Effects of Nitrogen Accumulation, Transportation, and Grain Nutritional Quality and Advances in Fungal Endophyte Research in Quinoa (Chenopodium quinoa Willd.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Test Site
2.1.1. Experimental Design
2.1.2. Determination of Dry Mass
2.1.3. Yield and Components
2.1.4. Methods for Determination of Endophytic Bacteria in Grains
2.1.5. Data Quality Control and Analysis
3. Results
3.1. Nitrogen Accumulation and Translocation in Quinoa Plants
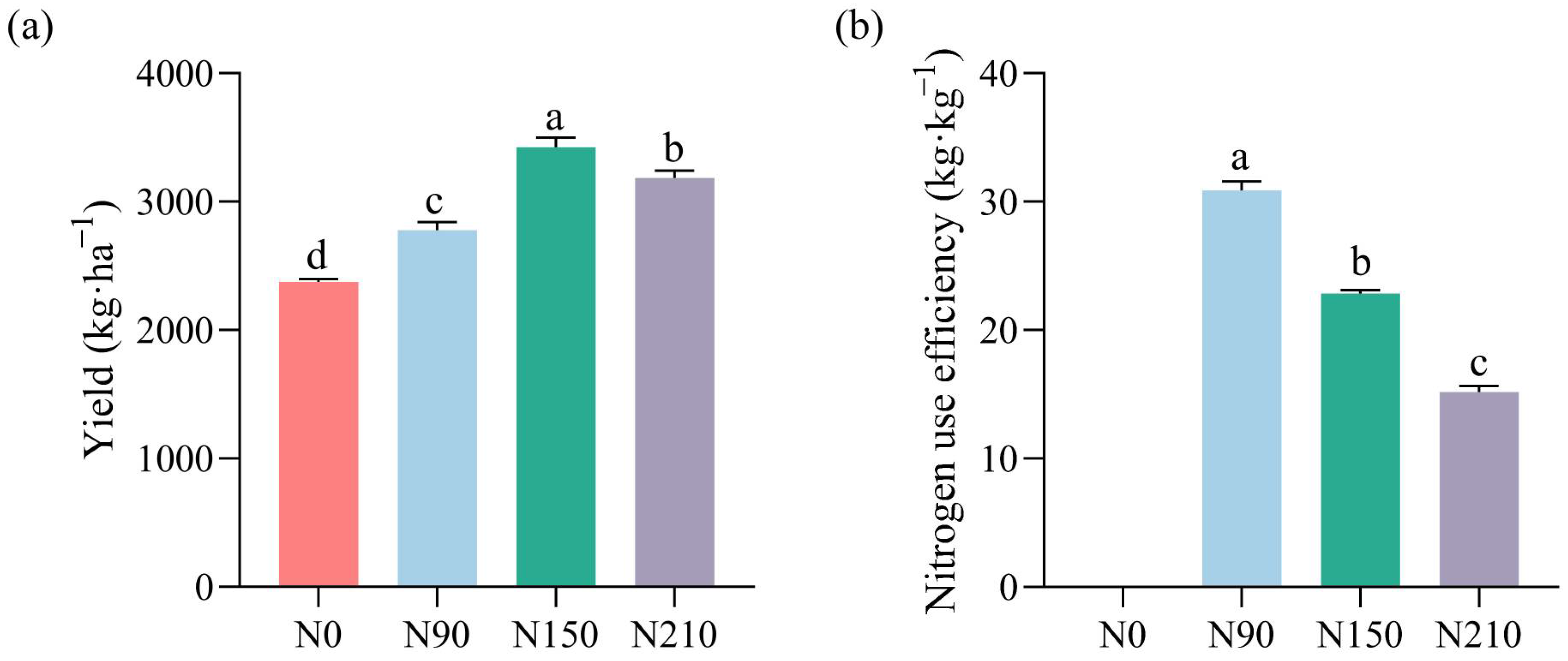
3.1.1. Effects of Nitrogen Application Rates on Quinoa Yield and Nitrogen Use Efficiency
3.1.2. Effects of Nitrogen Application Rates on Nutritional Quality of Quinoa Grains
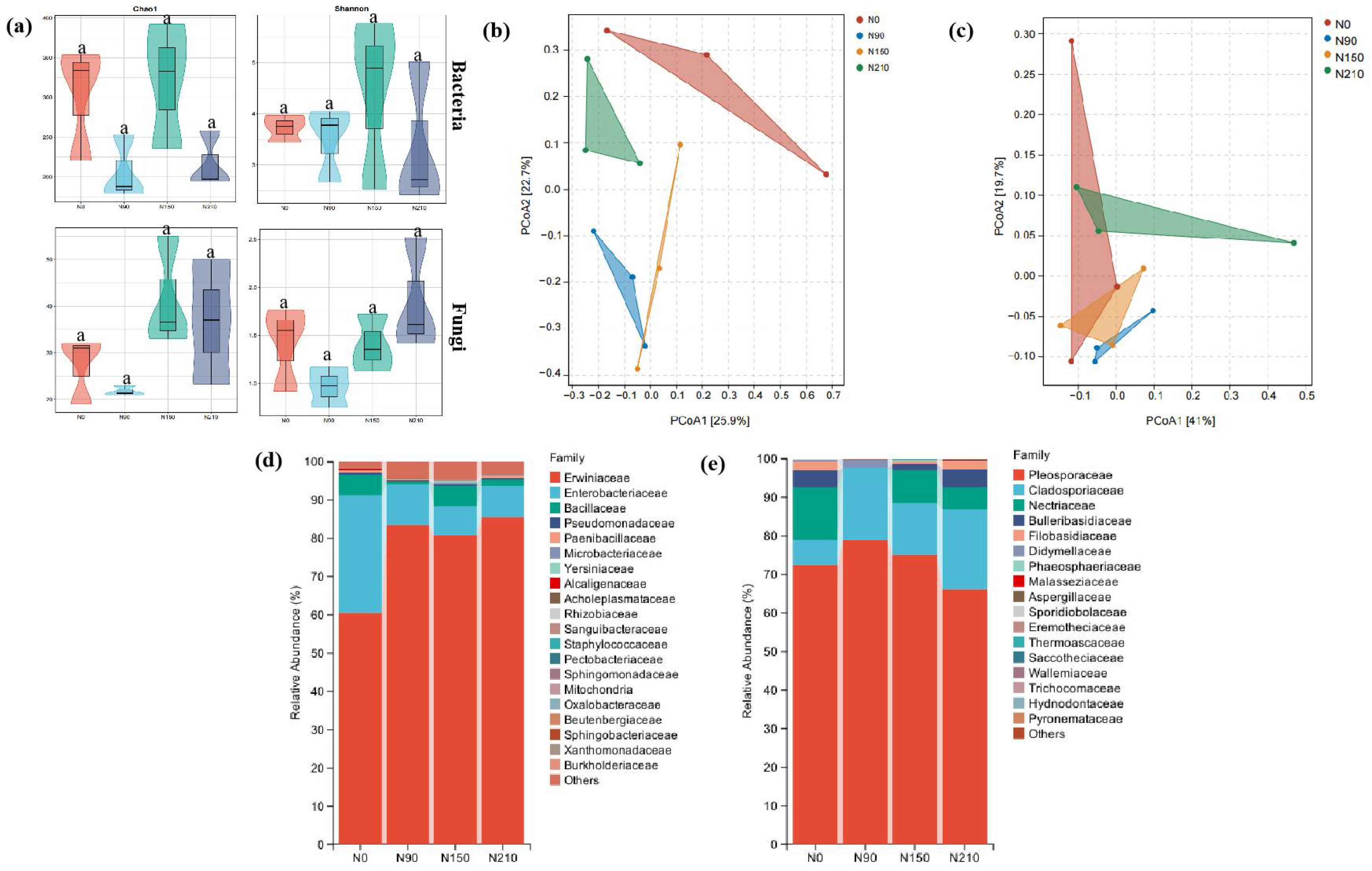
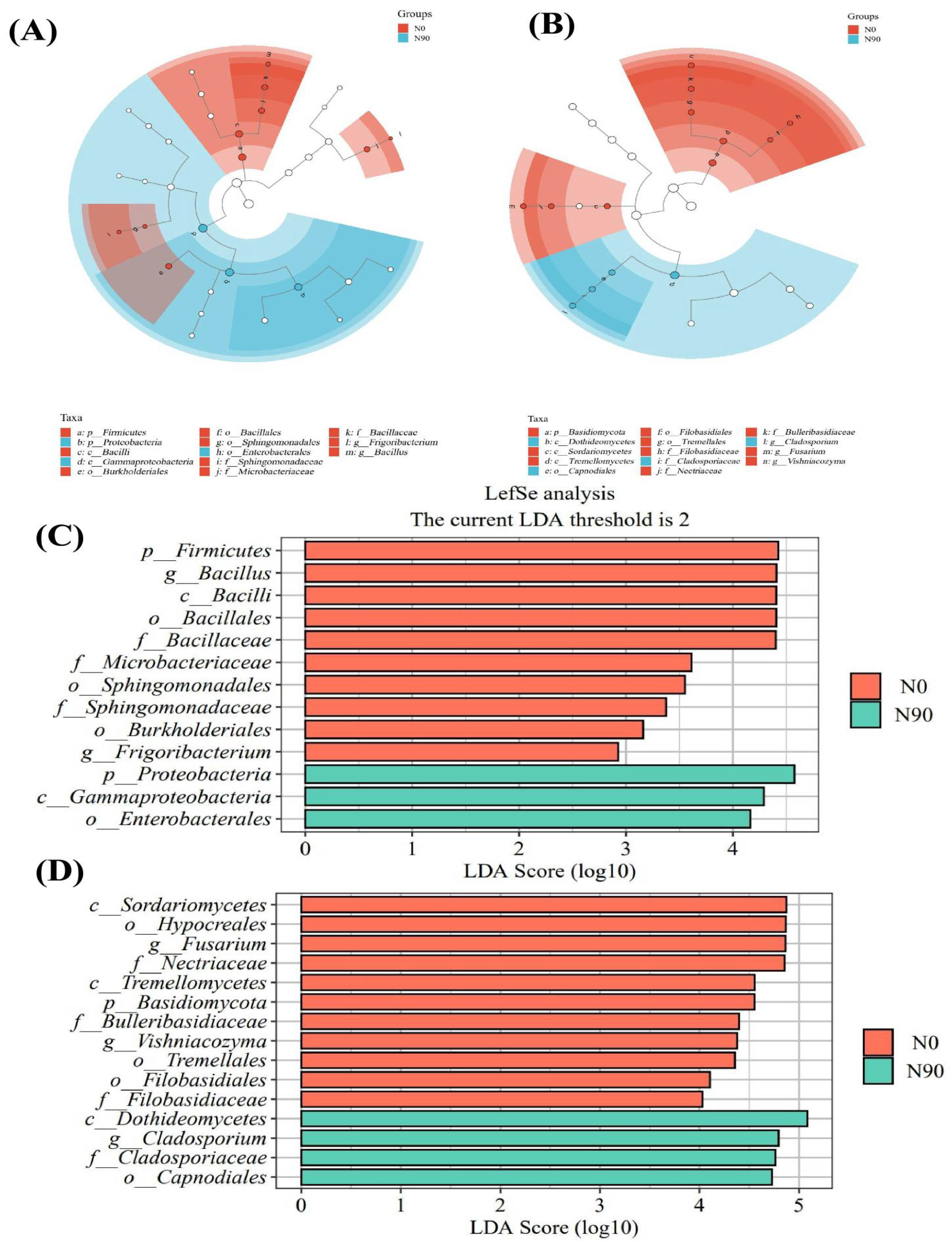
3.1.3. Effects of Nitrogen Application on Endophytic Microflora of Quinoa Grain
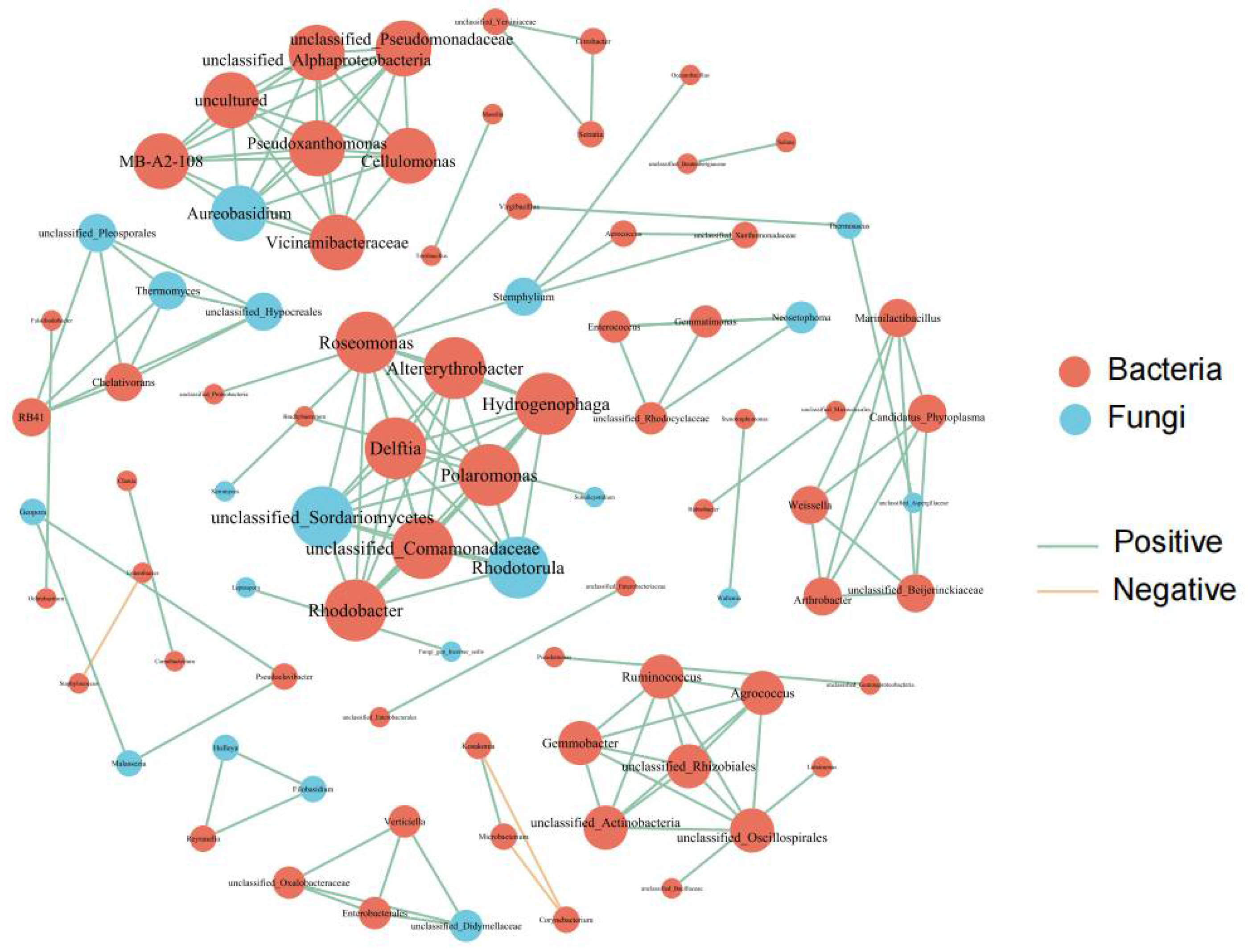
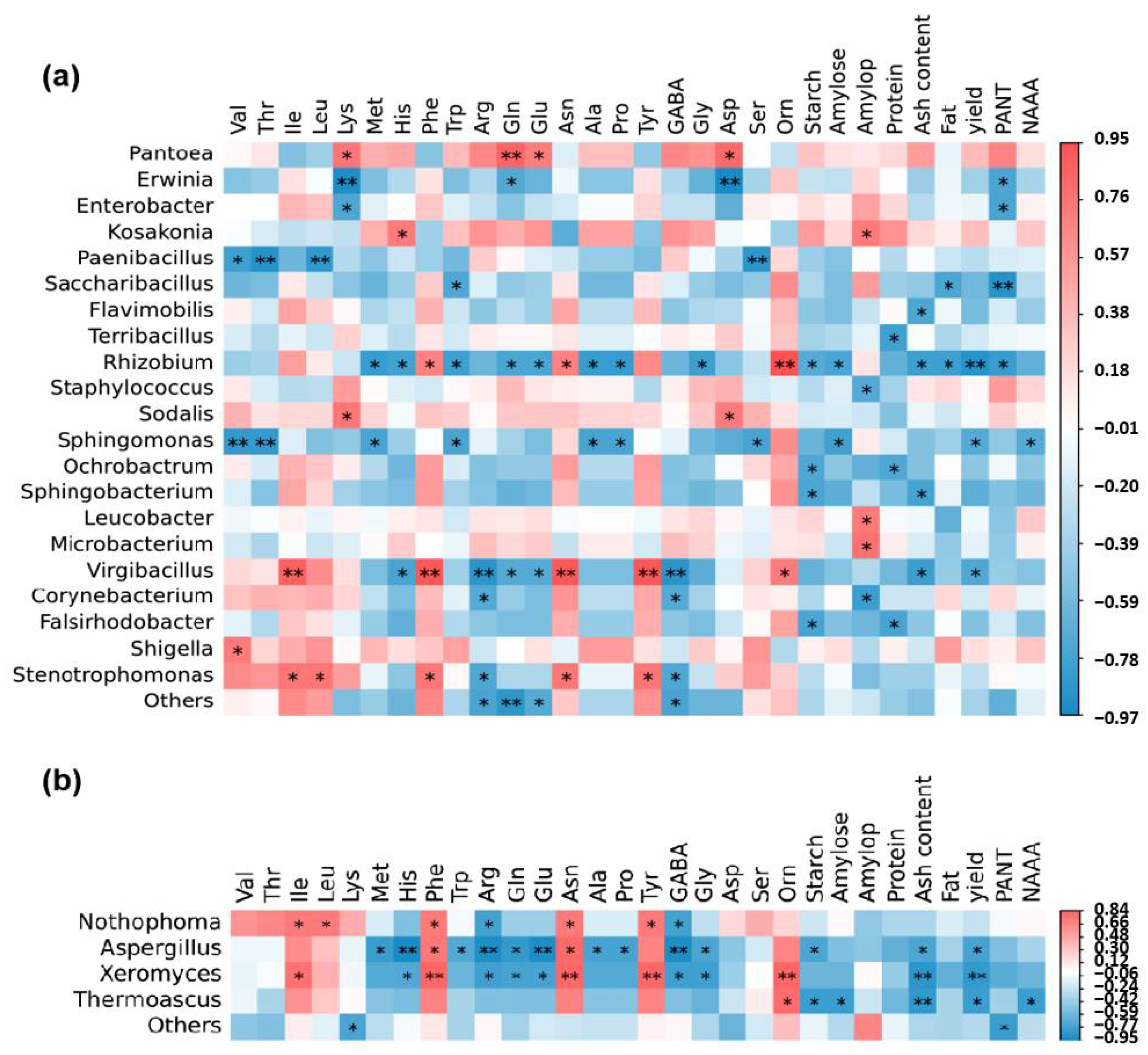
3.2. Correlation Analysis of Nitrogen Accumulation and Movement, Yield, Grain Quality, and Endophytic Flora
4. Discussion
4.1. Effects of Nitrogen Fertilizer on Nitrogen Accumulation, Transport Crop Quality
4.2. Interactions of Endophytic Bacteria in Seeds and Their Effects on Plant Growth and Nutritional Quality
4.3. Nitrogen Fertilizer Management Strategies to Ensure High Yield and Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Risi, J.; Galwey, N.W. The Chenopodium Grains of the Andes: Inca Crops for Modern Agriculture. Adv. Appl. Biol. 1984, 10, 145–216. [Google Scholar]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Izewska, A.; Krosniak, M.; Gawlik, M.; Gawlik, M.; Gorinstein, S. Effect of Diet Supplemented with Quinoa Seeds on Oxidative Status in Plasma and Selected Tissues of High Fructose-Fed Rats. Plant Foods Hum. Nutr. 2010, 65, 146–151. [Google Scholar] [CrossRef] [PubMed]
- White, P.L.; Alvistur, E.; White, H.S.; Collazos, C. Nutritive Values of Crops, Nutrient Content and Protein Quality of Quinua and Caihua, Edible Seed Products of the Andes Mountains. J. Agric. Food Chem. 1955, 3, 531–534. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Mujica, Á.; Jensen, C.R. The Resistance of Quinoa (Chenopodium quinoa Willd.) to Adverse Abiotic Factors. Food Rev. Int. 2003, 19, 109–199. [Google Scholar] [CrossRef]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.-E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): Potential and perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Wright, K.; Pike, O.; Fairbanks, D.; Huber, C. Composition of Atriplex hortensis, sweet and bitter Chenopodium quinoa seeds. J. Food Sci. 2002, 67, 1383–1385. [Google Scholar] [CrossRef]
- Bastidas, E.; Roura, R.; Rizzolo, D.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: An integrative review. J. Nutr. Food Sci. 2016, 6, 3. [Google Scholar]
- Liu, X.; Hu, B.; Chu, C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, L.; Hu, J.; Liu, X.; Jiang, D.; Cao, W.; Dai, T.; Tian, Z. Low nitrogen priming im proves nitrogen uptake and assimilation adaptation to nitrogen deficit stress in wheat seedling. Planta 2024, 259, 107. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Vadivel, R.; Das, T.K. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, N.; Wang, W.; Zhu, Y.; Liu, Y. Endophytic bacterial community diversity in genetically related hybrid rice seeds. Appl. Microbiol. Biotechnol. 2023, 107, 6911–6922. [Google Scholar] [CrossRef]
- Kang, X.; Shen, B.; Wang, H.; Zhang, J.; Hu, J.; Guo, M.; Li, Z.; Chen, X.; Ma, S.; Yuan, H. Effect of Different Amount Nitrogen Fertilizer and Ratio of Basic and Additional Fertilizers on Quinoa’s Yield and Economic Characters. J. Agric. 2017, 7, 34. [Google Scholar]
- Ni, R.; Zhang, Y.; Pang, C.; Wu, R.; Zhang, Z.; Tian, Y.; Liu, L. Plastic Responses of Quinoa Seedling to Change of Water and Nitrogen Coupling. Crops 2015, 31, 91–98. [Google Scholar]
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Schadt, C.W.; Chang, S.X. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Kavamura, V.N.; Hayat, R.; Clark, I.M.; Rossmann, M.; Mendes, R.; Hirsch, P.R.; Mauchline, T.H. Inorganic nitrogen application affects both taxonomical and predicted functional structure of wheat rhizosphere bacterial communities. Front. Microbiol. 2018, 9, 378965. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, K.; Zhang, X.; Du, J. Determination of amylose and amylopectin in wheat and wheat bud by dual wavelength method. J. Chin. Cereals Oils Assoc. 2009, 1, 137–140. [Google Scholar]
- GB 5009.5-2016; National Food Safety Standard—Determination of Protein in Foods. Standards Press of China: Beijing, China, 2016.
- GB 5009.6-2016; National Standard for Food safety, the Determination of Fat in Food Products. Standards Press of China: Beijing, China, 2016.
- GB 5009.4-2016; Determination of Ash in National Standard for Food Safety. Standards Press of China: Beijing, China, 2016.
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Prepr. 2018, 6, e27295v1. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing. Computing 2011, 1, 12–21. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Luo, F.; Zhong, J.; Yang, Y.; Scheuermann, R.H.; Zhou, J. Application of random matrix theory to biological networks. Phys. Lett. A 2006, 357, 420–423. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.; Gu, Q.; Liang, M.; Mu, S.; Zhou, B.; Huang, F.; Lin, B.; Zou, C. Analysis of the correlation between bacteria and fungi in sugarcane tops silage prior to and after aerobic exposure. Bioresour. Technol. 2019, 291, 121835. [Google Scholar] [CrossRef]
- Feng, X.; Pan, L.; Wang, C.; Zhang, H. Residue analysis and risk assessment of pyrethrins in open fiel d and greenhouse turnips. Environ. Sci. Pollut. Res. 2018, 25, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Acuña, J.J.; Inostroza, N.G.; Mora, M.L.; Radic, S.; Sadowsky, M.J.; Jorquera, M.A. Endophytic Bacterial Communities Associated with Roots and Leaves of Plants Growing in Chilean Extreme Environments. Sci Rep. 2019, 9, 4950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zeiss, M.R.; Geng, S. Agricultural pesticide use and food safety: California’s model. J. Integr. Agric. 2015, 14, 2340–2357. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z. Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: A review. Front. Microbiol. 2016, 7, 1463. [Google Scholar] [CrossRef] [PubMed]
- Pino-Otín, M.R.; Val, J.; Ballestero, D. Ecotoxicity of a new biopesticide produced by Lav andula luisieri on non-target soil organisms from different trophic levels. Sci. Total Environ. 2019, 671, 83–93. [Google Scholar] [CrossRef]
- Ipsilantis, I.; Samourelis, C.; Karpouzas, D.G. The impact of biological pesticides on arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2012, 45, 147–155. [Google Scholar] [CrossRef]
- Igarashi, Y.; Iida, T.; Yoshida, R.; Furumai, T. Pteridic acids aand b, novel plant growth promoters with auxin-like activityfrom Streptomyces hygroscopicus TP-A0451. J. Antibiot. 2002, 55, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Pullen, C.B.; Schmitz, P.; Meurer, K. New bioactive compounds from Streptomyces strains residing in the wood of Celastraceae. Planta 2002, 216, 162–167. [Google Scholar] [CrossRef]
- Mccormick, M.H.; Mcguire, J.M.; Pittenger, G.E. Vancomycin, a new antibiotic. Chemical and biologic properties. Antibiot. Annu. 1955, 3, 606–611. [Google Scholar] [PubMed]
- Zhang, Y.; Liao, Y.; Chen, S.W. Isolation, preliminaryidentification and nitrogen-fixation activity of endophytesfrom roots of one- and two-year-old Xanthoceras sorbifoliaplants. Chin. J. Plant Ecol. 2010, 34, 839–844. [Google Scholar]
- Gopal, M.; Gupta, A.; Arunachalam, V. Impact of azadirachtin, an insecticidal allelochemi cal from neem on soil microflora, enzyme and respiratory activities. Bioresour. Technol. 2007, 98, 3154–3158. [Google Scholar] [CrossRef]
- Kiran, U.; Patra, D.D. Medicinal and aromatic plant materials as nitrification inhibitors for au gmenting yield and nitrogen uptake of Japanese mint (Mentha arvensis L. Var. Piperascens). Bioresour. Technol. 2003, 86, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Topp, E.; Mulbry, W.M.; Zhu, H.; Nour, S.M.; Cuppels, D. Characterization of s-triazine herbicide metabolism by a Nocardioides sp. isolated from agricultural soil. Appl. Environ. Microbiol. 2000, 66, 3134–3141. [Google Scholar] [CrossRef]
- Walvekar, V.A.; Bajaj, S.; Singh, D.K. Ecotoxicological assessment of pesticides and their combination on rhizospheric microbial community structure and function of Vigna radiata. Environ. Sci. Pollut. Res. 2017, 24, 17175–17186. [Google Scholar] [CrossRef]
| Nitrogen Application | Grain Nitrogen Accumulation (kg ha−1) | Pre-Flowering Nitrogen Translocation (kg) | Pre-Flowering Contribution to Grain (%) | Post-Flowering Nitrogen Accumulatio (kg) | Post-Flowering Contribution to Grain (%) |
|---|---|---|---|---|---|
| N0 | 63.23 c | 41.86 c | 66.18 a | 21.38 c | 33.82 c |
| N90 | 96.60 b | 63.23 b | 65.24 a | 33.37 b | 34.76 c |
| N150 | 109.36 a | 69.67 a | 63.65 b | 39.69 a | 36.35 b |
| N210 | 106.19 a | 64.51 b | 60.87 c | 41.67 a | 39.13 a |
| Nitrogen Application | Protein (g/100 g) | Amylose (%) | Amylopectin (%) | Total Starch (%) | Ash (g/100 g) | Fat (g/100 g) |
|---|---|---|---|---|---|---|
| N0 | 12.32 b | 0.10 c | 0.47 a | 57.38 c | 3.97 b | 4.33 b |
| N90 | 12.47 b | 0.14 b | 0.47 a | 61.30 b | 4.04 b | 4.58 b |
| N150 | 15.06 a | 0.17 a | 0.48 a | 65.49 a | 5.08 a | 5.57 a |
| N210 | 13.34 ab | 0.14 b | 0.47 a | 61.59 b | 5.02 a | 4.68 b |
| Nitrogen Application | Essential Amino Acids (μg/g) | |||||||||||||
| TAA | Val * | Thr * | Ile * | Leu * | Lys * | Met * | His * | Phe * | Trp * | EAA | EAA /TAA | -- | ||
| N0 | 3669.31 b | 68.10 c | 28.69 d | 51.27 b | 62.74 b | 130.51 b | 34.64 b | 193.73 c | 55.22 b | 74.89 c | 699.78 c | 0.19 a | ||
| N90 | 3786.35 b | 79.51 a | 40.02 a | 58.65 a | 72.65 a | 141.20 a | 37.51 a | 190.68 c | 59.98 a | 81.89 b | 762.08 b | 0.20 a | ||
| N150 | 4255.96 a | 78.41 a | 37.97 b | 49.54 b | 66.52 b | 133.65 b | 39.87 a | 256.60 a | 47.22 c | 85.01 a | 794.79 a | 0.19 a | ||
| N210 | 4268.67 a | 72.45 b | 32.79 c | 46.70 c | 58.97 c | 143.01 a | 38.34 a | 232.96 b | 46.21 c | 81.30 b | 752.73 b | 0.18 a | ||
| Non-Essential Amino Acids (μg/g) | ||||||||||||||
| Nitrogen Application | Arg | Gln | Glu | Asn | Ala | Pro | Tyr | GABA | Gly | Asp | Ser | Orn | NEAA | EAA /NEAA |
| N0 | 883.61 b | 532.02 c | 827.60 b | 44.57 b | 123.01 d | 94.87 c | 97.51 b | 91.74 b | 34.74 d | 152.58 c | 70.00 b | 17.27 a | 2969.53 b | 0.24 ab |
| N90 | 813.77 c | 560.90 c | 838.86 b | 57.85 a | 138.90 c | 107.39 b | 105.20 a | 85.67 c | 42.12 c | 175.91 b | 82.22 a | 15.48 a | 3024.27 b | 0.25 a |
| N150 | 974.19 a | 650.69 b | 1012.82 a | 33.02 c | 154.89 a | 124.78 a | 92.02 c | 103.78 a | 48.91 b | 174.83 b | 83.07 a | 8.14 c | 3461.16 a | 0.23 ab |
| N210 | 990.87 a | 707.87 a | 1008.09 a | 38.31 c | 142.80 b | 109.93 b | 89.90 c | 107.09 a | 54.36 a | 185.01 a | 69.51 b | 12.21 b | 3515.94 a | 0.21 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Jiang, Z.; Yang, X.; Zhang, Y.; Huang, J.; Dai, J.; Noor, H.; Wu, X.; Ren, A.; Gao, Z.; et al. Effects of Nitrogen Accumulation, Transportation, and Grain Nutritional Quality and Advances in Fungal Endophyte Research in Quinoa (Chenopodium quinoa Willd.) Plants. J. Fungi 2024, 10, 504. https://doi.org/10.3390/jof10070504
Li L, Jiang Z, Yang X, Zhang Y, Huang J, Dai J, Noor H, Wu X, Ren A, Gao Z, et al. Effects of Nitrogen Accumulation, Transportation, and Grain Nutritional Quality and Advances in Fungal Endophyte Research in Quinoa (Chenopodium quinoa Willd.) Plants. Journal of Fungi. 2024; 10(7):504. https://doi.org/10.3390/jof10070504
Chicago/Turabian StyleLi, Linghong, Zhijun Jiang, Xinhui Yang, Yulai Zhang, Jianxun Huang, Jing Dai, Hafeez Noor, Xiangyun Wu, Aixia Ren, Zhiqiang Gao, and et al. 2024. "Effects of Nitrogen Accumulation, Transportation, and Grain Nutritional Quality and Advances in Fungal Endophyte Research in Quinoa (Chenopodium quinoa Willd.) Plants" Journal of Fungi 10, no. 7: 504. https://doi.org/10.3390/jof10070504







