Abstract
Davidsoniella virescens is so far only known in North America. However, recently in southern Poland, blackish growth consisting of fungal mycelia and sporulation structures was found on the wood of Fagus sylvatica. As a result of isolation, 17 cultures of this fungus were obtained. All cultures produced an intense sweet odor. This fungus, both in situ and in vitro, abundantly produced perithecia with long necks and asexual stage. Particularly characteristic was the production of variable endoconidia in two types of phialophores differing mainly in the width of the collarette. The nucleotide sequences for five gene fragments of representative cultures were used in phylogenetic analyses: 18S; the internal transcribed spacer regions ITS1 and ITS2, including the 5.8S gene (ITS); 28S region of the ribosomal RNA (rRNA), β-tubulin 2 (TUB2) and translation elongation factor 1-α (TEF1). Based on morphological and phylogenetic analyses, the fungus on European beech in Poland was identified as Davidsoniella virescens. The optimal temperature for radial colony growth was 20 °C. However, the differences between colony diameter at 25 °C compared to that at the optimal temperature were not statistically significant. Six D. virescens isolates were used for pathogenicity assay. They were inoculated into wounds on stems of two-year-old seedlings of Fagus sylvatica and Acer saccharum (36 seedlings of each tree species). Final evaluation was performed 4 months after inoculation. No external symptoms were observed in any A. saccharum seedling, neither in the crown nor on the stem. However, 13.9% of F. sylvatica seedlings showed wilting symptoms throughout the entire crown within 3–6 weeks after inoculation. Moreover, after 4 months on the stems of 30.6% beech seedlings, necrotic lesions with a length of 1.3 to 7.2 cm were formed, without any symptoms of wilting. The most noticeable internal symptom was the discoloration of the wood, which was observed in all inoculated seedlings of both tree species. All D. virescens isolates caused greater wood discoloration in F. sylvatica than in A. saccharum. Most of the differences found in the extent of discoloration between host plants were statistically significant. The discoloration caused by all D. virescens isolates in F. sylvatica was significantly greater than in the control. However, none of the isolates tested on A. saccharum caused significantly greater wood discoloration compared to the control. Pathogenicity tests showed that the D. virescens isolates identified in southern Poland may pose a greater threat to native European beech than to foreign sugar maple.
1. Introduction
Davidsoniella is a recently introduced new genus, which is a result of a revision in the family Ceratocystidaceae [1]. There are currently four species included in this genus, and Davidsoniella virescens (R.W. Davidson) Z.W. de Beer, T.A. Duong & M.J. Wingf. is designated as a type species [1,2]. Taxonomic affiliation and nomenclature of this species have undergone numerous changes [3]. It was originally described in the United States on hardwood logs and lumber as Endoconidiophora virescens by Davidson [4]. The author observed that this fungus possesses growth and morphological features that are very similar to those of Endoconidiophora coerulescens Münch, which was described much earlier in Europe [5]. However, he also pointed out some morphological and ecological differences between the two species [4]. Subsequently, both of these species were transferred to Ceratocystis, respectively, as C. virescens (R.W. Davidson) C. Moreau and C. coerulescens (Münch) Bakshi [6,7,8]. However, Hunt [9], in his monographic study, assigned C. virescens as synonym, under the name C. coerulescens. On the other hand, the author noted that C. coerulescens is an exceedingly variable species and recommended that the population on hardwoods should be treated separately, at least as a variety or form [9].
In three subsequent mycological monographs C. virescens has not been listed as a separate taxon, but considered synonymous to C. coerulescens [10,11,12]. However, further research began to provide evidence of differences between these species and the need to treat them as separate species. Re-examination of Davidson’s authentic material indicated that C. coerulescens and C. virescens manifest morphological differences in their conidial states [13,14]. Each of these fungal species produces different volatile metabolites, which is reflected in the different odor of cultures [15,16,17]. Isozyme analysis and molecular comparison also clearly separate these two species of fungi [18,19,20,21]. While C. virescens was transferred to Davidsoniella in the last taxonomic revision, the fungus C. coerulescens was placed in the genus Endoconidiophora [1].
Significant differences between both species of fungi also concern the distribution, the host plant spectrum and the ecological role in forest stands. E. coerulescens occurs in Europe, North America, and Asia, where it causes sapstain on many conifers [5,7,11,22]. D. virescens has so far been recorded only in the eastern areas of North America and in Canada [4,10,23,24]. It is reported mainly as a saprotroph on the logs of various hardwood species [4,25,26]. D. virescens also occurs as a pathogen, which causes sapstreak disease on Acer saccharum Marsh., and in scattered locations on Liriodendron tulipifera L. [23,26,27,28,29]. D. virescens has also been found sporadically on A. rubrum L., A. saccharinum L., and Betula alleghaniensis Britton, but the disease in this species is little known [24,25,30]. The most characteristic symptom of sapstreak disease is the distinctive stain of internal wood in the roots and at the base of the stem. Stained wood is greenish yellow with reddish streaks and appears watersoaked. In cross-section, the stain streaks have a radial arrangement and are surrounded by a dark-green zone [23,25,27]. The pH of A. saccharum wood is normally around 5.5, while wood in the watersoaked areas had pH of 8.5 or higher [27]. The stain significantly lowers the commercial value of affected lumber [23,31]. These symptoms inside the trunk are accompanied by the development of small leaves, as well as gradual branch dieback. In case of symptom progression, this lead to death of the tree within a few years [23,27,29,32,33,34].
D. virescens is able to survive and sporulate on air-dried boards for several months [23]. This indicates that it is possible to introduce the fungus together with wood or wood products. There are so far no reports of D. virescens from regions outside North America [24,26,35]. A. saccharum does not occur in European forests, but there are other maple species, A. campestre L., A. platanoides L. and A. pseudoplatanus L., that could be potentially affected by D. virescens. However, there is no convincing evidence that D. virescens poses a real threat to Acer species other than A. saccharum [24]. This results in different assessments of phytosanitary risk and quarantine regulations [30,36]. D. virescens has not been considered to be a quarantine pest by the European and Mediterranean Plant Protection Organization [35]. However, this species is listed in Annex II as a Union quarantine pest not known to occur in the EU territory [24]. Import restrictions into the EU mainly apply to wood of A. saccharum from Canada and the United States, but there are also other restrictions, and various phytosanitary certificates are required [24]. In this context, it should be noted that, so far, it is not certain whether the saprotrophic forms occurring on various hardwoods in the USA are the same species as the causal factor of sapstreak diseases on sugar maple trees [24]. A draft nuclear genome sequence was developed for the D. virescens isolate originated from A. saccharum, which should allow for some comparative studies with other isolates [2].
Recently, during research in southern Poland, an accumulation of mycelium and spore-forming structures of the fungus of the Davidsoniella genus was found on the cross sections of logs and branches of Fagus sylvatica. Research was carried out in order to (i) characterize this fungus in vivo and in vitro, and its identification based on morphology and phylogenetic analysis, (ii) determine the growth rate of its colonies depending on temperature, and (iii) determine pathogenicity towards two plant species, F. sylvatica and A. saccharum. On this basis, an attempt was made to assess whether D. virescens may pose a threat for European forests.
2. Materials and Methods
2.1. Study Material, Isolation and Microscopic Analyses
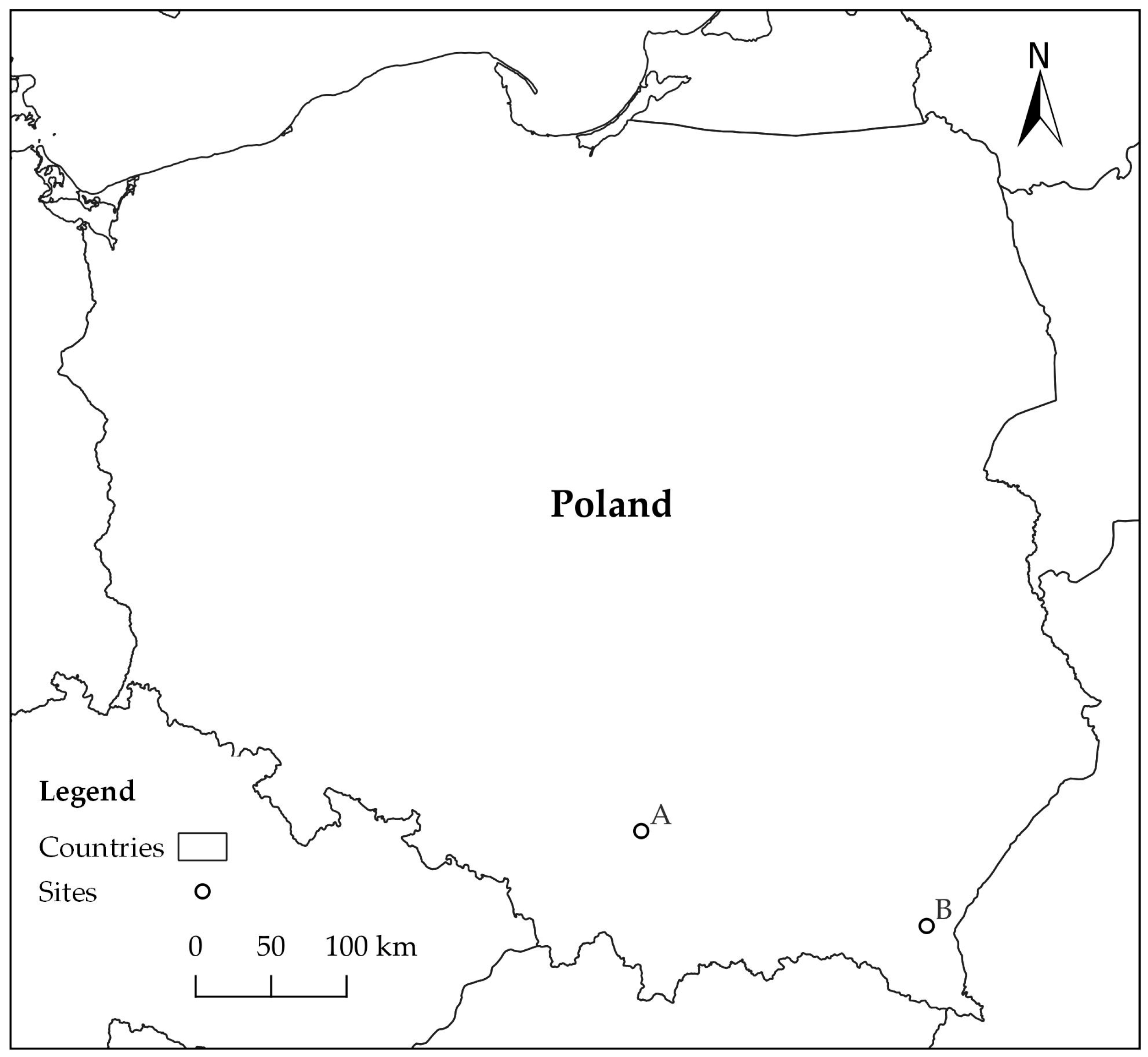
Study material was collected on two forest sites (A, B) in southern Poland, approximately 200 km apart (Figure 1). Site A was located in Ojców National Park (N 50°12′16″ E 19°48′48″), and site B in Rozpucie (Brzozów Forest District; N 49°35′24″ E 22°25′9″) (Figure 1). Study material consisted of fragments of logs and branches of Fagus sylvatica, on which the presence of blackish patches had been detected on cross sections as an effect of accumulation of mycelium and fungal sporulation structures (hereafter referred to as ‘blackish fungal growth’—BFG).
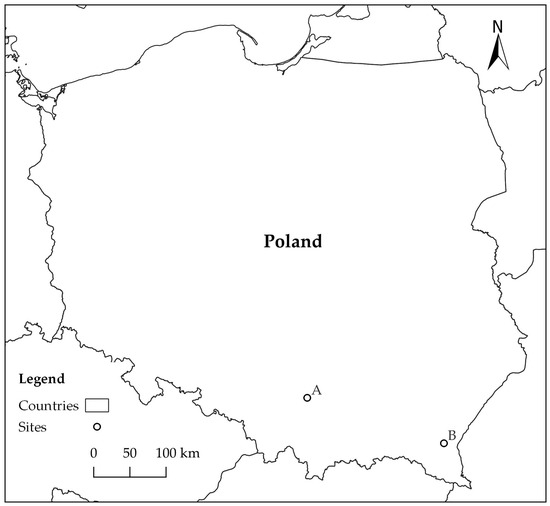
Figure 1.
Location of forest sites: A—Ojców National Park, B—Rozpucie (Brzozów Forest District).
Beech material on site A came from an old beech tree (over 90 years old, 75 cm in diameter at the base of the trunk), consisting of two trunks. One of them was broken under the influence of the wind at a height of 1.6 m (Figure 2a). Due to the forest road being blocked, the trunk was cut into several fragments, which were left on the side of the road. Beech material on site B remained as a result of previously carried out cuts of individual trees. On site A, 12 small wood slices together with fungal structures were sampled from 12 different places on cross sections of logs in August and October 2018 (Table 1). From site B, 12 fragments of logs and branch bases (length 4–15 cm, diameter 7–18 cm) were collected in August 2021 (Table 1). The samples were placed separately in plastic bags and brought to the laboratory, where they were stored in a cold room at 5 °C and subjected to microscopic analysis.

Figure 2.
Occurrence of blackish fungal growth (BFG) on Fagus sylvatica on two sites in Poland; Site A: (a) F. sylvatica trunk broken by the wind; arrow shows BFG on the surface of wood without bark; (b,c) BFG on cross section of logs. Site B: (d,e) BFG, uniform structures on the face of the logs; (f) numerous small areas of BFG on the face of the log; (g) wood discoloration 3 cm below the symptoms shown in (f); (h,i) wood discoloration in logs in which face was covered with BFG; scale bars: (h) = 1 cm, (i) = 10 cm.

Table 1.
Davidsoniella virescens isolates obtained from Fagus sylvatica wood and used in phylogenetic analyses.
From each sample, 4–6 slides were prepared, and individual elements constituting the BFG were measured. The aim was to initially identify the fungus species based on morphological features and to determine whether the BFG in all samples was created by the same fungus. Appropriate mycological keys and monographs were used for identification [1,4,9,10,11,12,13].
In vitro experiments were performed on malt extract agar (MEA: 20 gL−1 Biocorp malt extract, 20 gL−1 Biocorp agar (Biocorp Polska Sp. z.o.o., Warsaw, Poland), supplemented with 100 mL−1 streptomycin sulphate (Polfa S.A., Warsaw, Poland)) in Petri dishes (diam. 9 cm). Fungal isolations were performed within 24–72 h after samples had been collected from the forest sites. Samples were sectioned. The exposed surface was disinfected by washing with cotton wool soaked in 96% ethanol. Then, small fragments of wood were taken using a sterilized scalpel after removal of superficial tissue. From each sample, 6–12 pieces of wood were taken. From the samples from site B, which showed a grey-brown discoloration of the wood, 12–24 fragments were additionally taken for isolation, 3–12 cm below the BFG. These fragments were placed in Petri dishes on the surface of malt extract agar. The incubation took place at 20 °C in darkness. All Petri dishes were examined every 3–5 days for at least 3 weeks. Cultures showing morphological structures characteristic of BFG in vivo were isolated on MEA in new Petri dishes. One representative culture was selected for each sample. Then, their morphological and molecular characteristics were examined. Individual structures were mounted in distilled water, and 20 to 60 measurements at 400× or 1000× magnification were made of each structure; the extreme values and standard deviation (SD) are given. Observations were made with a Zeiss V12 Discovery stereomicroscope and with a Zeiss Axiophot light microscope (Zeiss, Göttingen, Germany), using differential interference contrast or phase contrast illumination. The specimens examined are preserved in the Department of Forest Ecosystems Protection, University of Agriculture in Kraków, Poland.
2.2. DNA Extraction, PCR, Sequencing and Phylogenetic Analyses
To verify the morphology-based identification, the nucleotide sequences for five gene fragments of representative cultures were determined: 18S; the internal transcribed spacer regions ITS1 and ITS2, including the 5.8S gene (ITS); 28S region of the ribosomal RNA (rRNA), β-tubulin 2 (TUB2) and translation elongation factor 1-α (TEF1). Genomic DNA extraction, polymerase chain reaction (PCR) amplification and sequencing reactions of the isolates were carried out according to the procedure described in detail by Kowalski et al. [37]. The 18S, ITS, 28S regions and the TUB2 and TEF1 genes were amplified using the primers [38,39,40,41,42,43] listed in Supplementary Materials (Table S1). Due to the difficulty in obtaining gene fragment sequences of TUB2 and TEF1 for the isolates studied, new primers were designed and used in PCR. For TUB2, it was the forward primer T10CerPB 5′-TCTATAGGTCCACCTCCAGAC-3′, and for TEF1, it was the reverse primer EFCF6PB 5′-CATRTCACGGACGGCGAARC-3′. The latter oligonucleotide was obtained by modifying the EFCF6 primer proposed by [43].
The resulting 18S, ITS and 28S sequences were concatenated because they contained overlapping fragments. Two adjacent fragments of the TEF1 gene were treated similarly. All obtained gene fragment sequences in this study were deposited in GenBank with the accession numbers shown in Table 1. The sequences obtained from representative cultures were used as queries in searches using the BLASTn algorithm [44] to retrieve sequences of taxa closely related to them from GenBank (http://www.ncbi.nlm.nih.gov, accessed on 7 May 2024) for phylogenetic analysis. Individual datasets for the five gene fragment regions were used for phylogenetic analyses using methods following Kowalski et al. [37]. The datasets contained all species of the genus Davidsoniella for which at least one of the gene sequences was available in the GenBank, with Endoconidiophora coerulescens and E. polonica included as an outgroup (Supplementary Table S2) following de Beer et al. [1]. The best evolutionary substitution model for dataset 18S was HKY+I; for ITS, it was GTR+I; for 28S, it was HKY+G; and for the protein-coding gene datasets TUB2 or TEF1, it was GTR+G and GTR+I+G, respectively. The resulting alignments and trees were deposited into TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S31400, accessed on 19 May 2024).
2.3. Colony Growth in Relation to Temperature
A temperature assay was performed in vitro on 2% MEA in Petri dishes. Six D. virescens isolates obtained from F. sylvatica wood in southern Poland were used for the test (Table 1). Mycelial plugs (8 mm in diameter) from the edge of actively growing 5-day-old colonies were transferred into the center of dishes and incubated at 5, 10, 15, 20, 25, 30 and 35 °C in the dark. Two replicated plates were prepared for each fungal isolate and temperature. Colony diameters were measured after 8 days with a millimeter ruler in directions perpendicular to each other. The average diameter from measurements in each replicate was calculated.
2.4. Pathogenicity Test
Fagus sylvatica and Acer saccharum seedlings were used for the pathogenicity test. Two-year-old seedlings of F. sylvatica were obtained in autumn 2021 from a forest nursery in the Daleszyce Forest District (N 50°47′39″ E 20°42′36″). They were immediately planted in sandy clay, moderately moist soil (30 × 30 cm spacing), in a fenced area on the outskirts of Kraków, where there was side shade from neighboring low trees and shrubs. In 2022, all individuals were developing well, without any disease symptoms. Dimensions of seedlings at the time of inoculation were as follows: height 0.5–0.8 m, diameter at the stem base 0.5–0.7 cm.
Two-year-old seedlings of A. saccharum were obtained in June 2022 at the ornamental tree nursery in Radomyśl Wielki near Mielec (eastern Poland). They were grown individually in pots (height 15 cm, diameter 18 cm) on a substrate composed of peat and perlite. They were located next to a plot with F. sylvatica. At the time of inoculation, their dimensions were height of 0.5–0.9 m and diameter at the stem base of 0.5–8 cm. Both plant species were regularly observed and watered as required.
Inoculation was carried out in the first days of August 2022 in ca. 7 mm long wounds made with a sterile scalpel on stem approximately 15 cm above the root collar. Six D. virescens isolates obtained from F. sylvatica wood in southern Poland were used for the test (Table 1). Six F. sylvatica and six A. saccharum seedlings were inoculated with each isolate. Inoculum production, inoculation and re-isolation were carried out according to the methods used by Kowalski et al. [45]. Briefly, isolates were grown on MEA for 5 days at 20 °C in darkness. Small sterile beech-wood sticks (ca. 5 × 2 × 2 mm) were then placed on the colonies and incubated further for 2 weeks. These beech-wood pieces used for inoculation were overgrown by mycelium along with endoconidia and ascospores and the structures producing them (phialophores, perithecia). For control inoculations, sterile beech-wood sticks were kept on fungus-free MEA for 2 weeks. In total, 72 stems were inoculated. Control inoculations were carried out with sterile wood pieces applied to wounds of six stems of each plant species. The symptoms were examined 4 months after inoculation. The lengths of superficially visible necrotic lesions were measured, and the occurrence of fungal fructification was recorded. After removal of the bark and superficial layers of wood, the extent of wood discoloration was determined. If a given seedling began to die earlier, the analysis was performed immediately after observing symptoms in the crown. Re-isolations were attempted from all inoculated and all control stems within 24 h from harvesting. Three to eight (over 1400 in total) tissue pieces were taken from inoculation wounds, the lesions and discolored wood. The isolation was considered positive for a given seedling if the tested fungus grew from at least one tissue sample.
2.5. Statistical Analyses
Differences between groups within the study variables were given an analysis of variance (ANOVA) after log10(x + 1) transformation of the data and checking that they met the assumptions of homogeneity of variance. If the data did not meet these assumptions, a post hoc test of multiple comparisons of the mean ranks for all groups of variables following Kruskal–Walis test was applied. For ANOVA, the Tukey HSD post hoc test was performed. All statistical analyses were performed using the program Statistica 13.1 [46].
3. Results
3.1. Symptoms on Fagus sylvatica Logs In Situ
D. virescens was first observed in August 2018 during research in Ojców National Park (site A) (Figure 1, Table 1). On a trunk of a broken beech (on the lying and standing parts), blackish fungal growths (BFGs) were found on the surface of bark-free wood (Figure 2a). In the remaining cases, they were formed on cross-sectional surfaces of the logs, 13 to 27 cm in diameter (Figure 2b,c). BFGs were of different sizes (Figure 2b,c); in only one log, fungal growth occupied the entire front surface. Observation in October 2018 showed that the blackish structures were covered with a fine white powder (Figure 2b).
Very similar symptoms were observed in August 2021 in Rozpucie (Brzozów Forest District, site B, Figure 1). They occurred on 12 log fragments and the bases of branches lying on the ground or in the heap. They always occurred on exposed wood, never on bark. They took the form of either uniform, quite extensive structures (Figure 2d,e) or numerous separate patches sometimes merging with each other (Figure 2f). In five logs, BFG covered over 75% of the cross-sectional area (Figure 2e). In some logs, BFG was covered with a fine white powder (Figure 2e). The wood under the BFG had grey-brown discoloration to a depth of 12 cm (Figure 2g–i). The shape of the wood discoloration corresponded to a large extent to the shape of BFG on the front face of the logs (Figure 2f–i). Of all analyzed logs, D. virescens cultures were obtained from 17 samples (Table 1).
3.2. Morphological Characterization of Davidsoniella virescens
3.2.1. In Vitro
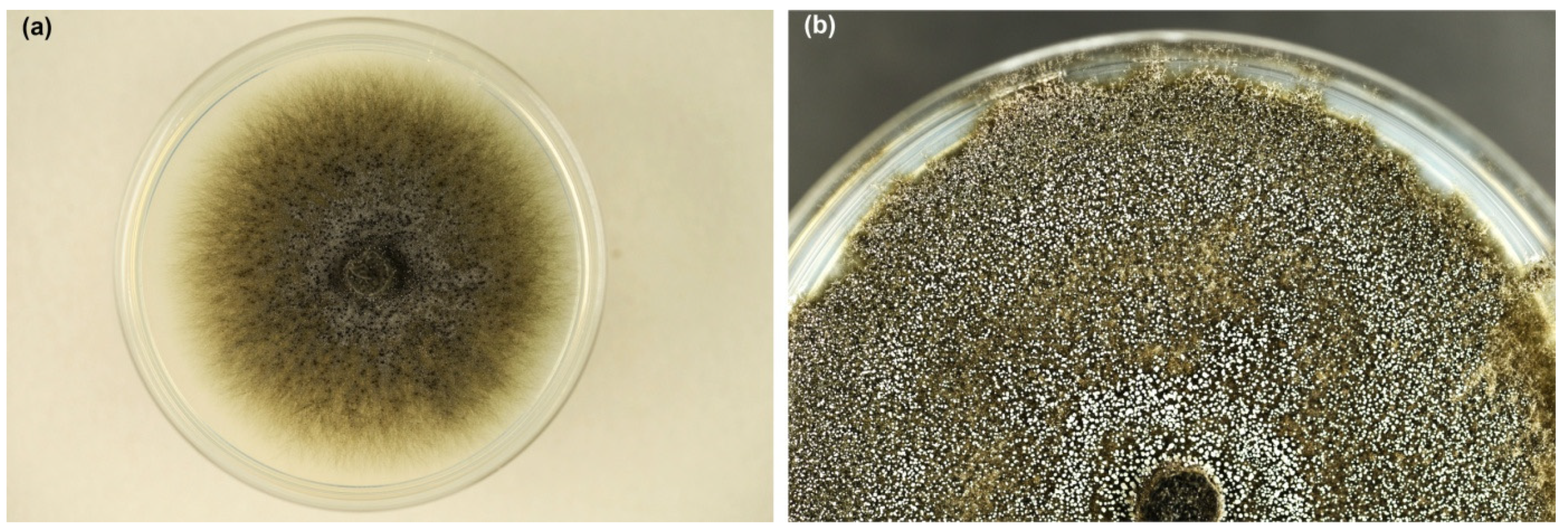
Colonies on MEA were growing rapidly; after 8 days at 20 °C, they reached 5.5–8.3 cm in diameter. Mycelium was dark greenish grey, initially quite fluffy (Figure 3a), later suppressed to the medium (Figure 3b). Superficial hyphae were subhyaline to greenish grey, smooth, septate, branched, 2.5 to 6.0 µm thick. Immersed hyphae were dark grey, smooth, 4–8 µm thick. The colony was covered with a white fine powder in places due to the production of asexual spores. Young colonies emitted an intense, sweet odor, which became less pleasant in older colonies.
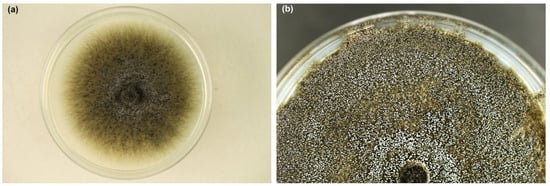
Figure 3.
Colonies of Davidsoniella virescens on MEA at 20 °C: (a) one week old, (b) three weeks old (Petri dishes 9 cm in diameter).
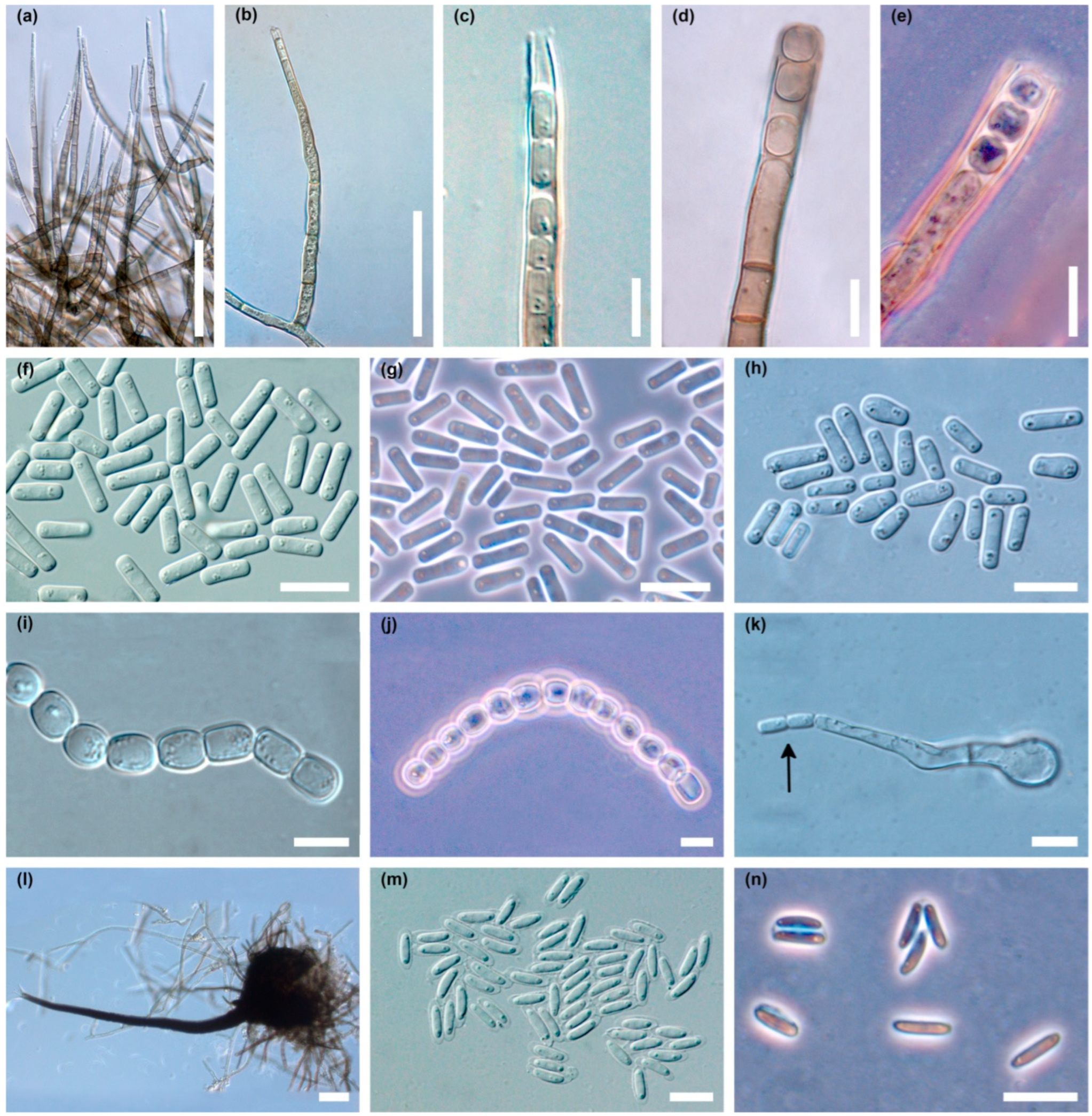
The colonies produced an abundant asexual stage, characteristic of the Chalara genus (Figure 4a–n). Two types of phialophores developed, differing in frequency, shape, length and width dimensions. Phialophores of the first type (type A) were simple, only occasionally with a branch at the base, cylindrical, olivaceous brown and 3–9 (14) septate, 42–325 µm (169.3 ± 74.7 µm; mean ± SD) long, and 4.5–6.2 µm wide at the base, terminating in a phialide (Figure 4a,b). The phialides were subcylindrical, 30–79 µm long, with hyalin, subcylindrical to conical, 2.5–3.7 µm wide collarette (Figure 4a–c). Endogenously formed conidia were often arranged in long chains. They were unicellular, hyaline, smooth, thin walled, cylindrical, initially with truncate, later with rounded ends, 5.0–17.5 µm (9.8 ± 2.8 µm) long and 2.0–5.0 µm (3.0 ± 0.8 µm) wide, often with polarly located small oil droplets (Figure 4c,f–h).
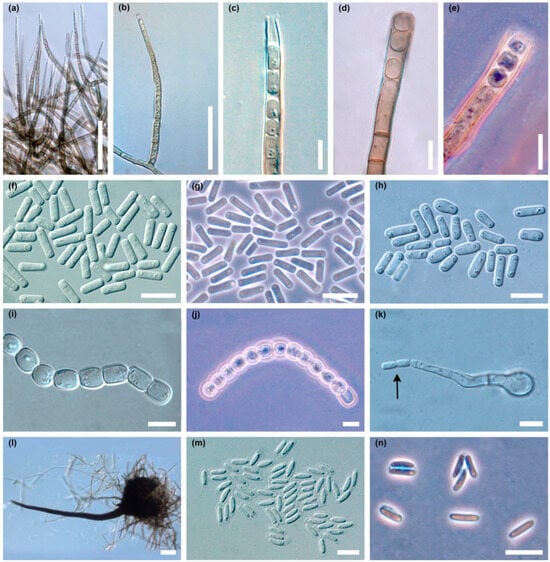
Figure 4.
Microscopic structures of Davidsoniella virescens in vitro on MEA: (a) very numerous phialophores of type A produced in a young colony, (b) a single phialophore of type A, (c) apical part of the phialophore type A with endoconidia, (d) phialophore type B with endoconidia, (e) phialophore type B with endoconidia, phase contrast, (f,g) endoconidia type A in a young colony, (f) = DIC, (g) = phase contrast, (h) endoconidia type A in two-week-old colony, (i,j) chains of type B endoconidia, (i) = DIC, (j) = phase contrast, (k) germinating type B endoconidium producing endoconidia (arrow), (l) perithecium, long neck with divergent ostiolar hyphae, (m,n) ascospores surrounded by transparent sheath, (m) = DIC, (n) = phase contrast; scale bars: (a,b) = 50 µm, (c–k) = 10 µm, (l) = 50 µm, (m,n) = 10 µm.
Phialophores of the second type (type B) were olivaceous brown, 3–10 septate, 60–225 µm (121.9 ± 43.0 µm) long, 5–7 µm wide at the base, and slightly enlarged to 6.2–7.5(–9.0) µm at the top (Figure 4d,e). Endoconidia were hyaline, unicellular, oval to barrel-shaped, and globose, 5–13 × 4.5–13.0 µm (8.1 ± 2.0 × 6.8 ± 1.8 µm), occurring singly or in long chains (Figure 4i,j). After germination of type B endoconidia, the germ hypha can transform into a type A phialide that produces type A endoconidia (Figure 4k). Phialophores of type A predominate quantitatively in young cultures (Figure 4a). Phialophores of type B are rare in young cultures, but quite common in older ones (Figure 4d,e).
In the cultures, after 3–5 days, on superficial mycelium or partly embedded in agar, perithecia began to be produced abundantly (Figure 3a), and over time appeared densely throughout the colony (Figure 3b). Perithecia were black, flask shaped to globose; 120–250 µm (181.2 ± 33.1 µm) high, 125–250 µm (180.5 ± 32.0 µm) wide; ornamented with numerous dark, septate, smooth hyphal hairs; 36–350 µm (179.3 ± 72.8 µm) long, and 4.0–5.0 µm wide (Figure 4l). Necks were black, becoming lighter towards the apex, straight or slightly curved, 480–1300 µm (801.7 ± 183.0 µm) long excluding ostiolar hyphae, 35–50 µm wide at the base, and 10–12(–15) µm wide at the top. Ostiolar hyphae were divergent, hyaline, 16–30 µm long, 1.2–1.5 µm wide at the base, tapered slightly to blunted tips (Figure 4l). Ascospores were collecting in sticky white spherical masses at the top of neck. Ascospores were unicellular, hyaline, cylindrical to oblong, straight to slightly curved with polar oil droplets, surrounded by transparent sheath, which appeared uniform or slightly thicker at the spore ends; 5.0–9.0 × 1.5–2.5 µm (7.0 ± 0.8 × 2.0 ± 0.2) without sheath and 7.0–11.0 × 2.0–4.0 µm (8.8 ± 0.8 × 2.7 ± 0.4) µm with sheath (Figure 4m,n).
3.2.2. In Situ
Each structure observed in vitro was produced by D. virescens in situ on F. sylvatica wood. They were similar in shape and size, hence only their main characteristics are given below. Perithecia formed on the surface of the wood or were slightly embedded in the substrate; brown-black, flask shaped, spherical or slightly flattened at the base; 107–200 µm (153.2 ± 21.0 µm) high, 120–180 µm (153.1 ± 17.8 µm) wide; ornamented with numerous dark, septate, hyphal hairs, 30–300 µm long (157.6 ± 67.9 µm) and 3.0–5.0 µm wide. Necks were black, becoming lighter toward the apex, 450–1100 µm (683.0 ± 181.7 µm) long excluding ostiolar hyphae, 27.5–42.5 µm at the base, 10–15(–22) µm wide at the top. Ostiolar hyphae were hyaline, divergent, 15–42.5 µm long. Ascospores were unicellular, hyaline, cylindrical to oblong, straight or slightly curved with oil droplets, 5.5–8.0 × 1.8–2.2 µm without sheath and 7.0–9.0 × 2.0–2.7 µm with transparent sheath.
Phialophores of type A were simple, occasionally with one branch at the base, olivaceous brown, 3–9 septate, 52–325 µm (172.4 ± 58.5 µm) long, (3.2–)5–7.5 µm wide at the base, terminating in a phialide. The phialides were hyalin to light olive, subcylindrical to obclavate, 55–77 µm long. Collarettes were 2.0–3.7 µm wide. Endogenously produced conidia (type A) were unicellular, hyaline, thin walled, cylindrical with truncate or rounded ends, 5–13 × 1.8–4.0 µm (8.0 ± 2.1 × 2.3 ± 0.4 µm), with small oil droplets, often arranged in long chains. Phialophores of type B were olivaceous brown, 3–5 septate, 45–125 µm long, 3.7–6.0 µm wide at the base, 5.0–7.0(–9.0) µm wide at the top. Endoconidia (type B) were hyaline, unicellular, oval to barrel-shaped, or spherical, 5.0–9.0 × 4.0–9.0 µm (7.6 ± 0.9 × 6.5 ± 1.3 µm), occurring singly or in short chains.
On sites A and B, it was observed that some blackish structures were entrained with a fine white powder. Microscopic analysis showed that this was the result of the formation of type A and B endoconidia. Moreover, in some samples, quite numerous conidiophores and conidia of another fungus of the Clonostachys genus were developed.
3.3. DNA Sequence Data and Phylogenetic Analysis
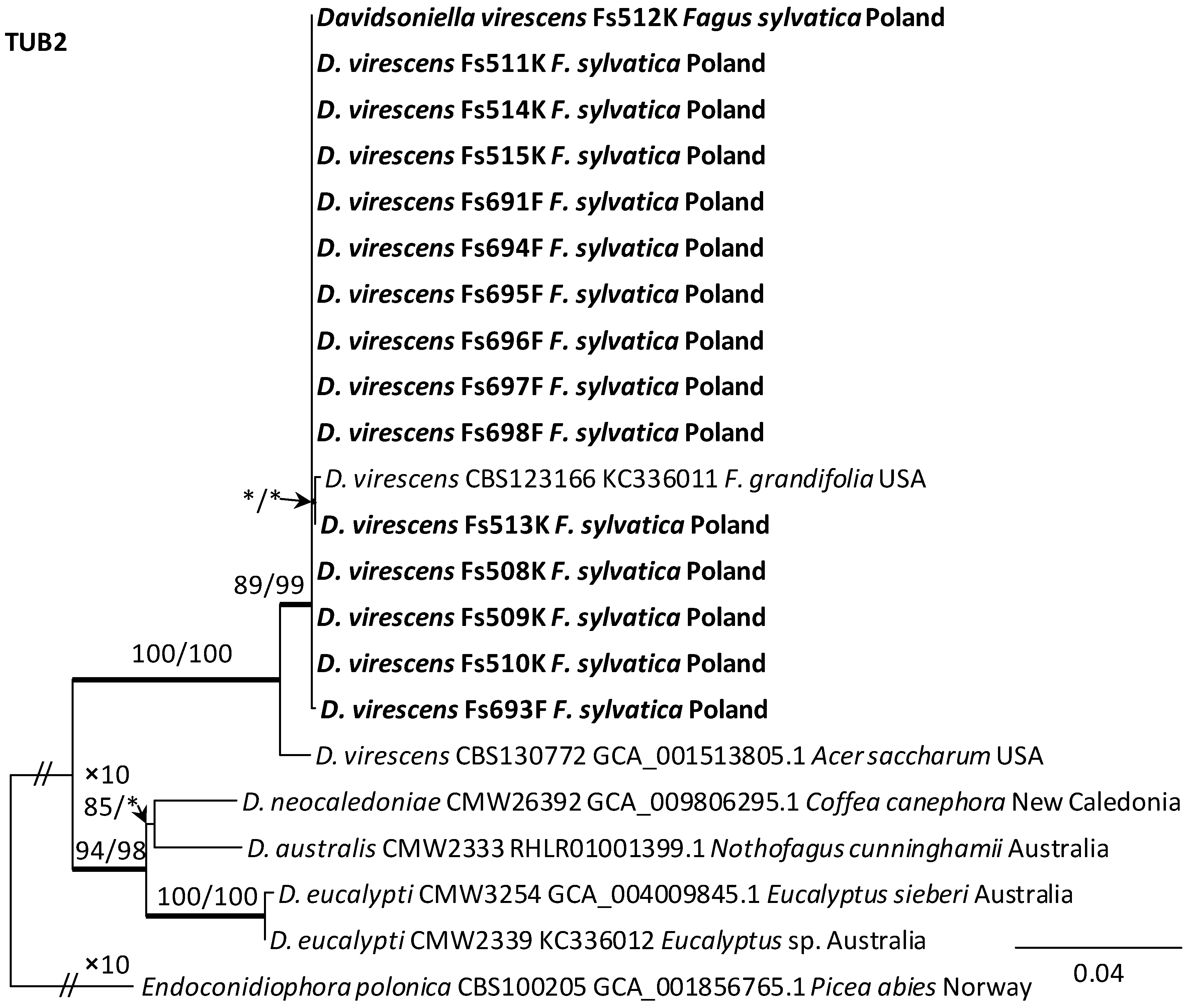
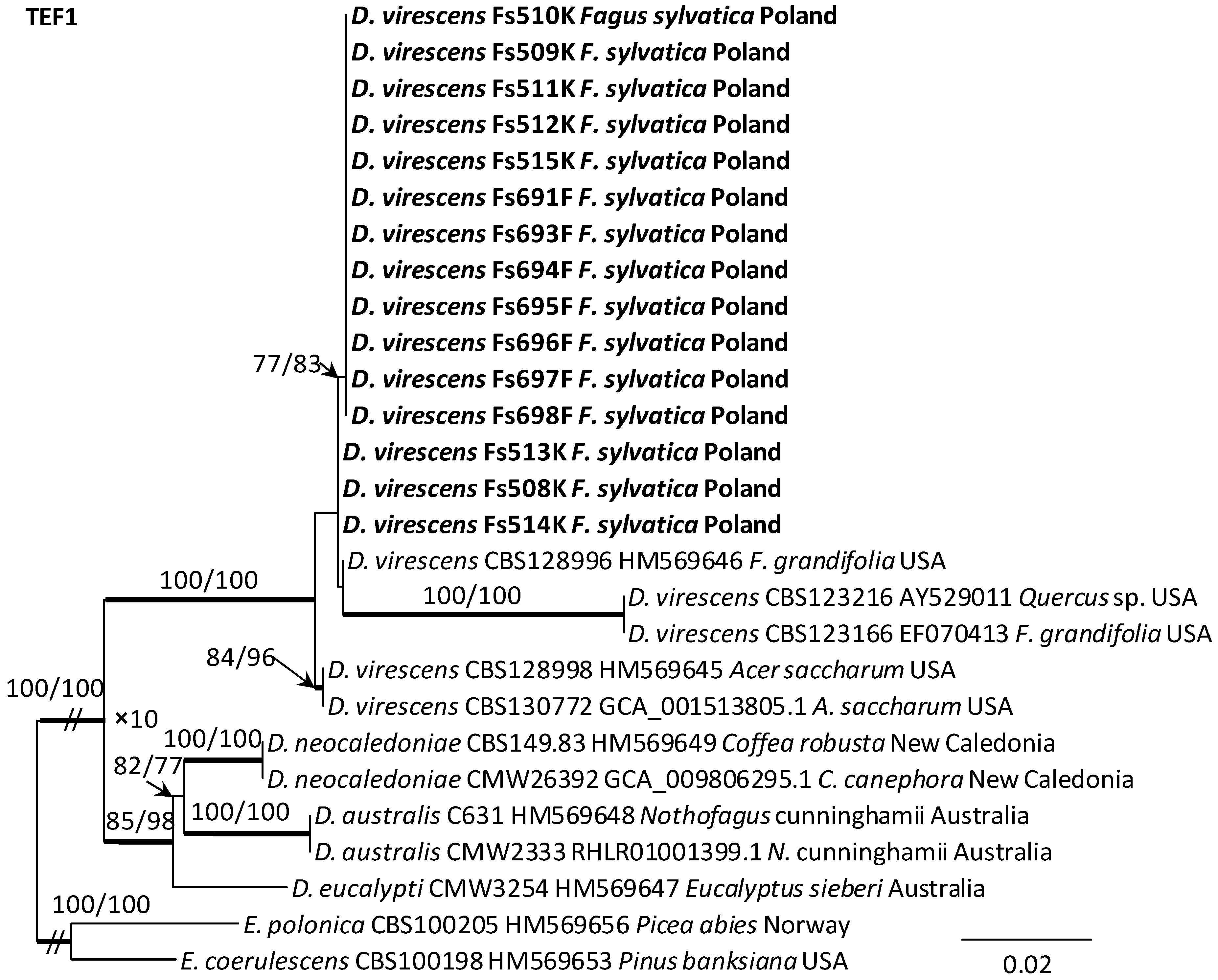
Alignments for the 18S, ITS, 28S, TUB2 and TEF1 sequences respectively contained 1736, 520, 825, 1106, and 1563 characters (including gaps). The datasets used in the phylogenetic analysis contained different numbers of variable characters: 18S—5, ITS—59, 28S—20; TUB2—298 and TEF1—273, of which 3, 51, 13, 108 and 219 were parsimoniously informative, respectively. The aligned TUB2 gene region consisted of introns 1–5 with exons 2–6. The exon/intron arrangement of the TEF1 data included exons 3, 4 and 5 interrupted with introns 3 and 4. The results of phylogenetic analysis of the nucleotide sequences of five gene fragments of isolates from Poland indicated that they were most similar phylogenetically to D. virescens isolates from the USA. The isolates of this species formed a distinct clade with strong statistical support on all phylogenetic trees except the tree obtained from the analysis of the 18S gene fragment (Figure 5, Figure 6 and Figures S1–S3). TEF1 was the most phylogenetically robust among all markers tested (Figure 6). The topology of the trees obtained for each of the three genes, i.e., 28S (Supplementary Figure S3), TUB2 and TEF1 (Figure 5 and Figure 6), within the D. virescens clade indicated the existence of two distinct phylogenetic lineages associated with different host plants. Distinguishing at least one of these in each of the three analyses was statistically significant. The first group of isolates was derived from trees of the genus Fagus or Quercus, while the second was associated with A. saccharum. All Polish isolates obtained from F. sylvatica showed affinity with North American isolates derived from F. grandifolia and formed a distinct phylogenetic lineage with them. Phylogenetic analysis of protein-coding gene sequences (TUB2, TEF1) indicated the presence of intraspecific variability among D. virescens isolates. Polish isolates were characterized by relatively low variability in these genes, in contrast to North American isolates, especially with regard to TEF1 sequences (Figure 5 and Figure 6).
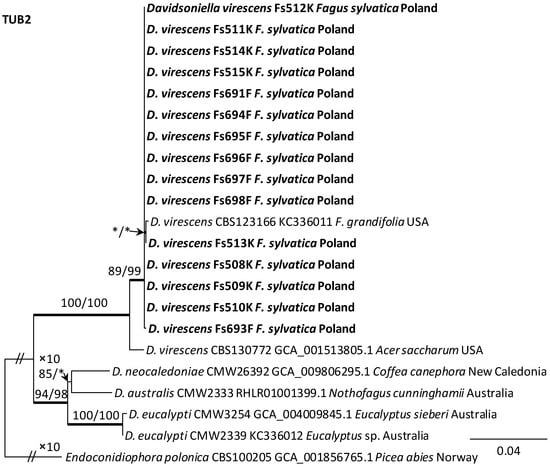
Figure 5.
Phylogram obtained from analyses of the beta-tubulin (TUB2) data for the genus Davidsoniella. Sequences obtained during this study are presented in bold type. The presented phylogram was obtained from Maximum Likelihood (ML) analyses. The bootstrap values ≥ 75% for ML and Maximum Parsimony (MP) analyses are presented at nodes as follows: ML/MP. Bold branches indicate posterior probability values ≥ 0.95 obtained from Bayesian Inference (BI) analyses. * Bootstrap values < 75%. The tree is drawn to scale (see bar), with branch length measured in the number of substitutions per site. Endoconidiophora polonica represents the outgroup.
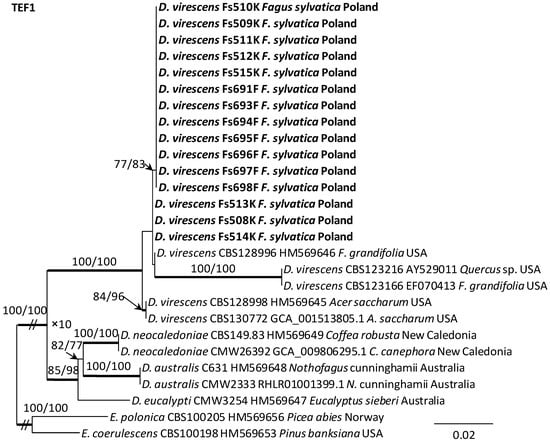
Figure 6.
Phylogram obtained from analyses of the translation elongation factor 1-alpha (TEF1) data for the genus Davidsoniella. Sequences obtained during this study are presented in bold type. The presented phylogram was obtained from Maximum Likelihood (ML) analyses. The bootstrap values ≥ 75% for ML and Maximum Parsimony (MP) analyses are presented at nodes as follows: ML/MP. Bold branches indicate posterior probability values ≥ 0.95 obtained from Bayesian Inference (BI) analyses. The tree is drawn to scale (see bar), with branch length measured in the number of substitutions per site. Endoconidiophora coerulescens and E. polonica represent the outgroup.
3.4. Colony Growth in Relation to Temperature
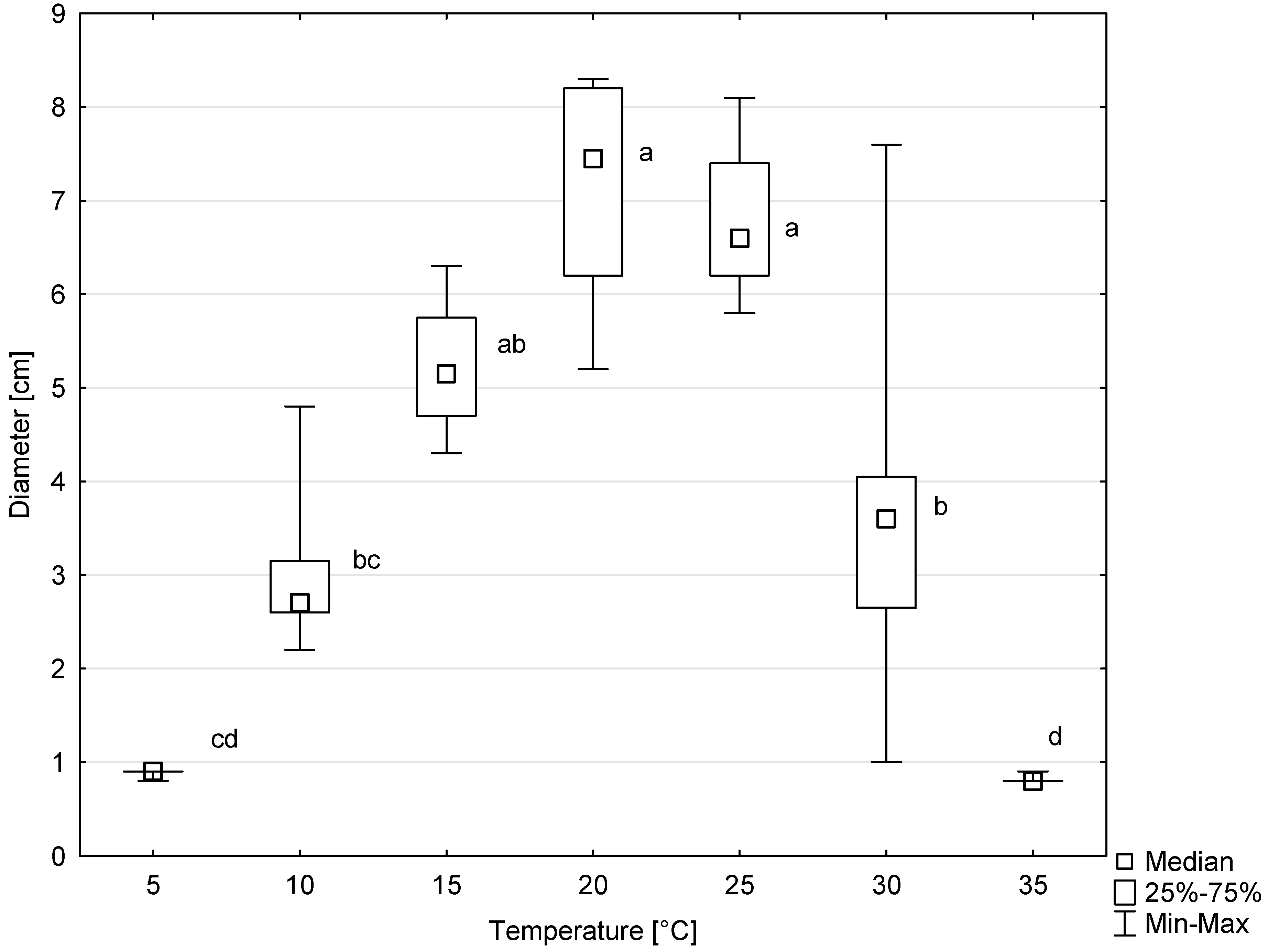
Colonies of all six tested isolates of D. virescens showed growth on MEA in the temperature range of 5 to 30 °C, and occasionally also at 35 °C (Figure 7). The optimal temperature for radial colony growth was 20 °C. However, at 25 °C, colony growth was only slightly slower. The differences between colony diameter at this temperature compared to the optimal temperature were not statistically significant (Figure 7). Above 25 °C, colony growth was significantly slower, and the differences between colony diameters growing at 25, 30 and 35 °C were statistically significant, according to the Kruskal–Wallis test (Figure 7).
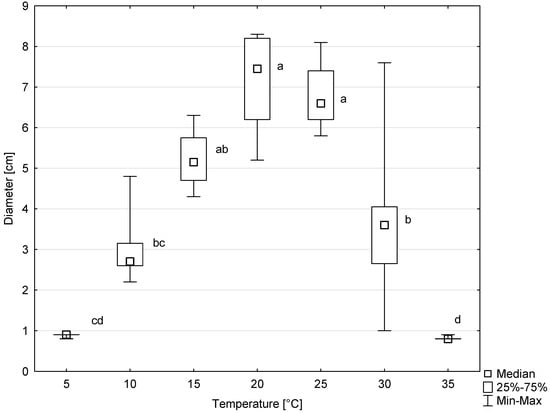
Figure 7.
Colony diameters of six Davidsoniella virescens isolates after 8 days of growth at different temperatures. Values indicated with different letters were determined to be significantly different at α = 0.05 in Kruskal–Wallis test.
3.5. Pathogenicity Test
3.5.1. Fagus sylvatica
Out of 36 seedlings of F. sylvatica, inoculated in stem wounds with D. virescens, five seedlings showed wilting symptoms throughout the entire crown after 3–6 weeks. The leaves dried quite suddenly, turned pale green or greyish-green, less often brown, and wrinkled or curled at the edges (Figure 8a–p). Symptoms in the crown were accompanied by bark necrotic lesions and wood discoloration within the stems, occasionally in the roots (Figure 8a–p). Synthetic descriptions of symptoms in these seedlings are given in Table 2.

Figure 8.
Symptoms in five Fagus sylvatica seedlings that manifested wilting 3–6 weeks post inoculation with Davidsoniella virescens (WD = wood discoloration): Seedling no. 5/Fs513F: (a) wilting symptoms, (b) inoculation site and local bark necrosis, (c) WD at the lower border with living tissue, inner bark is green, (d) WD up to 2.5 cm above the inoculation wound, inner bark is dying, Seedling no. 4/Fs697F: (e) wilting symptoms, (f) inoculation site and extended bark necrosis, (g) WD 3–5 cm above the inoculation wound, (h) streaky WD in the upper part of the stem, Seedling no. 2/Fs508K: (i) wilting symptoms, (j) WD 3–5 cm above the root collar, (k) WD 5–7 cm above the inoculation wound, Seedling no. 6/Fs697F: (l) inoculation site (arrow) and extended bark necrosis, (m) WD below the inoculation wound, (n) WD at the upper border with the living tissue, Seedlings no. 3/Fs509K: (o) WD 8–10 cm above the wound, (p) WD at the stem base, up to 2 cm above the root collar; scale bars: (a) = 50 mm, (b–d) = 5 mm, (e) = 50 mm, (f) = 20 mm, (g,h) = 5 mm, (i) = 50 mm, (j,k) = 5 mm, (l) = 10 mm, (m) = 1 mm, (n–p) = 5 mm.

Table 2.
Symptoms in five Fagus sylvatica seedlings that manifested wilting 3–6 weeks post inoculation with Davidsoniella virescens.
Among the remaining 31 seedlings, no symptoms of wilting or early leaf discoloration were observed. Natural discoloration of the leaves occurred only due to the arrival of autumn. During the evaluation 4 months after the inoculation, 11 seedlings had bark necrosis around the inoculation site with a length of 1.3 to 7.2 cm (Table 3). In 54.8% of the stems, the wounds were not healed; in the remaining cases, the wounds were partially healed. In 6.5% of stems in the wounded area, D. virescens produced perithecia. The most noticeable internal symptom was the discoloration of the wood (Figure 9a–l). On six stems, their length did not exceed 5 cm (Figure 9a–c). In the remaining stems, the wood was discolored over a length of 5.1 to 15.6 cm (Figure 9d–i, Table 3). On most stems within the wound inoculations, the wood was dark grey, while below and above this area, the wood was light brown or grey-brown (Figure 9a–i). Sometimes this discoloration was not uniform, but there were darker longitudinal streaks (Figure 9h,i). The longitudinal section of some stems clearly showed that wood discoloration was associated with bark necrosis (Figure 9h,i, Table 3). A hand-drawn tangential section of discolored wood showed few hyphae of D. virescens in the vessels and in ray parenchyma (Figure 9j,k).

Table 3.
Presence of bark necrosis (necr.) and discoloration (discol.) in stem wood of Fagus sylvatica and Acer saccharum 4 1 months post inoculation with Davidsoniella virescens.

Figure 9.
Internal symptoms in stems of Fagus sylvatica 4 months post inoculation with Davidsoniella virescens (WD = wood discoloration): (a–c) WD less than 5 cm long, (d–g) WD more than 5 cm long, (h,i) extensive grey-brown WD and dying of the bark above the inoculation site; in seedling no. 4/Fs508K (h) and no. 3/Fs513F (i), (j,k) hand-drawn tangential section through DW: D. virescens hyphae in the vessels, arrows (j), D. virescens hyphae in the vessels and in parenchyma cell, arrows (k), (l) WD in control seedling; scale bars: (a–i) = 5 mm, (j,k) = 50 µm, (l) = 5 mm.
3.5.2. Acer saccharum
None of the 36 inoculated seedlings showed any symptoms of early leaf discoloration or wilting, as well as bark necrosis around the wound. In 47.2% of the stems, the wounds were not healed, and in the remaining cases, the wounds were partially healed. Perithecia of D. virescens developed on two (5.6%) stems in the wound area. There was discoloration of the wood inside all stems, starting from the inoculation site. In two seedlings, the wood was discolored brown (Figure 10a); in the remaining ones, the wood was dark grey with an olive green tint (Figure 10b–j). The length of the discolored wood ranged from 1.2 to 6.1 cm (Table 3). In 20 (55.6%) seedlings, the length of the discolored wood did not exceed 2 cm (Figure 10b–d). In some stems, the discoloration was irregular, with dark streaks extending both upwards and downwards (Figure 10e–h). Sometimes they formed quite thin lines (Figure 10i,j). A hand-drawn tangential section of discolored wood showed few hyphae of D. virescens in the vessels (Figure 10k).

Figure 10.
Internal symptoms in stems of Acer saccharum 4 months post inoculation with Davidsoniella virescens (WD = wood discoloration): (a) brown WD above and below the wound, (b–d) limited dark grey WD, (e–h) dark grey irregular WD with dark streaks, (i,j) WD in form of thin lines, (k) hand-drawn tangential section through discolored wood, D. virescens hyphae in the vessels (arrows), (l) WD in control seedling; scale bars: (a–j) = 5 mm, (k) = 50 µm, (l) = 5 mm.
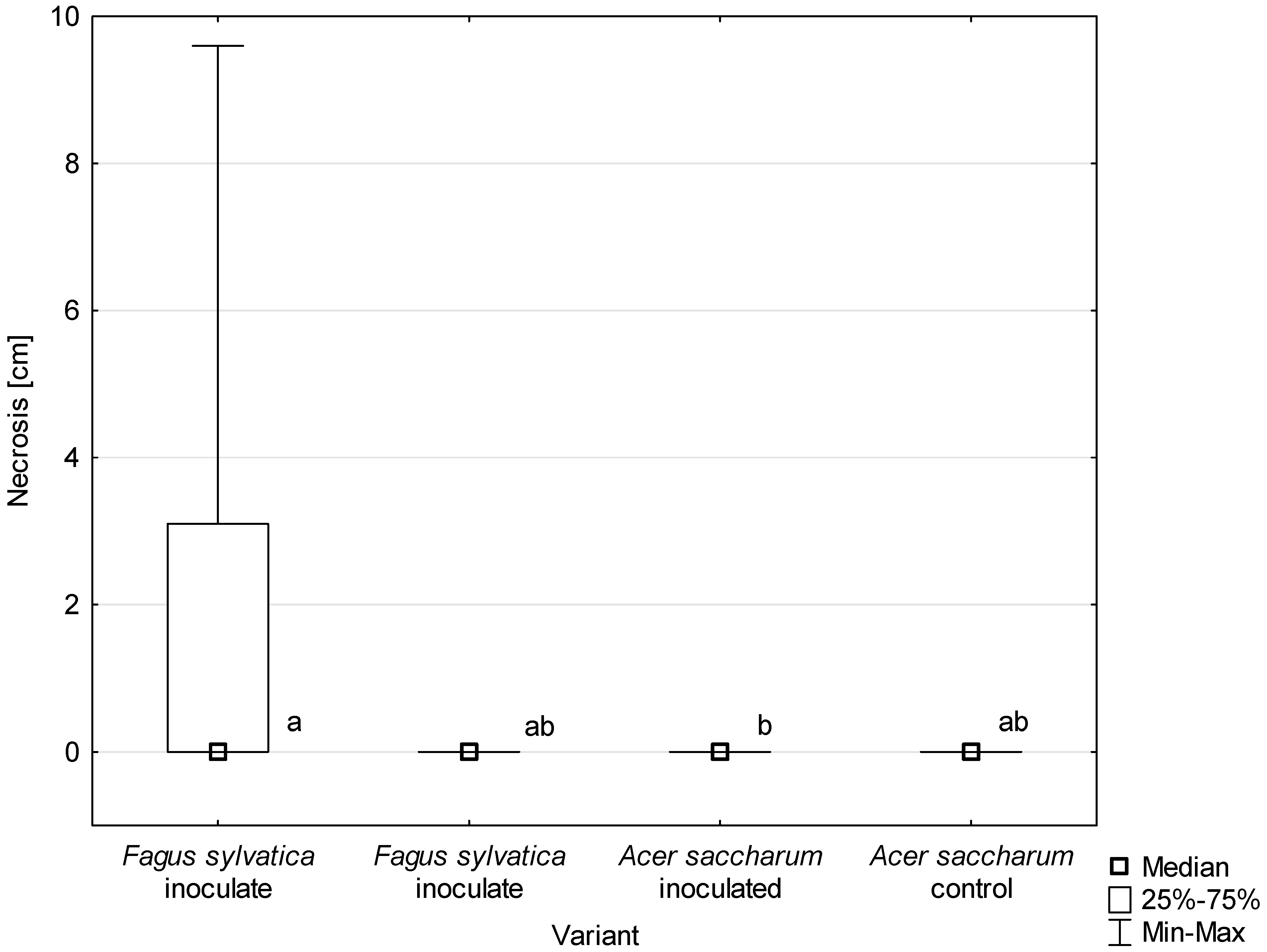
All D. virescens isolates caused greater wood discoloration and bark necrotic lesions in F. sylvatica than in A. saccharum (Figure 11, Table 3). Most of the differences found in the extent of discoloration between host plants were statistically significant (Table 3). The discoloration caused by all D. virescens isolates in F. sylvatica was significantly greater than in the control. However, none of the isolates tested on A. saccharum caused significantly greater discoloration compared to the control (Table 3).
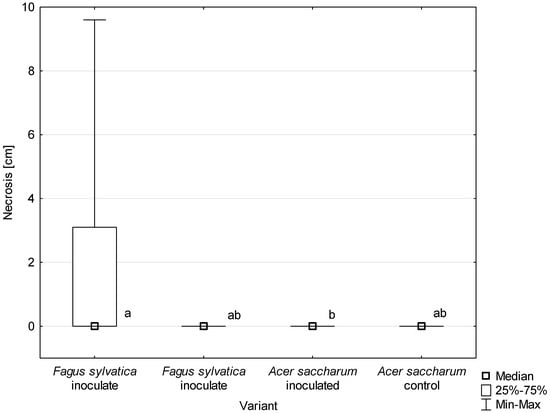
Figure 11.
Length of bark necrosis on stems of tested trees 4 months post inoculation with Davidsoniella virescens. Data are presented in total for all tested isolates. Values indicated with different letters were determined to be significantly different at α = 0.05 in Kruskal–Wallis test.
3.5.3. Re-Isolation and Control
D. virescens was re-isolated from all five seedlings of F. sylvatica showing wilt symptoms. Its colonies grew from a total of 40.6% of wood fragments (Table 4). Some differences were found in the frequency of its isolation depending on the place where wood fragments had been collected (Table 4). In the case of seedling no. 3/Fs509K, D. virescens was also re-isolated from discolored wood in roots. Of the 31 F. sylvatica seedlings without wilt symptoms, a positive re-isolation result was obtained in 26 of them (Table 4). D. virescens colonies grew from 19.0% of wood fragments (Table 4). From 36 inoculated stems of A. saccharum, 584 discolored wood fragments were placed on MEA. D. virescens was isolated from only 1.0% of such fragments from five (13.9%) seedlings (Table 4). Colonies of fungi of the genera Alternaria, Aureobasidium, Cadophora, Cladosporium, Clonostachys, Colletotrichum, Epicoccum, Fusarium, Paraconiothyrium, Pezicula, Phoma, Phomopsis, and Trichoderma were also isolated from wood fragments of both plant species. Among the F. sylvatica isolates, Clonostachys sp. predominated, while Fusarium spp. were the most frequently isolated colonies from A. saccharum.

Table 4.
Results of Davidsoniella virescens re-isolation 4 months after inoculation of Fagus sylvatica and Acer saccharum seedlings (from F. sylvatica seedlings with wilting symptoms, re-isolations were performed 3–6 weeks after inoculation).
No control seedlings of F. sylvatica and A. saccharum developed bark necrosis on stems. In the area of the wound in F. sylvatica, the wood became light brown in color at a length of 1.1–2.2 cm (Figure 9l, Table 3). In A. saccharum, the length of discolored wood was 0.9–1.8 cm (Figure 10l, Table 3). The wood was uniformly brown or dark grey with an olive tint (Figure 10l). D. virescens was not isolated from any control seedlings (Table 4).
4. Discussion
Davidsoniella virescens has so far been found only in North America [4,26,47]. The discovery of this species in Poland is the first report from outside the previously known area of occurrence. Fagus sylvatica has not yet been mentioned among the colonized spectrum of trees [4,26,47]. In some areas of the United States, D. virescens inoculum is readily available. The fungus often produces mycelium along with sporulating structures on recently cut surfaces of stumps and logs [4,23,27,34,48,49]. Current observations in Poland at two significantly distant forest sites led to similar conclusions. Mycelium and sporulation structures of D. virescens did not form on the bark of the currently analyzed logs. The same was observed in inoculated plant seedlings, in which perithecia developed only occasionally (11.1% of beech, 5.6% of sugar maple) at the site of injury. Regardless of whether colonization occurred naturally (logs) or as a result of artificial inoculation (seedlings), the wood showed symptoms of grey or grey-brown discoloration, which was uniform or marked with dark streaks.
Colonized wood plays a significant role in the development of D. virescens. First, spores formed very abundantly on the wood surface can be easily spread throughout the tree stand. Conidia, due to the lack of mucus, might be spread by the wind. However, ascospores are surrounded by mucus, so rain and insects are more likely involved factors. The great importance of insects in the spread of pathogenic and saprotrophic Ceratocystidaceae fungi is well documented [1,12,50,51,52]. Such suggestions also apply to D. virescens [26,29,53]. Secondly, on logs and stumps, where we observed sporulation, the fungus was most likely able to survive the winter, because its mycelium was growing deep into the wood, which was confirmed by isolation on agar medium. For a fungus that does not produce sclerotia, this is an important way to survive unfavorable external conditions.
4.1. Morphological, Phylogenetic and Physiological Aspects
So far, the morphological features of D. virescens have been given in detail when a new species is described (as Endoconidiophora virescens) [4]. These features were also partially described during the re-examination of authentic Davidson’s strain [13,14]. Davidson [4] indicated that a characteristic feature of D. virescens is the production of two types of phialophores, narrow (2.2–3.5 µm) and wide (5–6.5 µm), and the corresponding two types of endoconidia. The microscopic characteristics of the isolates now obtained from F. sylvatica are largely consistent with those described for D. virescens by Davidson [4]. However, some differences can be noticed, especially regarding the Chalara asexual stage. Due to the diversity of phialophores and the endoconidia they produce, for some systematization, the following terms have been introduced for them: ‘type A’ and ‘type B’. In addition, greater variation in the size and shape of type A and B endoconidia has now been found. In the isolates currently analyzed, type A endoconidia were 2–4(–5) µm wide, whereas according to Davidson [4] they were 2–3 µm wide. Davidson [4] reports that endoconidia of the second type (=type B) are short barrel-shaped with dimensions of 5–9 × 5–6.5 µm. Currently, in addition to such spores, also globose spores, even over 9 µm in diameter, have been found both in situ and in vitro. Present observations indicate that changes in size and shape occur over time after the endoconidia leave the phialides. Most likely, Davidson [4] gave the dimensions of the endoconidia when they were still inside the phialides or shortly after they left them. However, without a direct comparison of the current cultures with fresh cultures from A. saccharum, it is not possible to determine whether this interpretation is justified. If D. virescens colonizing A. saccharum did not form globose conidia under any conditions, this would be a significant feature distinguishing it from the fungus colonizing F. sylvatica. The increased diversity of asexual forms in D. virescens is also influenced by the production of endoconidia by germinating type B endoconidia. Such a phenomenon has not been reported in this fungus so far. High variability in the size of conidia in cultures of D. virescens derived from A. saccharum was pointed out by Richter [29]. However, this author did not provide a detailed description of either conidia or phialophores. It was only reported that conidiospores (approximately 6–25 × 3–6 μm in size) were formed directly from hyphae. According to this author, ascospores reached a length of 6–7 μm in vitro, while in the current cultures, they were 5–9 μm long (without gelatinous sheaths).
In the present work, a comprehensive phylogenetic analysis of fungi of the genus Davidsoniella was performed with the use of multiple fragment sequences of five selected genes. This study has shown that four of them, i.e., ITS, 28S, TUB2 and TEF1, are highly useful for the identification of species belonging to this genus. Phylogenetic analysis of three (28S, TUB2, TEF1) of the five gene fragments tested indicated the existence of two lines of D. virescens isolates dependent on host plants. This phenomenon was first pointed out by Harrington et al. [18] on the basis of a study of the variability of D. virescens isolates using 10 isozymes. According to another study by Harrington et al. [54], much of the variation in D. virescens nuclear markers appears to be due to differences between two forms of the species, one associated with Acer and Liriodendron and the second associated with Fagus and other hardwoods. These results were later confirmed by Witthuhn et al. [20], who performed a phylogenetic analysis of selected fungal species, including D. virescens, based on MAT-2 DNA sequences of isolates obtained from different host plants. Differences in TEF1 gene sequences between D. virescens isolates obtained from Acer and Fagus were also evident in the phylogenetic tree presented by Harrington [55]. Unfortunately, this author used single D. virescens sequences representing each lineage, which made it impossible to determine whether these groups differed statistically. In the present study, the identified two groups of isolates received high statistical support according to the results of each of the three phylogenetic analysis methods used (ML, MP and BI). According to Witthuhn et al. [20], since D. virescens isolates fall into two groups based on phylogenetic analysis and host plant specificity, they “should be recognised as distinct species”. However, resolution of this question requires further detailed research.
Numerous Ceratocystidaceae produce intense odor. It is assumed that this is an adaptation for fungal dispersal by insects [1,4,9,12,17,56,57,58]. D. virescens develops a distinctive musty odor on lumber and in culture, while a similar species, E. coerulescens, produces a banana-oil odor [4]. According to other authors, a colony of D. virescens obtained from sugar maple tree had a sweet, musty odor [27,28,29]. In the cultures of D. virescens from F. sylvatica, a sweet but not musty odor was noticeable. This requires further explanation because the production of some metabolites may be influenced by strain-dependent feature and culture conditions [17]. Birkinshaw and Morgan [15] identified the products which are responsible for the odors in D. virescens and E. coerulescens cultures. The first one produced the same methylheptenone and mixture of L- and DL-methylheptenol as E. coerulescens, but in different proportions. However, D. virescens did not produce isobutyl acetate. As in the case of Ceratocystis platani (Walter) Engelbr. & T.C. Harr., volatile organic compounds may be useful as biomarkers for comparison of various strains of D. virescens from Europe and North America [58].
One of the basic factors influencing the development of fungi and the establishment of alien fungi in new areas is temperature [50,59,60,61]. Temperature may also influence the rate at which vascular pathogenic fungi cause disease symptoms [62,63]. Current tests have shown that D. virescens colonies originating from F. sylvatica are able to grow in a wide temperature range, from 5 to 30 °C, and some isolates even up to 35 °C. This means that they can colonize wood in Poland at different times of the year. It is sometimes observed that D. virescens colonies show a variable growth rate in vitro at the same temperature. One of the reasons for this phenomenon may be the high sensitivity of D. virescens colonies to prolonged storage on agar media in vitro. They may lose not only the ability of rapid radial growth but also the ability to produce perithecia [4]. Webber [26] analyzed the temperature range in the distribution zones of D. virescens on A. saccharum in North America and concluded that this fungus would find suitable conditions in the event of an accidental introduction in Great Britain. This also applies to the possibility of development in the areas where the native Acer species occur in continental Europe [47].
4.2. Pathogenicity of D. virescens Isolates from F. sylvatica
Pathogenicity is the ability of a given fungus to cause disease, while virulence determines the degree of pathogenicity [64]. The life style of D. virescens originating from southern Poland is unknown. The current tests were primarily aimed at determining whether it is capable of causing disease symptoms in living plants. The factor that often differentiates the pathogenicity of fungi is the plant host [64]. Two plant species were used in the current tests, F. sylvatica and A. saccharum. The pathogenicity test was conducted by placing fungal inoculum in wounds formed on the stems of both tree species. This should be a favorable circumstance for D. virescens, because wounds play an important role in the process of infection of trees by this fungus. In North American forest stands, D. virescens infects A. saccharum usually through wounds at the root collar and at the base of the stems, which are formed during human activities such as forest thinning, logging, road building, or sap hauling [23,27,29,32,34,49,65].
Wood discoloration was observed in all currently inoculated seedlings, and its extension was very diverse. It should be taken into consideration that as a result of wounding living sapwood, even without the participation of fungi, wood discoloration occurs as a result of compartmentalization [27,66,67,68,69]. This could currently be observed in control plants. In case of infection by weak fungal pathogens through wounds, colonization is typically limited to a small area around the wound site [51]. Numerous pathogenic species from the Ceratocystidaceae may extensively colonize sapwood tissue and cause discoloration or staining as well as wilting and death of whole trees [51,70]. Some of them, e.g., C. platani on infected Platanus trees, after axial expansion in sapwood, can travel through the medullary rays back to the bark, causing its necrosis at various heights of the stem. That results in canker stain, a disease leading to mortality of plane trees [70,71]. However, other fungi, e.g., Bretziella fagacearum (Bretz) Z.W. de Beer et al., spread in the xylem vessel and induce the formation of tyloses and gums, which impair water transport, thus causing symptoms typical of true vascular disease [50,62]. According to Hepting [27], D. virescens develops very numerous hyphae in the vessels, and only rarely in the rays. This author suggests that watersoaking occurs as a result of the death of the surrounding ray and wood parenchyma cells. In places where the sapstreak comes into contact with the cambium, necrosis of this tissue occurs and may form elongated cankers [23,27]. Such a process cannot be excluded in some F. sylvatica seedlings showing stem bark necrosis. However, in the currently inoculated plant species, only a few hyphae were observed in the vessels.
Symptoms in both plant species were evaluated 4 months after inoculation. It is difficult to predict the further effects of the internal changes in the form of wood discoloration. In addition to wood discoloration, 30.6% of F. sylvatica seedlings developed bark necrosis, which would probably lead to their death in the following weeks. The emergence of wilt in the whole crown in five seedlings within 3–6 weeks after inoculation should be considered particularly disturbing. Based on comparison with other dangerous vascular pathogens, this rate of wilting can be considered as very fast [14,72,73]. It is difficult to indicate the factors that had a decisive influence on this. The involvement of certain biochemical compounds in causing wilt symptoms in inoculated F. sylvatica seedlings cannot be ruled out. In members of the Ceratocystidaceae, catechol dioxygenase (CDO) enzymes were identified as pathogenicity and virulence factors. These enzymes are involved in the degradation of phenolic plant defense compounds. It was shown that the genomes of most necrotrophic pathogens, including D. virescens, contained four different genes encoding CDOs, those of weak pathogens contained two to three genes, and those of saprotrophs had only a single gene [74].
The reason may lie in the high susceptibility of some host plant individuals that were unable to limit the rapid axial spread of the pathogen [75]. F. sylvatica is characterized by high genetic, biochemical and physiological diversity, which was the basis for distinguishing two (early and late) phenological forms. Individuals representing these forms differ in the timing of leaf development and autumn fall, frost resistance, level of reactive oxygen species production and profile of antioxidative enzyme activity [76,77,78]. In turn, late frosts are considered as important ecological events that strongly affect the genetic structure of beech, its radial growth, vitality and competitiveness [76,79]. Such aspects are important in the case of tree susceptibility to other pathogens. The differences in susceptibility of Quercus species towards B. fagacearum has generally been attributed to differences in anatomical features and physiological responses of the host to infection [50]. In the case of Dutch elm disease, trees of Ulmus minor Mill. susceptible to Ophiostoma novo-ulmi Brasier had significantly wider and longer vessels [80,81].
In the case of one wilting beech seedling, wood discoloration extended to the roots. This phenomenon is noteworthy because some pathogenic fungi, e.g., C. platani, B. fagacearum, and Davidsoniella australis, can move between neighboring trees through functional root anastomosis, which accelerates their spread in forest stands [50,51,70]. Such situations were also observed in the case of D. virescens in naturally infected sugar maple stands [27,82]. Development of the sapstreak disease is often more rapid and extensive in roots than in stems [23].
A. saccharum is one of the primary deciduous tree species exhibiting sapstreak disease caused by D. virescens in North America. Infected trees show generally symptoms in the crown, inside the base of the trunk and in the roots [23,28,35,47,83]. In currently tested seedlings of A. saccharum, after 4 months, the average vertical extension of stained wood was 2.08 to 2.90 cm. However, Smith and Houston [75] found mean vertical discoloration at a length of 2.8 cm 7 weeks after inoculation of A. saccharum seedlings with D. virescens. In the current experiment, neither bark necrosis on the stem nor disease symptoms in the crown were observed in A. saccharum. Houston [23] observed that older sugar maple trees, that died quickly, showed extensive vascular staining, whereas in trees that never developed severe foliar symptoms, discoloration was usually strongly restricted by the tree. In the currently inoculated A. saccharum seedlings, discoloration of xylem was quite limited, which could be seen by comparison with F. sylvatica. In addition, the study showed that D. virescens was significantly less likely to be re-isolated from stained xylem of inoculated A. saccharum seedlings than in the case of F. sylvatica. However, Hepting [27] regularly re-isolated D. virescens from inoculated sugar maple trees if the tissue was grey, while isolations from watersoaked or green-streaked tissue yielded either no organisms or different kinds of bacteria. D. virescens may affect the internal wood chemistry. In vitro tests have shown that this species can produce volatiles that enhance the growth of other fungi [2,84]. In the case of canker stain of plane trees, C. platani rapidly loses its isolability in vitro if wood has been colonized by saprotrophs [70]).
In North America, it has not yet been clarified whether strains colonizing logs of various hardwood species saprotrophically can cause disease symptoms on living tree species, including sugar maple. Only Harrington et al. [54] reported that an isolate from beech did not cause the disease of A. saccharum. However, the pathogenicity of isolates from sugar maple trees with sapstreak symptoms towards trees of the same species has been demonstrated [27,75]. The present results indicate that the D. virescens isolates found on F. sylvatica in Poland may pose a greater threat to F. sylvatica than to A. saccharum. This result is quite unexpected. When analyzing the phytosanitary risk from D. virescens in Europe, the main consideration was the threat to the native Acer species [26,47]. Many aspects related to the appearance of D. virescens in Europe require further scientific explanation in order to provide the basis for determining the phytosanitary risk for our forest stands.
5. Conclusions
In 2018 and 2021, the fungus Davidsoniella virescens was identified on the wood of Fagus sylvatica in southern Poland based on morphological and phylogenetic studies. This is the first record of this species outside North America. F. sylvatica was demonstrated for the first time as a host plant for this fungus. The current pathogenicity test showed that most F. sylvatica and all A. saccharum seedlings were able to limit the extension of wood discoloration, which prevented any further negative impact on the health of the seedlings. However, the high susceptibility of some beech seedlings turned out to be disturbing. It manifested as sudden wilting of the entire crown or the formation of extensive necrotic bark lesions. These results indicate that Polish isolates of D. virescens may pose a greater threat to F. sylvatica than to A. saccharum. There is a need for further research on the taxonomy and lifestyle of this fungus as well as an assessment of phytosanitary risk for the European forests. The rapid rate of production of ascospores and vegetative endoconidia by D. virescens as well as their huge abundance may be an important factor in the spread and in the establishment of the fungus in new areas. Moreover, based on the temperature assay, it can be concluded that the development of D. virescens mycelium can occur, although at different rates, throughout the year, excluding frosty winter periods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof10070465/s1, Figure S1: Phylogram obtained from analyses of small subunit (SSU) ribosomal DNA (18S) data for the genus Davidsoniella; Figure S2: Phylogram obtained from analyses of internal transcribed spacer (ITS) data for the genus Davidsoniella; Figure S3: Phylogram obtained from analyses of large subunit (LSU) ribosomal DNA (28S) data for the genus Davidsoniella; Table S1: Primers used in this study to amplify and sequence barcode regions in molecular identification; Table S2: Isolates and GenBank accession numbers for reference sequences used in the phylogenetic analyses. References [38,39,40,41,42,43] are cited in the supplementary materials.
Author Contributions
Conceptualization, T.K. and P.B.; methodology, T.K. (mycological aspects) and P.B. (molecular and statistical aspects); investigation and formal analysis, T.K. and P.B.; data curation, T.K. and P.B.; writing—original draft preparation, review and editing, T.K. and P.B.; software, P.B.; supervision, T.K. and P.B.; visualization, P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials or deposited in publicly available databases such as GenBank and TreeBASE.
Acknowledgments
The authors wish to thank Tomasz Gierat (Director of the Ojców National Park) and Paweł Biernacki (the Head of the Brzozów Forest District) for permission to conduct research in the designated area. The authors would also like to thank anonymous reviewers for constructive comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Beer, Z.W.; Duong, T.A.; Barnes, I.; Wingfield, B.D.; Wingfield, M.J. Redefining Ceratocystis and Allied Genera. Stud. Mycol. 2014, 79, 187–219. [Google Scholar] [CrossRef]
- Wingfield, B.D.; Barnes, I.; de Beer, Z.W.; De Vos, L.; Duong, T.A.; Kanzi, A.M.; Naidoo, K.; Nguyen, H.D.T.; Santana, Q.C.; Sayari, M.; et al. Draft Genome Sequences of Ceratocystis eucalypticola, Chrysoporthe cubensis, C. deuterocubensis, Davidsoniella virescens, Fusarium temperatum, Graphilbum fragrans, Penicillium nordicum, and Thielaviopsis musarum. IMA Fungus 2015, 6, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Index Fungorum—Search Page. Available online: http://www.indexfungorum.org/names/Names.asp (accessed on 22 May 2024).
- Davidson, R.W. Two American Hardwood Species of Endoconidiophora Described as New. Mycologia 1944, 36, 300–306. [Google Scholar] [CrossRef]
- Münch, E. Die Blaufäule des Nadelholzes. Naturwissenschaftliche Z. Forst Landwirtsch. 1907, 5, 531–573. [Google Scholar]
- Bakshi, B.K. Fungi Associated with Ambrosia Beetles in Great Britain. Trans. Br. Mycol. Soc. 1950, 33, 111–120. [Google Scholar] [CrossRef]
- Bakshi, B.K. Studies on Four Species of Ceratocystis, with a Discussion of Fungi Causing Sap-Stain in Britain. Mycol. Pap. 1951, 35, 1–16. [Google Scholar]
- Moreau, C. Coexistence Des Formes Thielaviopsis et Graphium Chez Une Souche de Ceratocystis Major (van Beyma) Comb. Nov. Remarques Sur Les Variations Des Ceratocystis. Rev. Mycol. Supplément Colon. 1952, 17, 17–25. [Google Scholar]
- Hunt, J. Taxonomy of the Genus Ceratocystis. Lloydia 1956, 19, 1–59. [Google Scholar]
- Griffin, H.D. The Genus Ceratocystis in Ontario. Can. J. Bot. 1968, 46, 689–718. [Google Scholar] [CrossRef]
- Olchowecki, A.; Reid, J. Taxonomy of the Genus Ceratocystis in Manitoba. Can. J. Bot. 1974, 52, 1675–1711. [Google Scholar] [CrossRef]
- Upadhyay, H.P. A Monograph of Ceratocystis and Ceratocystiopsis; The University of Georgia Press: Athens, Greece, 1981. [Google Scholar]
- Nag Raj, T.R.; Kendrick, B. A Monograph of Chalara and Allied Genera; Wilfrid Laurier University Press: Waterloo, ON, Canada, 1975. [Google Scholar]
- Kile, G.; Walker, J. Chalara australis sp. Nov. (Hyphomycetes), a Vascular Pathogen of Nothofagus cunninghamii (Fagaceae) in Australia and Its Relationship to Other Chalara Species. Aust. J. Bot. 1987, 35, 1–32. [Google Scholar] [CrossRef]
- Birkinshaw, J.H.; Morgan, E.N. Biochemistry of Wood-Rotting Fungi. 6. Volatile Metabolic Products of Species of Endoconidiophora. Biochem. J. 1950, 47, 55–59. [Google Scholar] [CrossRef]
- Koch, W.-G.; Sinnwell, V. Isopulegol from Liquid Cultures of the Fungus Ceratocystis coerulescens (Ascomycotina). Z. Für Naturforsch. C 1987, 42, 159–161. [Google Scholar] [CrossRef]
- Hanssen, H.-P. Volatile Metabolites Produced by Species of Ophiostoma and Ceratocystis. In Ceratocystis and Ophiostoma: Taxonomy, Ecology and Pathogenicity; Wingfield, M.J., Seifert, K.A., Webber, J.F., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 1993; pp. 117–125. [Google Scholar]
- Harrington, T.C.; Steimel, J.P.; Wingfield, M.J.; Kile, G.A. Isozyme Variation and Species Delimitation in the Ceratocystis coerulescens Complex. Mycologia 1996, 88, 104. [Google Scholar] [CrossRef]
- Witthuhn, R.C.; Wingfield, B.D.; Wingfield, M.J.; Wolfaardt, M.; Harrington, T.C. Monophyly of the Conifer Species in the Ceratocystis coerulescens Complex Based on DNA Sequence Data. Mycologia 1998, 90, 96–101. [Google Scholar] [CrossRef]
- Witthuhn, R.C.; Harrington, T.C.; Steimel, J.P.; Wingfield, B.D.; Wingfield, M.J. Comparison of Isozymes, RDNA Spacer Regions and MAT-2 DNA Sequences as Phylogenetic Characters in the Analysis of the Ceratocystis coerulescens Complex. Mycologia 2000, 92, 447–452. [Google Scholar] [CrossRef]
- Montoya, M.M.; Wingfield, M.J. A Review of Ceratocystis Sensu Stricto with Special Reference to the Species Complexes C. coerulescens and C. fimbriata. Rev. Fac. Nal. Agr. Medellín 2006, 59, 3045–3075. [Google Scholar]
- Lagerberg, T. Biological and Practical Researches into Blueing in Pine and Spruce. Sven Skogsvardsforen Tidskr 1927, 25, 145–272. [Google Scholar]
- Houston, D.R. Recognizing and Managing Sapstreak Disease of Sugar Maple; Research Paper—Northeastern Forest Experiment Station; USDA Forest Service: Washington, DC, USA, 1993. [Google Scholar]
- Gionni, A.; Pecori, F.; Santini, A.; Camilleri, M. Pest Survey Card on Davidsoniella virescens. EFSA Support. Publ. 2023, 20, 8186E. [Google Scholar] [CrossRef]
- Sinclair, W.A.; Lyon, H.H. Diseases of Trees and Shrubs, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 2005; ISBN 978-0-8014-4371-8. [Google Scholar]
- Webber, J. Abbreviated Pest Risk Analysis for Ceratocystis virescens. In Forest Research; 2018; pp. 1–8. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/downloadExternalPra.cfm?id=3513 (accessed on 19 May 2024).
- Hepting, G.H. Sapstreak, a New Killing Disease of Sugar Maple. Phytopathology 1944, 34, 1069–1076. [Google Scholar]
- Kessler, K.J.J. Sapstreak Disease of Sugar Maple. For. Pest Leafl. USDA For. Serv. 1972, 128, 1–4. [Google Scholar]
- Richter, D.L. The Sugar Maple Sapstreak Fungus (Ceratocystis virescens) (Davidson) Moreau, Ascomycota) in the Huron Mountains, Marquette County, Michigan. Mich. Bot. 2012, 51, 73–82. [Google Scholar]
- Kehr, R.; Pehl, L.; Wulf, A.; Schröder, T.; Kaminski, K. Zur Gefährdung von Bäumen und Waldökosystemen durch Eingeschleppte Krankheiten und Schädlinge. Nachrichtenblatt Dtsch. Pflanzenschutzdienstes 2004, 56, 217–238. [Google Scholar]
- Ohman, J.H.; Spike, A.B. Effect of Straining Caused by Sapstreak Disease on Sugar Maple Log and Lumber Values; Research Note NC-12; U.S. Dept. of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1966; Volume 12, pp. 1–4. [Google Scholar]
- Mielke, M.E.; Charette, D.A. The Incidence of Sapstreak Disease of Sugar Maple in Menominee County, Wisconsin, and Its Relationship to Wounds and Season of Logging. North. J. Appl. For. 1989, 6, 65–67. [Google Scholar] [CrossRef]
- Smith, K.T. Sapstreak Disease and Biodeterioration of Sugar Maple. Biodeterior. Res. 1990, 3, 303–310. [Google Scholar]
- Bal, T.L.; Richter, D.L.; Storer, A.J.; Jurgensen, M.F. The Relationship of the Sapstreak Fungus, Ceratocystis virescens, to Sugar Maple Dieback and Decay in Northern Michigan. Am. J. Plant Sci. 2013, 04, 436–443. [Google Scholar] [CrossRef]
- EPPO Global Database. Available online: https://gd.eppo.int/ (accessed on 21 April 2024).
- Zajonc, V.J.; Wulf, A. European Maple Species Endangered by Sapstreak (Ceratocystis virescens)? Nachrichtenblatt Dtsch. Pflanzenschutzdienstes 1997, 49, 297–300. [Google Scholar]
- Kowalski, T.; Bilański, P.; Grad, B. The Occurrence of Apiognomonia hystrix and Its Pathogenicity towards Acer Pseudoplatanus and Fraxinus Excelsior under Field Conditions. Forests 2021, 13, 35. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of Primer Sets Designed for Use with the PCR to Amplify Conserved Genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Hausner, G.; Reid, J.; Klassen, G.R. On the Phylogeny of Ophiostoma, Ceratocystis s.s., and Microascus, and Relationships within Ophiostoma Based on Partial Ribosomal DNA Sequences. Can. J. Bot. 1993, 71, 1249–1265. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Rehner, S.A.; Samuels, G.J. Taxonomy and Phylogeny of Gliocladium Analysed from Nuclear Large Subunit Ribosomal DNA Sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA from Several Cryptococcus Species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Oliveira, L.S.S.; Harrington, T.C.; Ferreira, M.A.; Damacena, M.B.; Al-Sadi, A.M.; Al-Mahmooli, I.H.S.; Alfenas, A.C. Species or Genotypes? Reassessment of Four Recently Described Species of the Ceratocystis Wilt Pathogen, Ceratocystis fimbriata, on Mangifera indica. Phytopathology 2015, 105, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.; Bilański, P.; Kraj, W. Pathogenicity of Fungi Associated with Ash Dieback towards Fraxinus excelsior. Plant Pathol. 2017, 66, 1228–1238. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Data Science Workbench. Statistica, Version 13.1; TIBCO Software Inc.: Palo Alto, CA, USA, 2020. Available online: https://tibco.com (accessed on 1 May 2024).
- Jeger, M.; Bragard, C.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Gregoire, J.; Jaques Miret, J.A.; MacLeod, A.; et al. Pest Categorisation of Davidsoniella virescens. EFSA J. 2017, 15, e05104. [Google Scholar] [CrossRef]
- Shigo, A.L. Observations on the Succession of Fungi on Hardwood Pulpwood Bolts. Plant Dis. Rep. 1962, 46, 379–380. [Google Scholar]
- Ohman, J.H.; Kessler, K.J. Current Status of the Sapstreak Disease of Sugar Maple in the Lake States; Research Note LS-10; Lake States Forest Experiment Station, U.S. Dept. of Agriculture: St. Paul, MN, USA, 1963; Volume 11, pp. 1–4. [Google Scholar]
- Juzwik, J.; Appel, D.N.; MacDonald, W.L.; Burks, S. Challenges and Successes in Managing Oak Wilt in the United States. Plant Dis. 2011, 95, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Harrington, T.C. Ceratocystis Diseases. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 230–255. [Google Scholar]
- Jankowiak, R.; Bilański, P. Ophiostomatoid Fungi Associated with Root-feeding Bark Beetles on Scots Pine in Poland. For. Pathol. 2013, 43, 422–428. [Google Scholar] [CrossRef]
- Walters, J.W. Sapstreak Disease of Sugar Maple. In Northern Hardwood Notes; U.S. Department of Agriculture, Forest Service, North Central Research Station: St. Paul, MN, USA, 1992. [Google Scholar]
- Harrington, T.C.; Steimel, J.; Kile, G. Genetic Variation in Three Ceratocystis Species with Outcrossing, Selfing and Asexual Reproductive Strategies. Eur. J. For. Pathol. 1998, 28, 217–226. [Google Scholar] [CrossRef]
- Harrington, T.C. The Genus Ceratocystis: Where Does the Oak Wilt Fungus Fit? In Proceedings of the 2nd National Oak Wilt Symposium; Appel, D.N., Billings, R.F., Eds.; USDA Forest Service, Forest Health Protection: St. Paul, MN, USA, 2009; pp. 27–42. [Google Scholar]
- Collins, R.P.; Morgan, M.E. Identity of Fruit-like Aroma Substances Synthesized by Endoconidial-Forming Fungi. Phytopathology 1962, 52, 407–409. [Google Scholar]
- Kowalski, T.; Butin, H. Taxonomie Bekannter Und Neuer Ceratocystis-Arten an Eiche (Quercus robur L.). J. Phytopathol. 1989, 124, 236–248. [Google Scholar] [CrossRef]
- Brilli, F.; Luchi, N.; Michelozzi, M.; Calamai, L.; Cencetti, G.; Pecori, F.; Nigrone, E.; Santini, A. Volatile Organic Compounds (VOC) as Biomarkers for Detection of Ceratocystis platani. For. Pathol. 2020, 50, e12618. [Google Scholar] [CrossRef]
- Kirisits, T.; Krumböck, S.; Konrad, H.; Pennerstorfer, J.; Halmschlager, E. Untersuchungen Über das Auftreten der Erreger der Holländischen Ulmenwelke in Österreich. Forstwiss. Cent. 2001, 120, 231–241. [Google Scholar] [CrossRef]
- Hauptman, T.; Piškur, B.; de Groot, M.; Ogris, N.; Ferlan, M.; Jurc, D. Temperature Effect on Chalara fraxinea: Heat Treatment of Saplings as a Possible Disease Control Method. For. Pathol. 2013, 43, 360–370. [Google Scholar] [CrossRef]
- Brasier, C.M.; Webber, J.F. Is There Evidence for Post-Epidemic Attenuation in the Dutch Elm Disease Pathogen Ophiostoma novo-ulmi? Plant Pathol. 2019, 68, 921–929. [Google Scholar] [CrossRef]
- Fenn, P.; Durbin, R.D.; Kuntz, J.E. Wilt Development in Red Oak Seedlings: A New System for Studying Oak Wilt. Phytopathology 1975, 65, 1381–1386. [Google Scholar] [CrossRef]
- Brasier, C.M.; Webber, J.F. Positive Correlations between in Vitro Growth Rate and Pathogenesis in Ophiostoma Ulmi. Plant Pathol. 1987, 36, 462–466. [Google Scholar] [CrossRef]
- Dickman, M.B. Subversion or Coersion? Pathogenic Determinants in Fungal Phytopathogens. Fungal Biol. Rev. 2007, 21, 125–129. [Google Scholar] [CrossRef]
- Houston, D.R. Importance of Buttress Root and Taphole Wounds as Infection Courts from the Sugar Maple (Acer saccharum) Sapstreak Pathogen, Ceratocystis coerulescens. Phytopathology 1992, 82, 244. [Google Scholar]
- Shigo, A.L. Compartmentalization: A Conceptual Framework for Understanding How Trees Grow and Defend Themselves. Annu. Rev. Phytopathol. 1984, 22, 189–214. [Google Scholar] [CrossRef]
- Shortle, W.C.; Smith, K.T.; Dudzik, K.R.; Parker, S. Response of Maple Sapwood to Injury and Infection. Eur. J. For. Pathol. 1995, 25, 241–252. [Google Scholar] [CrossRef]
- Woodward, S.; Pocock, S. Formation of the Ligno-suberized Barrier Zone and Wound Periderm in Four Species of European Broad-leaved Trees. Eur. J. For. Pathol. 1996, 26, 97–105. [Google Scholar] [CrossRef]
- Dujesiefken, D.; Liese, W.; Shortle, W.; Minocha, R. Response of Beech and Oaks to Wounds Made at Different Times of the Year. Eur. J. For. Res. 2005, 124, 113–117. [Google Scholar] [CrossRef]
- Panconesi, A. Canker Stain of Plane Trees: A Serious Danger to Urban Plantings in Europe. J. Plant Pathol. 1999, 81, 3–15. [Google Scholar]
- Butin, H. Krankheiten der Wald-und Parkbäume. Diagnose-Biologie-Bekämpfung, 2nd ed.; Eugen Ulmer Verlag: Stuttgart, Germany, 2019; ISBN 978-3-8186-0728-9. [Google Scholar]
- Urban, J.; Dvořák, M. Sap Flow-Based Quantitative Indication of Progression of Dutch Elm Disease after Inoculation with Ophiostoma Novo-Ulmi. Trees 2014, 28, 1599–1605. [Google Scholar] [CrossRef]
- Lechner, Y.; Maschek, O.; Kirisits, T.; Halmschlager, E. Further Pathogenicity Testing of Verticillium nonalfalfae, a Biocontrol Agent against the Invasive Tree of Heaven (Ailanthus altissima), on Non-Target Tree Species in Europe. Phytoparasitica 2023, 51, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Soal, N.C.; Coetzee, M.P.A.; van der Nest, M.A.; Hammerbacher, A.; Wingfield, B.D. Phenolic Degradation by Catechol Dioxygenases Is Associated with Pathogenic Fungi with a Necrotrophic Lifestyle in the Ceratocystidaceae. G3 Genes Genomes Genet. 2022, 12, jkac008. [Google Scholar] [CrossRef]
- Smith, K.T.; Houston, D.R. Metal Concentrations in Wood of Sugar Maple Infected with Sapstreak Disease. Can. J. For. Res. 1994, 24, 185–188. [Google Scholar] [CrossRef]
- Kraj, W.; Sztorc, A. Genetic Structure and Variability of Phenological Forms in the European Beech (Fagus sylvatica L.). Ann. For. Sci. 2009, 66, 203. [Google Scholar] [CrossRef]
- Kraj, W. Reactive Oxygen Species and Antioxidant Levels as the Factors of Autumn Senescence in Phenological Forms of Beech (Fagus sylvatica L.). Acta Physiol. Plant. 2016, 38, 32. [Google Scholar] [CrossRef]
- Kraj, W. Antioxidative Enzyme Activity as the Factor Causing Differential Autumn Senescence in Phenological Forms of Beech (Fagus sylvatica L.). Acta Physiol. Plant. 2017, 39, 16. [Google Scholar] [CrossRef]
- Dittmar, C.; Fricke, W.; Elling, W. Impact of Late Frost Events on Radial Growth of Common Beech (Fagus sylvatica L.) in Southern Germany. Eur. J. For. Res. 2006, 125, 249–259. [Google Scholar] [CrossRef]
- Solla, A.; Gil, L. Xylem Vessel Diameter as a Factor in Resistance of Ulmus Minor to Ophiostoma Novo-ulmi. For. Pathol. 2002, 32, 123–134. [Google Scholar] [CrossRef]
- Venturas, M.; López, R.; Martín, J.A.; Gascó, A.; Gil, L. Heritability of Ulmus minor Resistance to Dutch Elm Disease and Its Relationship to Vessel Size, but Not to Xylem Vulnerability to Drought. Plant Pathol. 2014, 63, 500–509. [Google Scholar] [CrossRef]
- Houston, D.R. Spread of the Sugar Maple Sapstreak Disease Pathogen, Ceratocystis coerulescens, via Root Grafts between Acer saccharum. Phytopathology 1991, 81, 122. [Google Scholar]
- Baral, S.K.; Schneider, R.; Pothier, D.; Berninger, F. Predicting Sugar Maple (Acer saccharum) Discoloured Wood Characteristics. Can. J. For. Res. 2013, 43, 649–657. [Google Scholar] [CrossRef]
- Wargo, P.M.; Harrington, T.C. Host Stress and Susceptibility. In Armillaria Root Disease. Agriculture Handbook; Shaw, C.G., Kile, G.A., Eds.; USDA Forest Service: Washington, DC, USA, 1991; Volume 691, pp. 88–101. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).