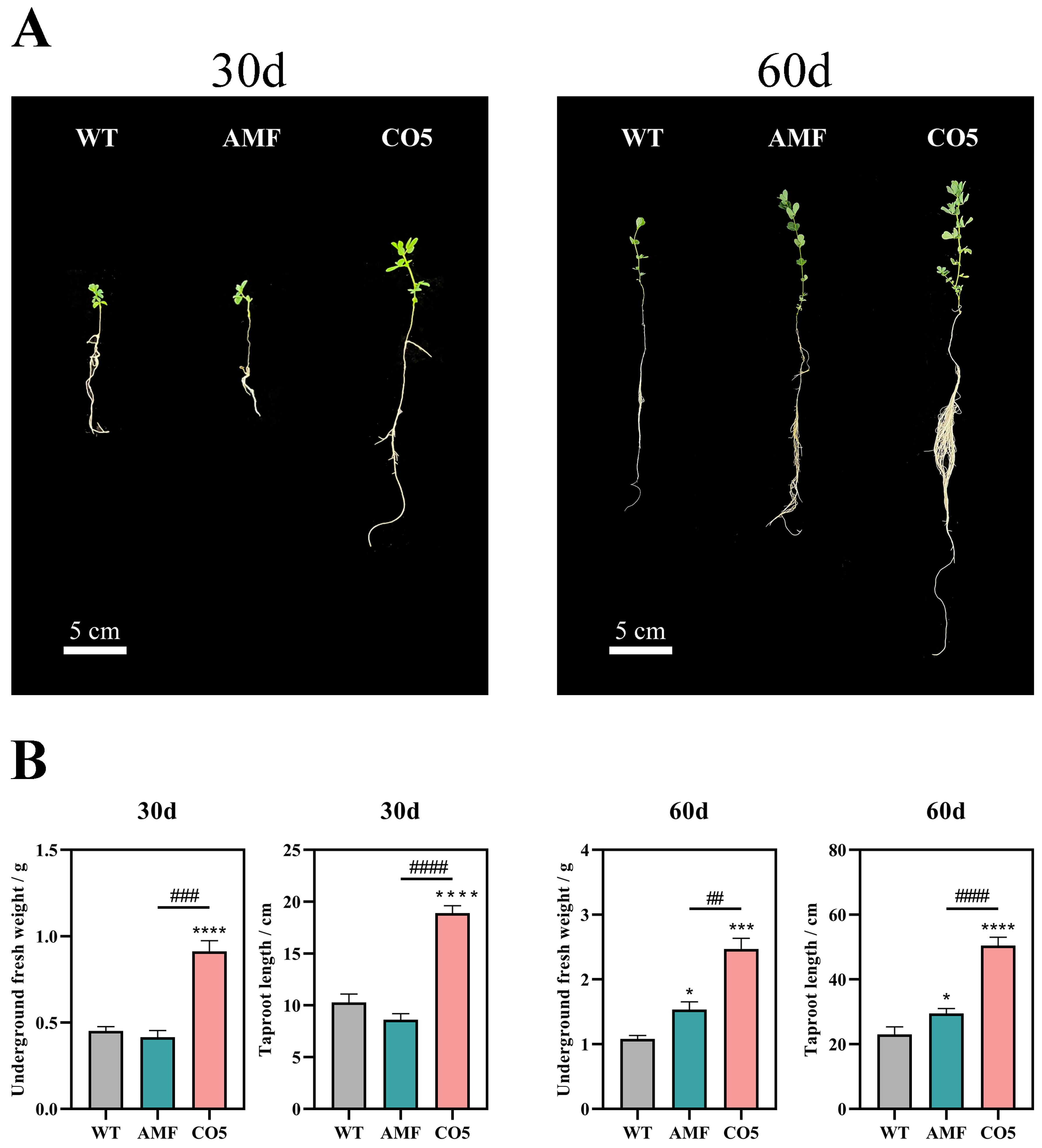
3.1. Analysis of Growth-Promoting Effects of CO5 on Lotus japonicus
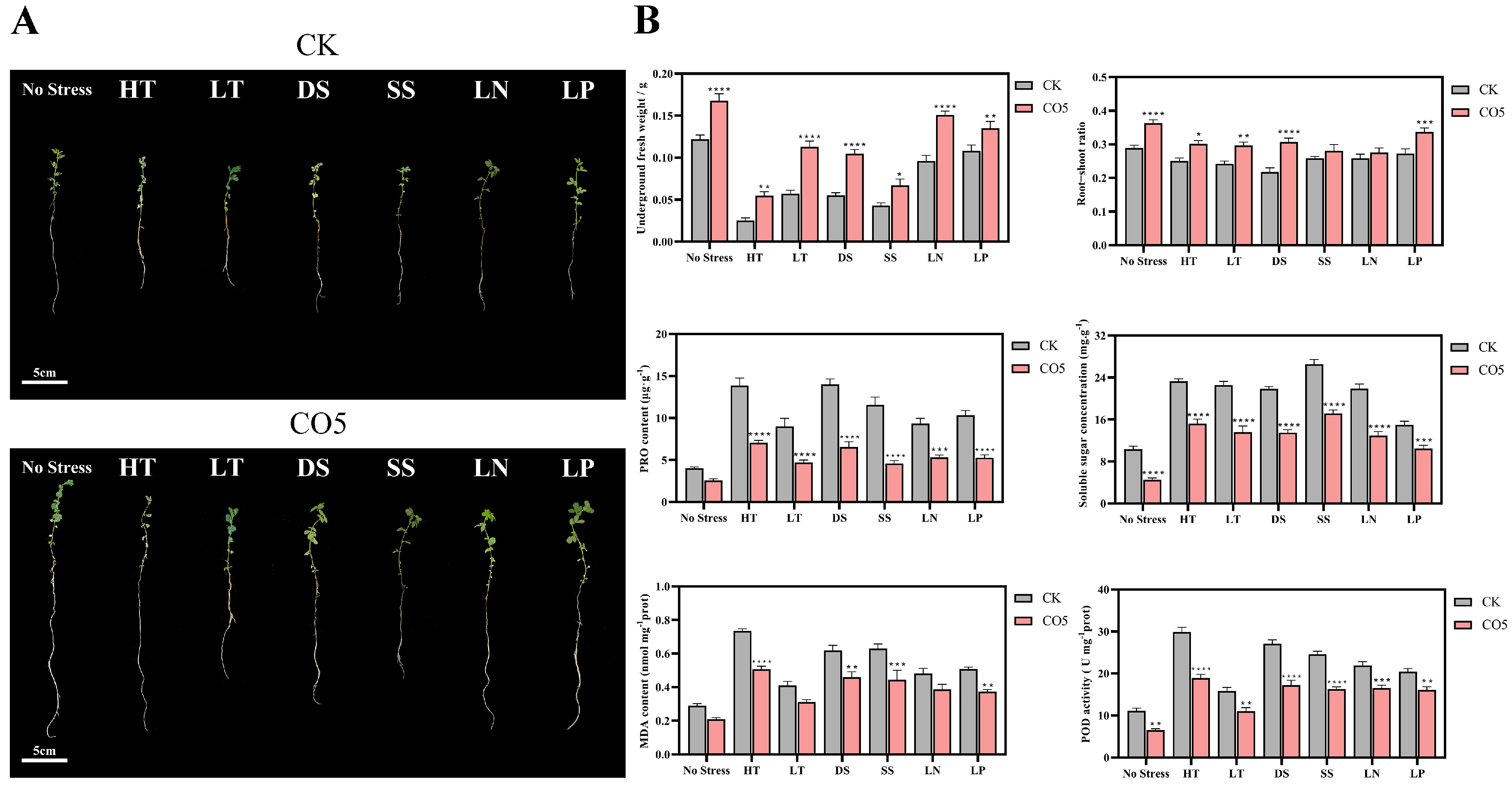
3.2. Analysis of Stress Resistance Enhancement Effects of CO5 on Lotus japonicus
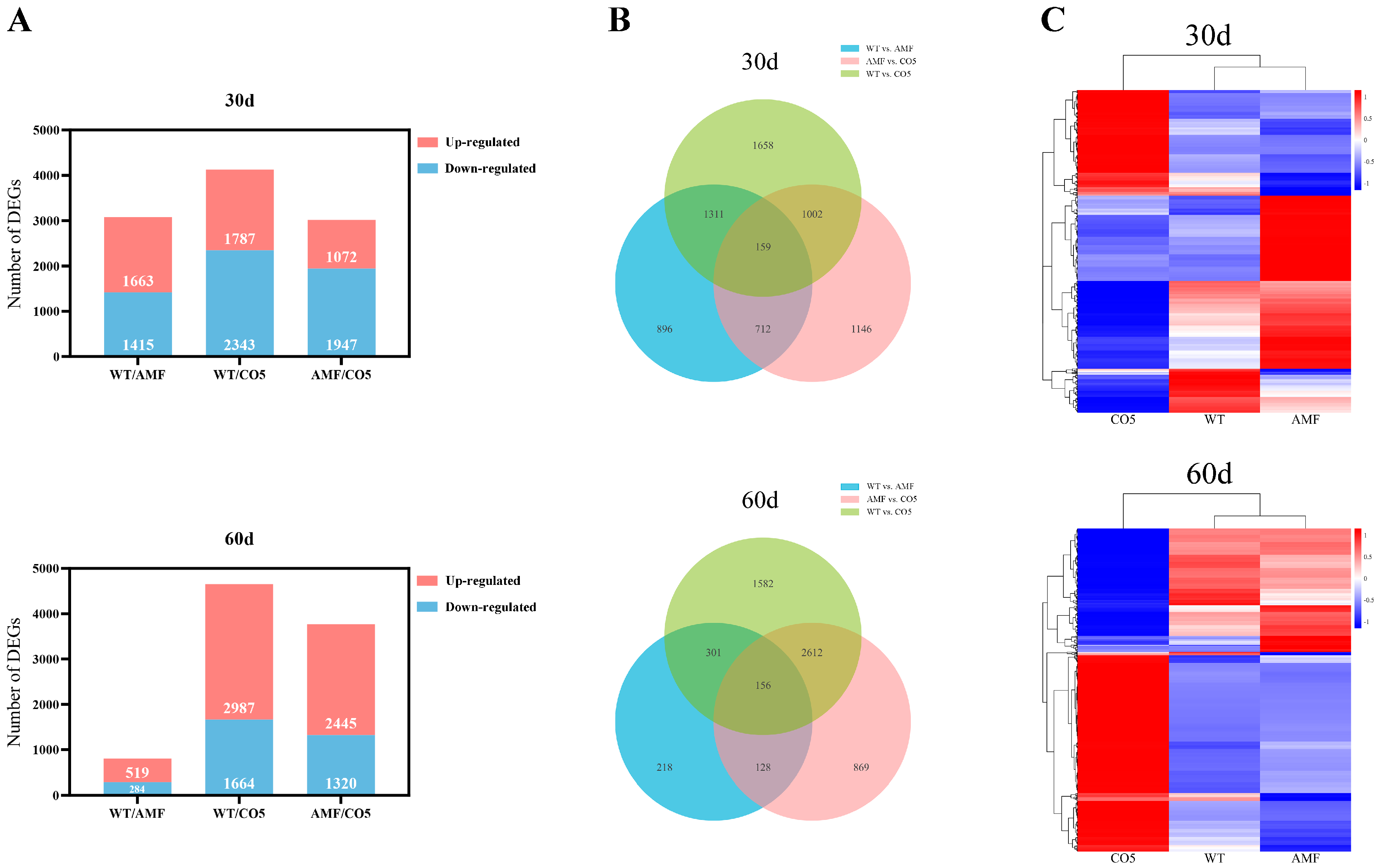
3.3. Dynamic Changes and Comparative Analysis of DEGs
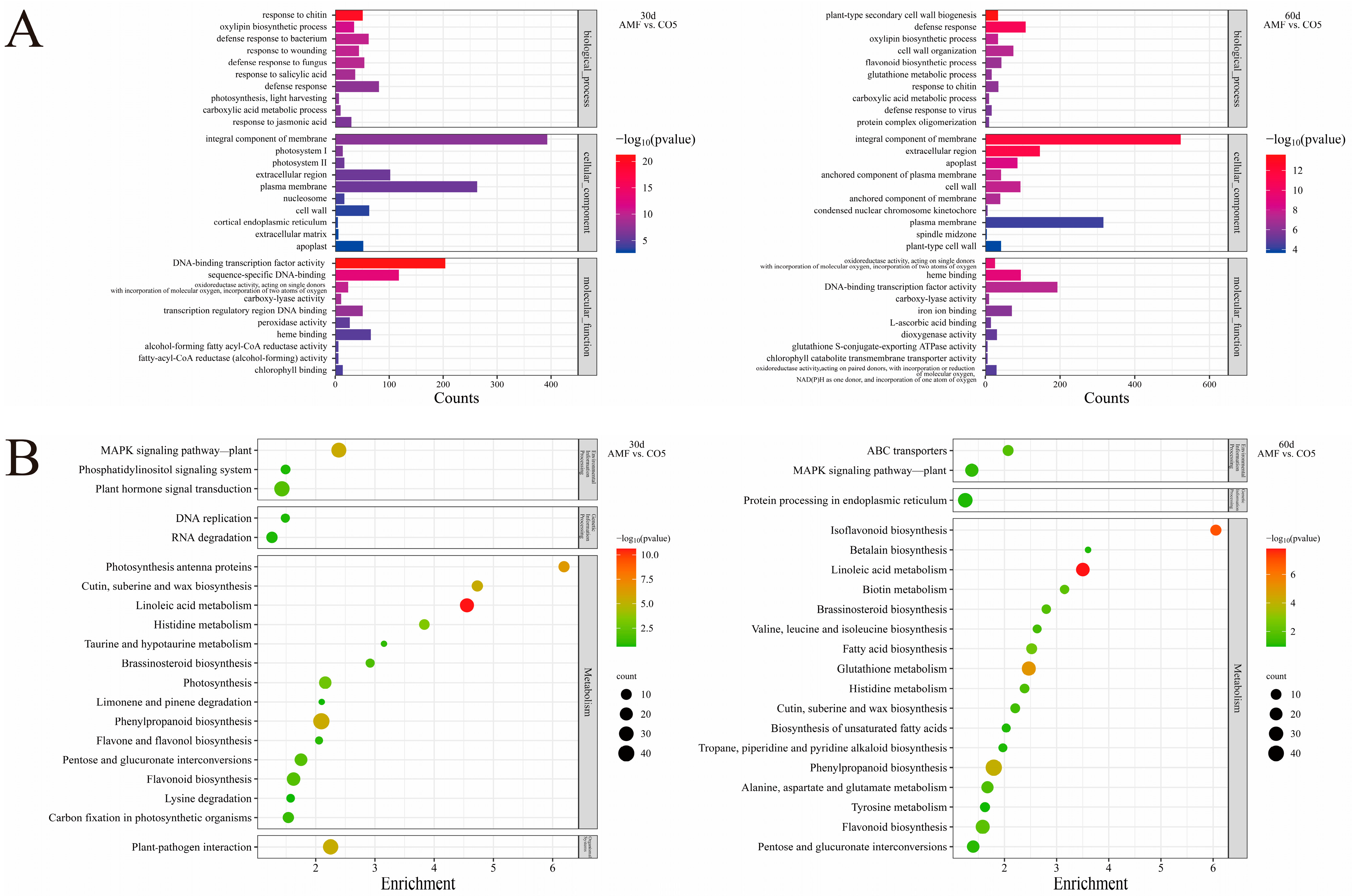
3.4. Gene Ontology Classification and Pathway Enrichment Analysis of DEGs
3.5. CO5 Activates the Symbiotic Pathway, Nitrogen and Phosphorus Absorption, and Disease-Resistant and Stress-Related Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Zhang, Z.; Liu, P.; Zhou, L.; Tan, S.; Kuang, S. Arbuscular Mycorrhizal Fungi Diversity in Sophora japonica Rhizosphere at Different Altitudes and Lithologies. J. Fungi 2024, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Limpens, E.; Geurts, R. Transcriptional Regulation of Nutrient Exchange in Arbuscular Mycorrhizal Symbiosis. Mol. Plant 2018, 11, 1421–1423. [Google Scholar] [CrossRef] [PubMed]
- Barto, E.K.; Weidenhamer, J.D.; Cipollini, D.; Rillig, M.C. Fungal superhighways: Do common mycorrhizal networks enhance below ground communication? Trends Plant Sci. 2012, 17, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Terrer, C.; Vicca, S.; Hungate, B.A.; Phillips, R.P.; Prentice, I.C. Mycorrhizal association as a primary control of the CO(2) fertilization effect. Science 2016, 353, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, L.B.; De Deyn, G.B.; Pugnaire, F.I.; Kothamasi, D.; van der Heijden, M.G.A. Symbiotic soil fungi enhance ecosystem resilience to climate change. Glob. Chang. Biol. 2017, 23, 5228–5236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, Y.; Jiang, H.; Jiang, C.; Du, Y.; Gong, C.; Wang, W.; Zhu, S.; Han, G.; Cheng, B. Systematic Identification, Evolution and Expression Analysis of the Zea mays PHT1 Gene Family Reveals Several New Members Involved in Root Colonization by Arbuscular Mycorrhizal Fungi. Int. J. Mol. Sci. 2016, 17, 930. [Google Scholar] [CrossRef] [PubMed]
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 233. [Google Scholar] [CrossRef]
- Chen, A.; Gu, M.; Wang, S.; Chen, J.; Xu, G. Transport properties and regulatory roles of nitrogen in arbuscular mycorrhizal symbiosis. Semin. Cell Dev. Biol. 2018, 74, 80–88. [Google Scholar] [CrossRef]
- Cheng, X.F.; Wu, H.H.; Zou, Y.N.; Wu, Q.S.; Kuca, K. Mycorrhizal response strategies of trifoliate orange under well-watered, salt stress, and waterlogging stress by regulating leaf aquaporin expression. Plant Physiol. Biochem. 2021, 162, 27–35. [Google Scholar] [CrossRef]
- Seneviratne, M.; Rajakaruna, N.; Rizwan, M.; Madawala, H.; Ok, Y.S.; Vithanage, M. Heavy metal-induced oxidative stress on seed germination and seedling development: A critical review. Environ. Geochem. Health 2019, 41, 1813–1831. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Wu, Q.S.; Kuca, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23 (Suppl. 1), 50–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kong, X.; Long, Y.; Liu, S.; Zhang, H.; Jia, J.; Cui, W.; Zhang, Z.; Song, X.; Qiu, L.; et al. Integrated single-nucleus and spatial transcriptomics captures transitional states in soybean nodule maturation. Nat. Plants 2023, 9, 515–524. [Google Scholar] [CrossRef]
- Delaux, P.M.; Schornack, S. Plant evolution driven by interactions with symbiotic and pathogenic microbes. Science 2021, 371, eaba6605. [Google Scholar] [CrossRef]
- Maillet, F.; Poinsot, V.; Andre, O.; Puech-Pages, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formey, D.; Niebel, A.; et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 2011, 469, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.J.; Dominguez-Nunez, J.A.; Aranaz, I.; Poza-Carrion, C.; Ramonell, K.; Somerville, S.; Berrocal-Lobo, M. Short-Chain Chitin Oligomers: Promoters of Plant Growth. Mar. Drugs 2017, 15, 40. [Google Scholar] [CrossRef]
- Genre, A.; Chabaud, M.; Balzergue, C.; Puech-Pages, V.; Novero, M.; Rey, T.; Fournier, J.; Rochange, S.; Becard, G.; Bonfante, P.; et al. Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytol. 2013, 198, 190–202. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, F.; Han, G.; Wang, W.; Zhu, S.; Li, X. Improvement of Lotus japonicus hairy root induction and development of a mycorrhizal symbiosis system. Appl. Plant Sci. 2018, 6, e1141. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, F.; Wu, F.; Zhao, M.; Zou, R.; Wu, J.; Li, X. A novel SCARECROW-LIKE3 transcription factor LjGRAS36 in Lotus japonicus regulates the development of arbuscular mycorrhizal symbiosis. Physiol. Mol. Biol. Plants 2022, 28, 573–583. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, W.; Wu, J.; Xie, K.; Li, X. LjAMT2;2 Promotes Ammonium Nitrogen Transport during Arbuscular Mycorrhizal Fungi Symbiosis in Lotus japonicus. Int. J. Mol. Sci. 2022, 23, 9522. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, M.; Genre, A.; Sieberer, B.J.; Faccio, A.; Fournier, J.; Novero, M.; Barker, D.G.; Bonfante, P. Arbuscular mycorrhizal hyphopodia and germinated spore exudates trigger Ca2+ spiking in the legume and nonlegume root epidermis. New Phytol. 2011, 189, 347–355. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Volpe, V.; Dell’Aglio, E.; Giovannetti, M.; Ruberti, C.; Costa, A.; Genre, A.; Guether, M.; Bonfante, P. An AM-induced, MYB-family gene of Lotus japonicus (LjMAMI) affects root growth in an AM-independent manner. Plant J. 2013, 73, 442–455. [Google Scholar] [CrossRef]
- Kosuta, S.; Chabaud, M.; Lougnon, G.; Gough, C.; Denarie, J.; Barker, D.G.; Becard, G. A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiol. 2003, 131, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Shamsy, R.; Liu, A.; Chen, S. Arbuscular mycorrhizal fungi-induced tolerance to chromium stress in plants. Environ. Pollut. 2023, 327, 121597. [Google Scholar] [CrossRef]
- Begum, N.; Xiao, Y.; Wang, L.; Li, D.; Irshad, A.; Zhao, T. Arbuscular mycorrhizal fungus Rhizophagus irregularis alleviates drought stress in soybean with overexpressing the GmSPL9d gene by promoting photosynthetic apparatus and regulating the antioxidant system. Microbiol. Res. 2023, 273, 127398. [Google Scholar] [CrossRef]
- Han, Y.; Lou, X.; Zhang, W.; Xu, T.; Tang, M. Arbuscular Mycorrhizal Fungi Enhanced Drought Resistance of Populus cathayana by Regulating the 14-3-3 Family Protein Genes. Microbiol. Spectr. 2022, 10, e0245621. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, H.; Lu, G.; Zhou, X.; Wang, J.; Li, J.; Zheng, K.; Fan, Y.; Zhou, H.; Wang, J.; et al. Non-targeted metabolomics analysis reveals the mechanism of arbuscular mycorrhizal symbiosis regulating the cold-resistance of Elymus nutans. Front. Microbiol. 2023, 14, 1134585. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The central role of transcription factors in bridging biotic and abiotic stress responses for plants’ resilience. New Crops 2024, 1, 100005. [Google Scholar] [CrossRef]
- Khokhani, D.; Carrera Carriel, C.; Vayla, S.; Irving, T.B.; Stonoha-Arther, C.; Keller, N.P.; Ane, J.M. Deciphering the Chitin Code in Plant Symbiosis, Defense, and Microbial Networks. Annu. Rev. Microbiol. 2021, 75, 583–607. [Google Scholar] [CrossRef]
- Vit, O.; Petrak, J. Integral membrane proteins in proteomics. How to break open the black box? J. Proteom. 2017, 153, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Mattison, C.P.; Rai, R.; Settlage, R.E.; Hinchliffe, D.J.; Madison, C.; Bland, J.M.; Brashear, S.; Graham, C.J.; Tarver, M.R.; Florane, C.; et al. RNA-Seq Analysis of Developing Pecan (Carya illinoinensis) Embryos Reveals Parallel Expression Patterns among Allergen and Lipid Metabolism Genes. J. Agric. Food Chem. 2017, 65, 1443–1455. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Sun, J.; Li, B.; Zhu, Q.; Chen, S.; Zhang, H. Arabidopsis fatty acid desaturase FAD2 is required for salt tolerance during seed germination and early seedling growth. PLoS ONE 2012, 7, e30355. [Google Scholar] [CrossRef]
- Gobbato, E.; Wang, E.; Higgins, G.; Bano, S.A.; Henry, C.; Schultze, M.; Oldroyd, G.E. RAM1 and RAM2 function and expression during arbuscular mycorrhizal symbiosis and Aphanomyces euteiches colonization. Plant Signal. Behav. 2013, 8, e26049. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Summers, W.; Paszkowski, U. Mechanisms Underlying Establishment of Arbuscular Mycorrhizal Symbioses. Annu. Rev. Phytopathol. 2018, 56, 135–160. [Google Scholar] [CrossRef]
- Pimprikar, P.; Carbonnel, S.; Paries, M.; Katzer, K.; Klingl, V.; Bohmer, M.J.; Karl, L.; Floss, D.S.; Harrison, M.J.; Parniske, M.; et al. A CCaMK-CYCLOPS-DELLA Complex Activates Transcription of RAM1 to Regulate Arbuscule Branching. Curr. Biol. 2016, 26, 1126. [Google Scholar] [CrossRef]
- Yu, N.; Luo, D.; Zhang, X.; Liu, J.; Wang, W.; Jin, Y.; Dong, W.; Liu, J.; Liu, H.; Yang, W.; et al. A DELLA protein complex controls the arbuscular mycorrhizal symbiosis in plants. Cell Res. 2014, 24, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jing, J.L.; Liu, L.; He, Y. ZmRAD17 Is Required for Accurate Double-Strand Break Repair During Maize Male Meiosis. Front. Plant Sci. 2021, 12, 626528. [Google Scholar] [CrossRef] [PubMed]
- Searle, J.S.; Wood, M.D.; Kaur, M.; Tobin, D.V.; Sanchez, Y. Proteins in the nutrient-sensing and DNA damage checkpoint pathways cooperate to restrain mitotic progression following DNA damage. PLoS Genet. 2011, 7, e1002176. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Rich, M.K.; Yoda, A.; Shimazaki, S.; Xie, X.; Akiyama, K.; Mizuno, Y.; Komatsu, A.; Luo, Y.; Suzuki, H.; et al. An ancestral function of strigolactones as symbiotic rhizosphere signals. Nat. Commun. 2022, 13, 3974. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Smith, S.M.; Huang, J. Origins of strigolactone and karrikin signaling in plants. Trends Plant Sci. 2022, 27, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Sun, J.; Albinsky, D.; Zarrabian, D.; Hull, R.; Lee, T.; Jarratt-Barnham, E.; Chiu, C.H.; Jacobsen, A.; Soumpourou, E.; et al. Nutrient regulation of lipochitooligosaccharide recognition in plants via NSP1 and NSP2. Nat. Commun. 2022, 13, 6421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Luo, W.; Li, R.; He, Q.; Fang, X.; Michele, R.D.; Ast, C.; von Wiren, N.; Lin, J. Single-particle analysis reveals shutoff control of the Arabidopsis ammonium transporter AMT1;3 by clustering and internalization. Proc. Natl. Acad. Sci. USA 2013, 110, 13204–13209. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, N.; Courty, P.E.; Brule, D.; Kunze, R. Identification of arbuscular mycorrhiza-inducible Nitrate Transporter 1/Peptide Transporter Family (NPF) genes in rice. Mycorrhiza 2018, 28, 93–100. [Google Scholar] [CrossRef]
- Han, C.; Wang, H.; Shi, W.; Bai, M. The molecular associations between the SnRK1 complex and carbon/nitrogen metabolism in plants. New Crops 2024, 1, 100008. [Google Scholar] [CrossRef]
- Ye, L.; Yang, P.; Zeng, Y.; Li, C.; Jian, N.; Wang, R.; Huang, S.; Yang, R.; Wei, L.; Zhao, H.; et al. Rhizobium symbiosis modulates the accumulation of arsenic in Medicago truncatula via nitrogen and NRT3.1-like genes regulated by ABA and linalool. J. Hazard. Mater. 2021, 415, 125611. [Google Scholar] [CrossRef]
- Fan, C.; Wang, X.; Hu, R.; Wang, Y.; Xiao, C.; Jiang, Y.; Zhang, X.; Zheng, C.; Fu, Y.F. The pattern of Phosphate transporter 1 genes evolutionary divergence in Glycine max L. BMC Plant Biol. 2013, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Gronlund, M.; Jakobsen, I.; Grotemeyer, M.S.; Rentsch, D.; Miyao, A.; Hirochika, H.; Kumar, C.S.; Sundaresan, V.; Salamin, N.; et al. Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the phosphate transporter1 gene family. Plant Cell 2012, 24, 4236–4251. [Google Scholar] [CrossRef] [PubMed]
- Fakher, B.; Ashraf, M.A.; Wang, L.; Wang, X.; Zheng, P.; Aslam, M.; Qin, Y. Pineapple SWEET10 is a glucose transporter. Hortic. Res. 2023, 10, uhad175. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, R.; Ping, W.; Sun, J.; Cain, M.; Li, X.; Li, L. AtQQS orphan gene and NtNF-YC4 boost protein accumulation and pest resistance in tobacco (Nicotiana tabacum). Plant Sci. 2022, 317, 111198. [Google Scholar] [CrossRef] [PubMed]
- Leng, H.; Jiang, C.; Song, X.; Lu, M.; Wan, X. Poplar aquaporin PIP1;1 promotes Arabidopsis growth and development. BMC Plant Biol. 2021, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Mishra, A. Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root-architecture, and photosynthesis. Physiol. Plant. 2021, 172, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, C.; Dai, H.; Liu, H.; Zhang, X.; Yang, J.; Chen, X.; Zhu, Y.; Wang, D.; Qi, X.; et al. A LysM Receptor Heteromer Mediates Perception of Arbuscular Mycorrhizal Symbiotic Signal in Rice. Mol. Plant 2019, 12, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Kun, Y.; Zhang, H.; Yu, C.; Luo, N.; Yan, J.; Zheng, S.; Hu, Q.; Zhang, D.; Kou, L.; Meng, X.; et al. Low phosphorus promotes NSP1-NSP2 heterodimerization to enhance strigolactone biosynthesis and regulate shoot and root architecture in rice. Mol. Plant 2023, 16, 1811–1831. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, X.; Bao, X.; Zhu, X. In the symbiosome: Cross-kingdom dating under the moonlight. New Crops 2024, 1, 100015. [Google Scholar] [CrossRef]

| Gene Symbol | Description |
|---|---|
| NSP1 | Nitrile-specifier protein |
| NSP2 | Protein Nodulation Signaling Pathway |
| NIN3 | Neutral/alkaline invertase, chloroplastic |
| RAM1 | GRAS family protein |
| RAD17 | Cell cycle checkpoint protein |
| RAD23B | Ubiquitin receptor |
| RAD1 | Cell cycle checkpoint protein |
| RAD5A | DNA repair protein |
| RAD52-2 | DNA repair RAD52-like protein 2, chloroplastic |
| RAD5B | DNA repair protein |
| MYB1 | Transcription factor |
| MYB2 | |
| MYB11 | |
| MYB17 | |
| RMS3 | Strigolactone esterase |
| DELLA1 | DELLA protein 1 |
| DELLA2 | |
| DAD1 | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit |
| CCD7 | Carotenoid cleavage dioxygenase, chloroplastic |
| CCD8 |
| Related Signal Pathway | Gene Symbol | Description |
|---|---|---|
| Phosphorus absorption | PHT1;1 | Inorganic phosphate transporter |
| PHT2 | ||
| PTS1 | Pterocarpan synthase | |
| PT1 | Low-affinity inorganic phosphate transporter | |
| PHR2 | Blue-light photoreceptor | |
| HAM1 | Histone acetyltransferase of the MYST family | |
| SHR | Protein SHORT-ROOT | |
| PAP2 | Poly(A) RNA polymerase protein | |
| SPS3 | Solanesyl diphosphate synthase, chloroplastic/mitochondrial | |
| PHO1 | Alpha-1,4 glucan phosphorylase L isozyme, chloroplastic/amyloplastic | |
| Nitrogen absorption | NLP3 | Omega-amidase, chloroplastic |
| NLP7 | Omega-amidase, chloroplastic | |
| NRT3.1 | High-affinity nitrate transporter | |
| SPX4 | SPX domain-containing protein | |
| CHL | Chloroplastic lipocalin | |
| ZFP1 | Zinc finger protein | |
| ZFP3 | ||
| NPF6.3 | Protein NRT1/PTR FAMILY | |
| NPF8.3 | ||
| NPF3.1 | ||
| Disease resistance | AMT1;3 | Ammonium transporter |
| AMT2 | ||
| CWINV3 | Beta-fructofuranosidase, insoluble isoenzyme | |
| FBL3 | Putative F-box/LRR-repeat protein | |
| FBL8 | ||
| SAG20 | Senescence-associated gene | |
| SWEET5 | Protein SWEETIE | |
| SWEET10 | ||
| Stress resistance | AQP1 | Probable aquaporin TIP-type |
| bHLH110 | Transcription factor | |
| TIP1;3 | Aquaporin | |
| HVA22 | Protein | |
| P5CS | Gamma-glutamyl phosphate reductase | |
| LEA14-A | Late embryogenesis abundant protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Jiang, J.; Zhou, J.; Chen, J.; Cheng, B.; Li, X. MyC Factor Analogue CO5 Promotes the Growth of Lotus japonicus and Enhances Stress Resistance by Activating the Expression of Relevant Genes. J. Fungi 2024, 10, 458. https://doi.org/10.3390/jof10070458
Luo X, Jiang J, Zhou J, Chen J, Cheng B, Li X. MyC Factor Analogue CO5 Promotes the Growth of Lotus japonicus and Enhances Stress Resistance by Activating the Expression of Relevant Genes. Journal of Fungi. 2024; 10(7):458. https://doi.org/10.3390/jof10070458
Chicago/Turabian StyleLuo, Xinhao, Jiaqing Jiang, Jing Zhou, Jin Chen, Beijiu Cheng, and Xiaoyu Li. 2024. "MyC Factor Analogue CO5 Promotes the Growth of Lotus japonicus and Enhances Stress Resistance by Activating the Expression of Relevant Genes" Journal of Fungi 10, no. 7: 458. https://doi.org/10.3390/jof10070458
APA StyleLuo, X., Jiang, J., Zhou, J., Chen, J., Cheng, B., & Li, X. (2024). MyC Factor Analogue CO5 Promotes the Growth of Lotus japonicus and Enhances Stress Resistance by Activating the Expression of Relevant Genes. Journal of Fungi, 10(7), 458. https://doi.org/10.3390/jof10070458





