Temporal Dynamics of Airborne Concentrations of Ganoderma Basidiospores and Their Relationship with Environmental Conditions in Oil Palm (Elaeis guineensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Site
2.2. Monitoring of Basidiospore Concentrations
2.3. Meteorological Data and Statistical Analysis
2.4. Field Disease Monitoring
3. Results
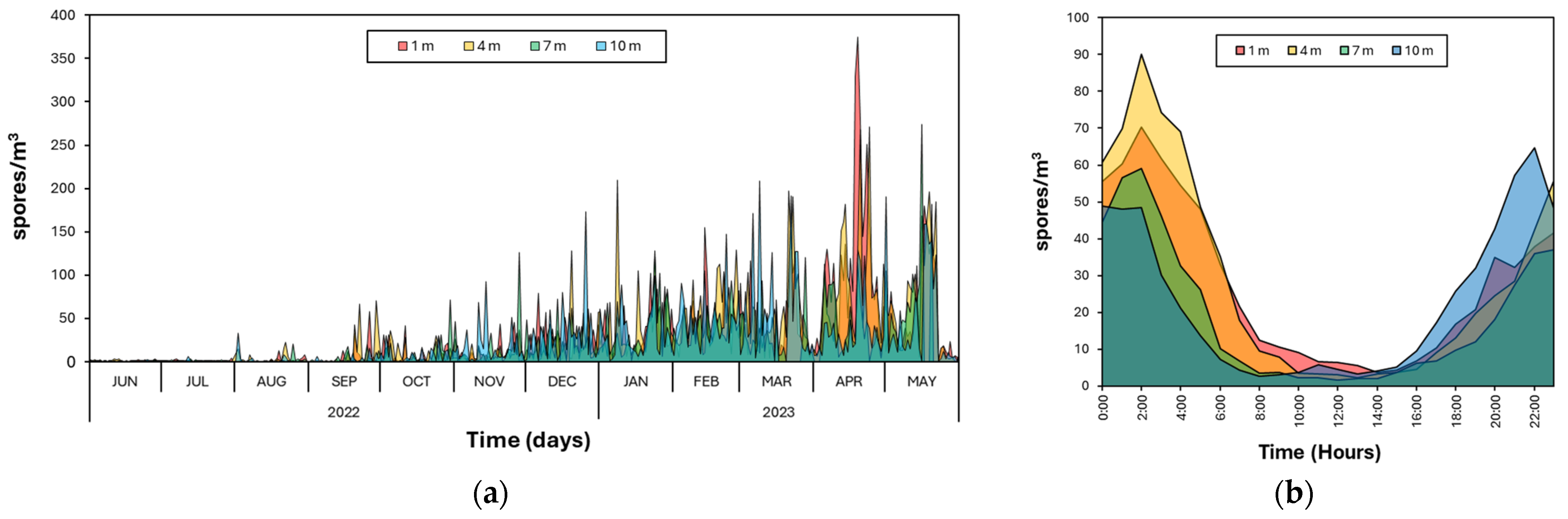
3.1. Capture Height
3.2. Capture Periodicity
3.3. Climatic Correlation
3.4. Field Disease Monitoring with Respect to the Sampling Point
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA—Foreign Agricultural Service Oilseeds Global Production (2023/2024). Available online: https://fas.usda.gov/ (accessed on 25 May 2024).
- Federación Nacional de Cultivadores de Palma de Aceite, Fedepalma. Anuario Estadístico 2023: Principales Cifras de La Agroindustria de La Palma de Aceite y En El Mundo 2018–2022. In Anuario Estadístico; Fedepalma: Bogota, Colombia, 2023; p. 235. [Google Scholar]
- Dishington, J.M. Innovación y Sostenibilidad En Agroindustria de La Palma de Aceite En Colombia. Rev. Palmas 2019, 40, 9–18. [Google Scholar]
- CABI—Technical Factsheet. Ganoderma boninense (Basal Stem Rot of Oil Palm); CABI: Wallingford, UK, 2022. [Google Scholar]
- Corley, R.; Tinker, P. The Oil Palm, 4th ed.; Ltda, B.S., Ed.; John Wiley & Sons: Oxford, UK, 2003; ISBN 9780470750971. [Google Scholar]
- Paterson, R.R.M. Ganoderma boninense Disease Deduced from Simulation Modelling with Large Data Sets of Future Malaysian Oil Palm Climate. Phytoparasitica 2019, 47, 255–262. [Google Scholar] [CrossRef]
- Ho, Y.W.; Nawawi, A. Ganoderma boninense Pat. from Basal Stem Rot of Oil Palm (Elaeis guineensis) in Peninsular Malaysia. Pertanika 1985, 8, 425–428. [Google Scholar] [CrossRef]
- Haryadi, D.; Hendra, H.; Singh Sidhu, M.; Panjaitan, T.; Chong, K.P. The First Report on Basal Stem Rot Disease Causal Pathogen in Asian Agri Group, North Sumatra, Indonesia. Asian Agri Res. Dev. Cent. 2019, 6, 141–149. [Google Scholar]
- Steyaert, R.L. Species of Ganoderma and Related Genera Mainly of the Bogor and Leiden Herbaria. Persoonia 1972, 7, 55–118. [Google Scholar]
- Castillo, S.Y.; Rodríguez, M.C.; González, L.F.; Zúñiga, L.F.; Mestizo, Y.A.; Medina, H.C.; Montoya, C.; Morales, A.; Romero, H.M.; Sarria, G.A. Ganoderma zonatum Is the Causal Agent of Basal Stem Rot in Oil Palm in Colombia. J. Fungi 2022, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, L. Basal Stem Rot of Oil Palm: The Pathogen, Disease Incidence, and Control Methods. Plant Dis. 2023, 107, 603–615. [Google Scholar] [CrossRef]
- Castillo, S.Y. Descripción de La Pudrición Alta Del Estípite En Colombia. In Informe de Labores—Cenipalma; Cenipalma, Ed.; Cenipalma: Bogotá, Colombia, 2023; p. 117. [Google Scholar]
- Rees, R.W.; Flood, J.; Hasan, Y.; Cooper, R.M. Effects of Inoculum Potential, Shading and Soil Temperature on Root Infection of Oil Palm Seedlings by the Basal Stem Rot Pathogen Ganoderma boninense. Plant Pathol. 2007, 56, 862–870. [Google Scholar] [CrossRef]
- Hasan, Y.; Foster, H.L.; Flood, J. Investigations on the Causes of Upper Stem Rot (USR) on Standing Mature Oil Palms. Mycopathologia 2005, 159, 109–112. [Google Scholar] [CrossRef]
- Pilotti, C.A. Stem Rots of Oil Palm Caused by Ganoderma boninense: Pathogen Biology and Epidemiology. Mycopathologia 2005, 159, 129–137. [Google Scholar] [CrossRef]
- Chung, G.F. Management of Ganoderma Diseases in Oil Palm to Minimise Spreading in the Fields. Planter 2005, 81, 765–773. [Google Scholar]
- Miller, R.N.G.; Holderness, M.; Bridge, P.D.; Chung, G.F.; Zakaria, M.H. Genetic Diversity of Ganoderma in Oil Palm Plantings. Plant Pathol. 1999, 48, 595–603. [Google Scholar] [CrossRef]
- Rees, R.W.; Flood, J.; Hasan, Y.; Wills, M.A.; Cooper, R.M. Ganoderma boninense Basidiospores in Oil Palm Plantations: Evaluation of Their Possible Role in Stem Rots of Elaeis guineensis. Plant Pathol. 2012, 61, 567–578. [Google Scholar] [CrossRef]
- Castillo, S.Y. Interacciones de Compatibilidad Somática de Aislamientos de Ganoderma zonatum. In Informe de Labores—Cenipalma; Cenipalma, Ed.; Cenipalma: Bogota, Colombia, 2022; p. 110. [Google Scholar]
- Pilotti, C.A.; Gorea, E.A.; Bonneau, L. Basidiospores as Sources of Inoculum in the Spread of Ganoderma boninense in Oil Palm Plantations in Papua New Guinea. Plant Pathol. 2018, 67, 1841–1849. [Google Scholar] [CrossRef]
- Flood, J.; Hasan, Y.; Foster, H. Ganoderma Diseases of Oil Palm: An Interpretation from Bah Lias Research Station. Planter 2002, 78, 689–710. [Google Scholar]
- Karunarathna, S.C.; Patabendige, N.M.; Lu, W.; Asad, S.; Hapuarachchi, K.K. An In-Depth Study of Phytopathogenic Ganoderma: Pathogenicity, Advanced Detection Techniques, Control Strategies, and Sustainable Management. J. Fungi 2024, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Jazuli, N.A.; Kamu, A.; Chong, K.P.; Gabda, D.; Hassan, A.; Abu Seman, I.; Ho, C.M. A Review of Factors Affecting Ganoderma Basal Stem Rot Disease Progress in Oil Palm. Plants 2022, 11, 2462. [Google Scholar] [CrossRef] [PubMed]
- Flood, J.; Bridge, P.D.; Pilotti, C.A. Basal Stem Rot of Oil Palm Revisited. Ann. Appl. Biol. 2022, 181, 160–181. [Google Scholar] [CrossRef]
- López-Vásquez, J.M. Estudios Epidemiológicos de La Pudrición Basal Del Estípite En Colombia. In Informe de Labores—Cenipalma; Cenipalma, Ed.; Cenipalma: Bogota, Colombia, 2023; p. 117. [Google Scholar]
- Grant-Smith, E. Sampling and Identifying Allergenic Pollens and Molds. An Illustrated Identification Manual for Air Samplers; Blewstone Press: San Antonio, TX, USA, 1986. [Google Scholar]
- Hirst, J.M. Changes in Atmospheric Spore Content: Diurnal Periodicity and the Effects of Weather. Trans. Br. Mycol. Soc. 1953, 36, 375-IN8. [Google Scholar] [CrossRef]
- Sparks, A.H. Nasapower: A NASA POWER Global Meteorology, Surface Solar Energy and Climatology Data Client for R Summary and Statement of Need. J. Open Source Softw. 2018, 3, 1035. [Google Scholar] [CrossRef]
- Jedryczka, M.; Strzelczak, A.; Grinn-Gofron, A.; Nowak, M.; Wolski, T.; Siwulski, M.; Sobieralski, K.; Kaczmarek, J. Advanced Statistical Models Commonly Applied in Aerobiology Cannot Accurately Predict the Exposure of People to Ganoderma Spore-Related Allergies. Agric. For. Meteorol. 2015, 201, 209–217. [Google Scholar] [CrossRef]
- Sanderson, F.R. An Insight into Spore Dispersal of Ganoderma boninense on Oil Palm. Mycopathologia 2005, 159, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Lim, C.C.; Chia, C.C.; Teo, K.W. Studies on Ganoderma Spread and Control. Planter 2003, 79, 367–383. [Google Scholar]
- Ho, Y.W.; Nawawi, A. Diurnal Periodicity of Spore Discharge in Ganoderma boninense Pat. from Oil Palm in Malaysia. Pertanika J. Trop. Agric. Sci. 1986, 9, 147–150. [Google Scholar]
- Sreeramulu, T. Observations on the Periodicity in the Air-Borne Spores of Ganoderma Applanatum. Mycologia 1963, 55, 371–379. [Google Scholar] [CrossRef]
- Haard, R.T.; Kramer, C.L. Periodicity of Spore Discharge in the Hymenomycetes. Mycologia 1970, 62, 1145–1169. [Google Scholar] [CrossRef]
- Ingold, C.T. Fungal Spores. Their Liberation and Dispersal; Oxford University Press: Oxford, UK, 1971. [Google Scholar]
- Hasnain, S.M.; Al-Frayh, A.; Khatija, F.; Al-Sedairy, S. Airborne Ganoderma Basidiospores in a Country with Desert Environment. Grana 2004, 43, 11–115. [Google Scholar] [CrossRef][Green Version]
- Grinn-Gofroń, A.; Bogawski, P.; Bosiacka, B.; Nowosad, J.; Camacho, I.; Sadyś, M.; Skjøth, C.A.; Pashley, C.H.; Rodinkova, V.; Çeter, T.; et al. Abundance of Ganoderma sp. in Europe and SW Asia: Modelling the Pathogen Infection Levels in Local Trees Using the Proxy of Airborne Fungal Spore Concentrations. Sci. Total Environ. 2021, 793, 148509. [Google Scholar] [CrossRef] [PubMed]
- Grinn-Gofroń, A.; Bosiacka, B.; Bednarz, A.; Wolski, T. A Comparative Study of Hourly and Daily Relationships between Selected Meteorological Parameters and Airborne Fungal Spore Composition. Aerobiologia 2018, 34, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.M. Influence of Meteorological Factors on the Air Spora. Grana 1993, 32, 184–188. [Google Scholar] [CrossRef]
- Kasprzyk, I.; Grinn-Gofroń, A.; Strzelczak, A.; Wolski, T. Hourly Predictive Artificial Neural Network and Multivariate Regression Trees Models of Ganoderma Spore Concentrations in Rzeszów and Szczecin (Poland). Sci. Total Environ. 2011, 409, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Sadyś, M.; Skjøth, C.A.; Kennedy, R. Back-Trajectories Show Export of Airborne Fungal Spores (Ganoderma sp.) from Forests to Agricultural and Urban Areas in England. Atmos. Environ. 2014, 84, 88–99. [Google Scholar] [CrossRef]
- Li, D.-W.; Kendrick, B. A Year-Round Outdoor Aeromycological Study in Waterloo, Ontario, Canada. Grana 1995, 34, 199–207. [Google Scholar] [CrossRef]
- Kadowaki, K.; Leschen, R.; Beggs, J. Periodicity of Spore Release from Individual Ganoderma Fruiting Bodies in a Natural Forest. Australas. Mycol. 2010, 29, 17–23. [Google Scholar]
- De la Cruz Buelvas, J.; Valencia Ochoa, G.; Vanegas Chamorro, M. Estudio Estadístico de La Velocidad y La Dirección Del Viento En Los Departamentos de Atlántico y Bolívar En Colombia. Ingeniare Rev. Chil. Ing. 2018, 26, 319–328. [Google Scholar] [CrossRef]



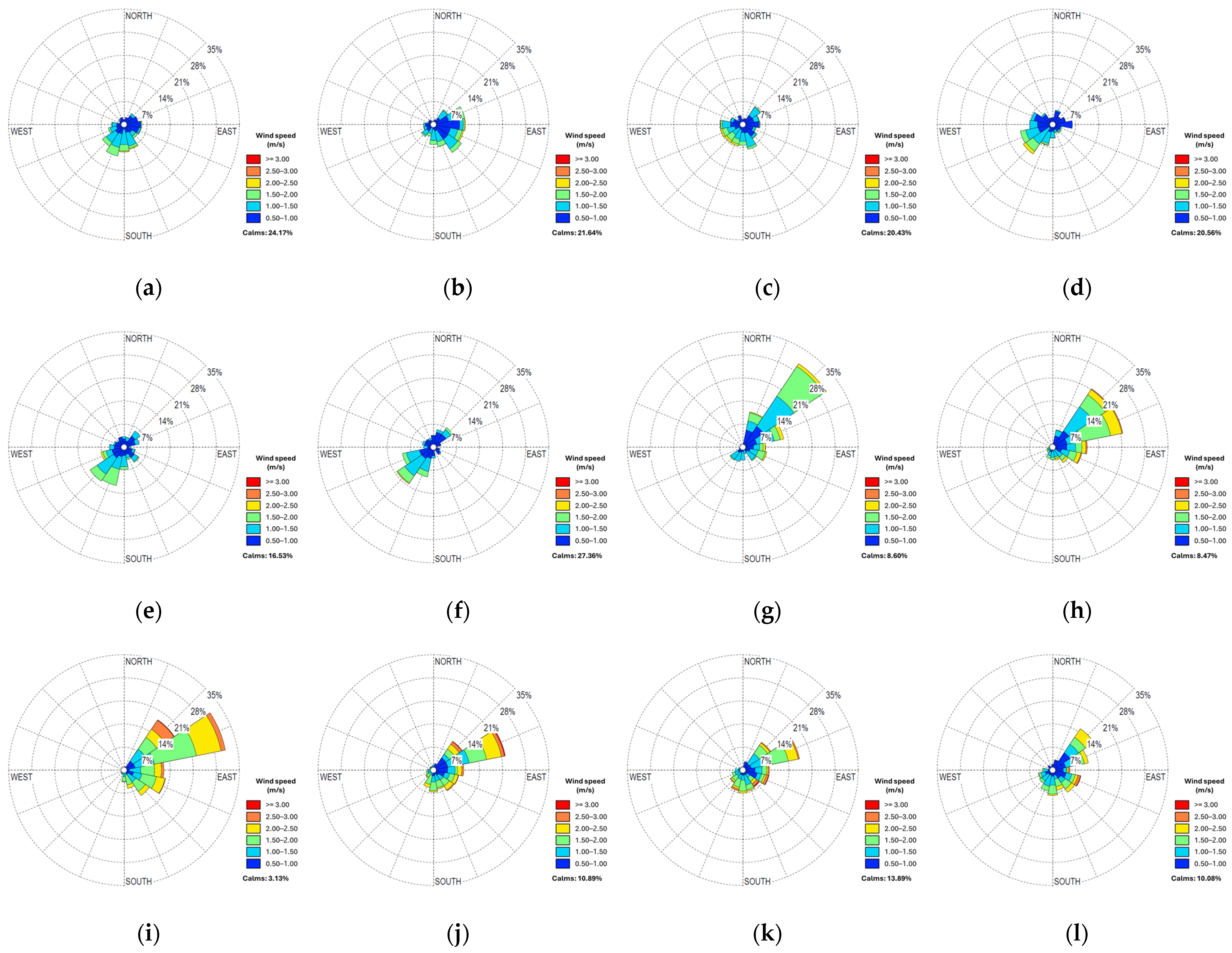

| Date | Solar Radiation | Temperature | Relative Humidity | Precipitation | Wind Speed at 2 m | Wind Speed at 10 m | |
|---|---|---|---|---|---|---|---|
| Year | Month | (Mj/month) | (°C) | (%) | (mm) | (m/s) | (m/s) |
| 2022 | June | 0.02 | 0.03 | −0.01 | −0.02 | −0.18 | −0.17 |
| July | 0.13 | 0.13 | −0.17 | −0.25 | 0.04 | 0.13 | |
| August | −0.16 | −0.14 | 0.10 | −0.01 | 0.07 | −0.02 | |
| September | −0.26 | −0.41 | 0.22 | −0.19 | 0.05 | 0.11 | |
| October | 0.32 | 0.01 | −0.15 | −0.03 | 0.16 | 0.14 | |
| November | 0.25 | 0.22 | −0.18 | −0.13 | −0.06 | −0.35 | |
| December | 0.02 | 0.28 | −0.48 | −0.25 | −0.04 | 0.54 ** | |
| 2023 | January | 0.15 | 0.17 | −0.26 | 0.05 | 0.42 * | 0.46 ** |
| February | 0.21 | 0.23 | −0.20 | 0.06 | 0.30 | −0.29 | |
| March | 0.24 | 0.01 | −0.07 | 0.15 | 0.24 | 0.22 | |
| April | −0.15 | −0.24 | 0.18 | 0.13 | −0.04 | −0.06 | |
| May | 0.01 | 0.24 | −0.34 | −0.18 | −0.22 | 0.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Vásquez, J.M.; Castillo, S.Y.; Zúñiga, L.F.; Sarria, G.A.; Morales-Rodríguez, A. Temporal Dynamics of Airborne Concentrations of Ganoderma Basidiospores and Their Relationship with Environmental Conditions in Oil Palm (Elaeis guineensis). J. Fungi 2024, 10, 479. https://doi.org/10.3390/jof10070479
López-Vásquez JM, Castillo SY, Zúñiga LF, Sarria GA, Morales-Rodríguez A. Temporal Dynamics of Airborne Concentrations of Ganoderma Basidiospores and Their Relationship with Environmental Conditions in Oil Palm (Elaeis guineensis). Journal of Fungi. 2024; 10(7):479. https://doi.org/10.3390/jof10070479
Chicago/Turabian StyleLópez-Vásquez, Juan Manuel, Sandra Yulieth Castillo, León Franky Zúñiga, Greicy Andrea Sarria, and Anuar Morales-Rodríguez. 2024. "Temporal Dynamics of Airborne Concentrations of Ganoderma Basidiospores and Their Relationship with Environmental Conditions in Oil Palm (Elaeis guineensis)" Journal of Fungi 10, no. 7: 479. https://doi.org/10.3390/jof10070479
APA StyleLópez-Vásquez, J. M., Castillo, S. Y., Zúñiga, L. F., Sarria, G. A., & Morales-Rodríguez, A. (2024). Temporal Dynamics of Airborne Concentrations of Ganoderma Basidiospores and Their Relationship with Environmental Conditions in Oil Palm (Elaeis guineensis). Journal of Fungi, 10(7), 479. https://doi.org/10.3390/jof10070479






