A Countermeasure Strategy against Peramine Developed by Chilesia rudis in the Endophyte–Ryegrass–Herbivore Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Larval Rearing
2.3. Evaluation, Isolation and Characterization of Endophytic Fungus in Lolium perenne
2.4. No-Choice Assay
2.5. Pupal Development Time and Adult Performance Evaluation
2.6. Carcasses, Feces and Guts Peramine Extraction
2.7. Plant Material Extraction
2.8. HPLC-DAD Analysis
2.9. Survival Rate Test
2.10. Statistical Analysis
3. Results
3.1. Evaluation, Isolation and Characterization of Endophytic Fungus in Lolium perenne
3.2. No-Choice Assay
3.3. Pupal Development Time and Adult Performance Evaluation and HPLC-DAD Analysis
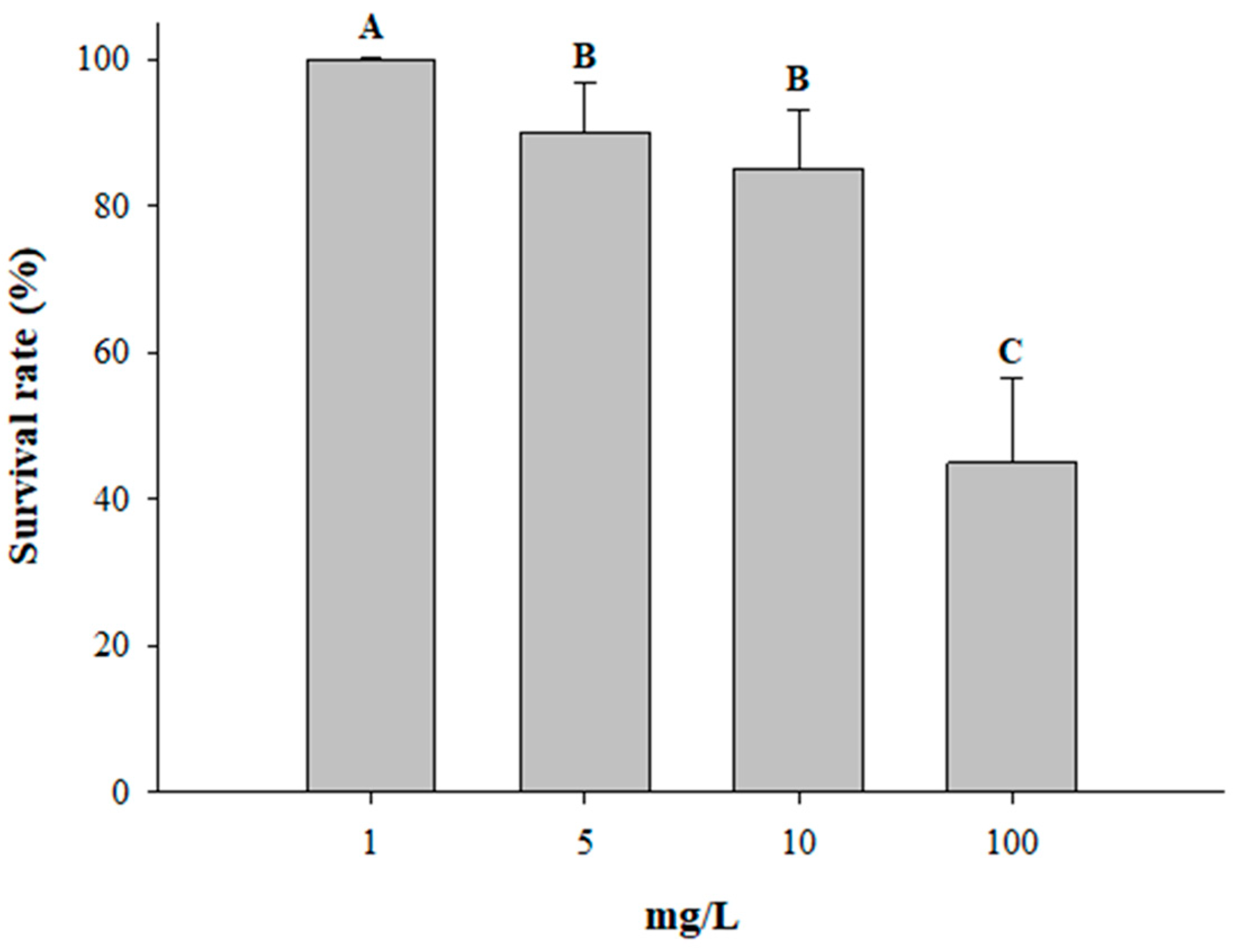
3.4. Survival Rate Test
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, W.; Liu, W.; Chen, H.; Yuan, Q.; Wang, Z.; Liu, H. Endophytic Beauveria bassiana of Tomato Resisted the Damage from Whitefly Bemisia tabaci by Mediating the Accumulation of Plant-Specialized Metabolites. J. Agric. Food Chem. 2023, 71, 13244–13254. [Google Scholar] [CrossRef] [PubMed]
- Hoogshagen, M.; Hastings, A.P.; Chavez, J.; Duckett, M.; Pettit, R.; Pahnke, A.A.; Agrawal, A.A.; de Roode, J.C. Mixtures of Milkweed Cardenolides Protect Monarch Butterflies against Parasites. J. Chem. Ecol. 2023, 50, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Zhou, S.; Shah, A.; Arafat, Y.; Arif Hussain Rizvi, S.; Shao, H. Plant Allelopathy in Response to Biotic and Abiotic Factors. Agronomy 2023, 13, 2358. [Google Scholar] [CrossRef]
- Erb, M.; Robert, C.A.M. Sequestration of plant secondary metabolites by insect herbivores: Molecular mechanisms and ecological consequences. Curr. Opin. Insect Sci. 2016, 14, 8–11. [Google Scholar] [CrossRef]
- Petschenka, G.; Agrawal, A. How herbivores coopt plant defenses: Natural selection, specialization, and sequestration. Curr. Opin. Insect Sci. 2016, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Fuentes, M.; Martínez-Cisterna, D.; Vera, W.; Ortega-Klose, F.; Reyes, C.; Matamala, I.; Bardehle, L. Feeding Performance of Argentine Stem Weevil Is Reduced by Peramine from Perennial Ryegrass Infected with Endophyte Fungus. Insects 2024, 15, 410. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Gao, C.; Wang, J.; Xu, W.; Wang, M.; Li, M.; Ma, B.; Tian, P. Effects of Drought Stress on Peramine and Lolitrem B in Epichloë-Endophyte-Infected Perennial Ryegrass. Life 2022, 12, 1207. [Google Scholar] [CrossRef] [PubMed]
- Shymanovich, T.; Musso, A.M.; Cech, N.B.; Faeth, S.H. Epichloë endophytes of Poa alsodes employ alternative mechanisms for host defense: Insecticidal versus deterrence. Arthropod-Plant Interact. 2019, 13, 79–90. [Google Scholar] [CrossRef]
- Nelli, M.R. Total Synthesis of Peramine, a Defensive Alkaloid Produced by Endophytic Fungi of Cool Season Grasses Possessing Anti-Insect Properties. J. Nat. Prod. 2016, 71, 1370–1377. [Google Scholar]
- Nelli, M.R.; Scheerer, J.R. Synthesis of peramine, an anti-insect defensive alkaloid produced by endophytic fungi of cool season grasses. J. Nat. Prod. 2016, 79, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.D.; Dymock, J.J.; Brimble, M.A. Effect of fungal metabolite peramine and analogs on feeding development of Argentine stem weevil (Listronotus bonariensis). J. Chem. Ecol. 1990, 16, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Hume, D.; McCulley, R. Fungal endophytes of tall fescue and perennial ryegrass: Pasture friend or foe? J. Anim. Science. 2013, 91, 2379–2394. [Google Scholar] [CrossRef] [PubMed]
- Keogh, R.G.; Tapper, B.A.; Fletcher, R.H. Distributions of the fungal endophyte Acremonium lolii, and of the alkaloids Lolitrem B and Peramine, within perennial ryegrass. N. Z. J. Agricutural Res. 1996, 39, 121–127. [Google Scholar] [CrossRef]
- Siegel, M.R.; Bush, L.P. Defensive chemicals in grass-fungal endophyte associations. Recent Adv. Phytochem. 1996, 30, 81–118. [Google Scholar]
- Fuchs, B.; Krischke, M.; Mueller, M.J.; Krauss, J.J. Peramine and Lolitrem B from Endophyte-Grass Associations Cascade Up the Food Chain. Chem. Ecol. 2013, 39, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Parra, L.; Mutis, A.; Chacón, M.; Lizama, M.; Rojas, C.; Catrileo, A.; Rubilar, O.; Tortella, G.; Birkett, M.; Quiroz, A. Horn fly larval survival in cattle dung is reduced by endophyte infection of tall fescue pasture. Pest Manag. Sci. 2016, 72, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Faeth, S.H.; Saari, S. Fungal grass endophytes and arthropod communities: Lessons from plant defence theory and multitrophic interactions. Fungal Ecol. 2012, 5, 364–371. [Google Scholar] [CrossRef]
- Zust, T.; Agrawal, A.A. Population growth and sequestration of plant toxins along a gradient of specialization in four aphid species on the common milkweed, Asclepias syriaca. Funct. Ecol. 2016, 30, 547–556. [Google Scholar] [CrossRef]
- Nishida, R. Sequestration of defensive substances from plants by lepidoptera. Annu. Rev. Entomol. 2002, 47, 57–92. [Google Scholar] [CrossRef]
- Opitz, S.E.W.; Müller, C. Plant chemistry and insect sequestration. Chemoecology 2009, 1, 71. [Google Scholar] [CrossRef]
- Weintraub, J.D. Host plant association patterns and phylogeny in the tribe Troidini (Lepidoptera: Papilionidae). In Swallowtail Butterflies: Their Ecology and Evolutionary Biology; Scriber, J.M., Tsubaki, Y., Lederhouse, R.C., Eds.; Scientific Publishers: Gainesville, FL, USA, 1995; pp. 307–316. [Google Scholar]
- Pinto, C.F.; Troncoso, A.J.; Urzua, A.; Niemeyer, H.M. Use of volatiles of Aristolochia chilensis (aristolochiaceae) in host searching by fourth-instar larvae and adults of battus Polydamas archidamas (Lepidoptera: Papilionidae: Troidini). Eur. J. Entomol. 2009, 106, 63–68. [Google Scholar] [CrossRef]
- Pinto, C.F.; Urzúa, A.; Niemeyer, H.M. Sequestration of aristolochic acids from meridic diets by larvae of Battus Polydamas archidamas (Papilionidae: Troidini). Eur. J. Entomol. 2011, 108, 41–45. [Google Scholar] [CrossRef]
- Ángulo, A.; Ruiz, V. Maenas rudis (Butler): Cuncuna colorada de prados y jardines; biología y estados inmaduros (Lepidoptera: Arctiidae). Boletín Soc. Biológica Concepción 1975, 139–147. [Google Scholar]
- Chacón-Fuentes, M.; Parra, L.; Rodriguez-Saona, C.; Seguel, I.; Ceballos, R.; Quiroz, A. Domestication in murtilla (Ugni molinae) reduced defensive flavonol levels but increased resistance against a native herbivorous insect. Environ. Entomol. 2015, 44, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Fuentes, M.; Parra, L.; Lizama, M.; Seguel, I.; Urzúa, A.; Quiroz, A. Plant flavonoid content modified by domestication. Environ. Entomol. 2017, 46, 1080–1089. [Google Scholar] [CrossRef]
- Chacon-Fuentes, M.; Bardehle, L.; Seguel, I.; Rubilar, F.; Martínez-Cisterna, D.; Quiróz, A. Domestication of Plants of Ugni molinae Turcz (Myrtaceae) Interferes in the Biology of Chilesia rudis (Lepidoptera: Erebidae) Larvae. Molecules 2021, 26, 2063. [Google Scholar] [CrossRef] [PubMed]
- Nickisch-Rosenegk, E.; Wink, M. Sequestration of pyrrolizidine alkaloids in several arctiid moths (Lepidoptera: Arctiidae). J. Chem. Ecol. 1993, 19, 1189–1903. [Google Scholar] [CrossRef] [PubMed]
- Dickel, F.; Freitak, D.; Mappes, J. Long-term prophylactic antibiotic treatment: Effects on survival, immunocompetence and reproduction success of Parasemia plantaginis (Lepidoptera: Erebidae). J. Insect Sci. 2016, 16, 46. [Google Scholar] [CrossRef]
- Dombrowski, J.; Baldwin, J.; Azevedo, M.; Banowetz, G. A sensitive PCR-based assay to detect Neotyphodium fungi in seed and plant tissue of tall, fescue and ryegrass species. Crop Sci. 2006, 46, 1064–1070. [Google Scholar] [CrossRef]
- Chacon-Fuentes, M.; Martínez-Cisterna, D.; Reyes, C.; Vera, W.; Fincheira, P.; Lizama, M.; Quiroz, A.; Bardehle, L. Infection of perennial ryegrass (Lolium perenne) by an endophyte fungus (Neotyphodium lolii) decreases the abundance and diversity of predators and parasitoids. Rev. Bras. De Entomol. 2023, 67, e20230012. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Kholghahmadi, M.; Karimi-Malati, A.; Jalali Sendi, J. Ecophysiological responses of individually and group reared Cydalima perspectalis (Lepidoptera: Crambidae) to alkaloid-containing host plants. Environ. Entomol. 2023, 52, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Ait Lhaj, R.; Saffaj, T.; Belmir, H.; Ihssane, B. The uncertainty profile used for full validation of the HPLC Method to determine 22 azo amines in fabrics. J. AOAC Int. 2023, 106, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Fuentes, M.; Bardehle, L.; Lizama, M.; Seguel, I.; Quiroz, A. Restoration of flavonols and isoflavonoids in Ugni molinae subjected to a reciprocal transplant experiment in a domestication framework. Chem. Ecol. 2019, 35, 115–127. [Google Scholar] [CrossRef]
- Guerre, P. Ergot alkaloid produced by endophytic fungi of the genus Epichloe. Toxins 2015, 7, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Young, C.A.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet 2013, 9, e1003323. [Google Scholar] [CrossRef] [PubMed]
- Shymanovich, T.; Faeth, S.H. Environmental factors affect the distribution of two Epichloë fungal endophyte species inhabiting a common host grove bluegrass (Poa alsodes). Ecol. Evol. 2019, 9, 6624–6642. [Google Scholar] [CrossRef]
- Newton, E.; Bullock, J.M.; Hodgson, D. Bottom-up effects of glucosinolate variation on aphid colony dynamics in wild cabbage populations. Ecol. Entomol. 2009, 34, 614–623. [Google Scholar] [CrossRef]
- Kos, M.; Broekgaarden, C.; Kabouw, P.; Lenferink, K.O.; Poelman, E.H.; Vet, L.E.M.; Dicke, M.; Van Loon, J.J.A. Relative importance of plant mediated bottom-up and top-down forces on herbivore abundance on Brassica oleracea. Funct. Ecol. 2011, 25, 113–1124. [Google Scholar] [CrossRef]
- Cibils-Stewart, X.; Putra, R.; Islam, T.; Fanna, D.J.; Wuhrer, R.; Mace, W.J.; Johnson, S.N. Silicon and Epichloë-endophyte defences in a model temperate grass diminish feeding efficiency and immunity of an insect folivore. Funct. Ecol. 2023, 37, 3177–3192. [Google Scholar] [CrossRef]
- Lampert, E.C.; Bowers, M.D. Host plant influences on iridoid glycoside sequestration of generalist and specialist caterpillars. J. Chem. Ecol. 2010, 36, 1101–1104. [Google Scholar] [CrossRef]
- Lampert, E.C.; Dyer, L.A.; Bowers, M.D. Dietary specialization and the effects of plant species on potential multitrophic interactions of three species of nymphaline caterpillars. Entomol. Exp. Appl. 2014, 153, 207–216. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T.; Honda, K.; Omura, H.; Hayashi, N. Sequestration of phenanthroindolizidine alkaloids by an Asclepiadaceae-feeding danaid butterfly, Ideopsis similis. Phytochemistry 2001, 56, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Spiering, M.J.; Wilkinson, H.H.; Blankenship, J.D.; Schardl, C.L. Expressed sequence tags and genes associated with loline alkaloid expression by the fungal endophyte Neotyphodium uncinatum. Fungal Genet. Biol. 2002, 36, 242–254. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Schmidt, D.; Bush, L.P. Different levels of protective alkaloids in grasses with stroma-forming and seed-transmitted Epichloë/Neotyphodium endophytes. J. Chem. Ecol. 2000, 26, 1025–1036. [Google Scholar] [CrossRef]

| Line Experimental | N° of Plants Tested | (E+) | (E−) | Endophyte (%) |
|---|---|---|---|---|
| L161 | 120 | 120 | 0 | 100 *a |
| L162 | 120 | 89 | 31 | 74 *d |
| L163 | 120 | 12 | 108 | 10 *e |
| L164 | 120 | 110 | 10 | 91.6 *b |
| L165 | 120 | 100 | 20 | 83.3 *c |
| L166 | 120 | 110 | 10 | 91.6 *b |
| L167 | 120 | 13 | 107 | 11 *e |
| JUMBO (E−) | 120 | 0 | 120 | 0 *f |
| ALTO AR1 (E+) | 120 | 120 | 0 | 100 *a |
| PCR (Tub2 Intron) | ||
|---|---|---|
| Experimental Lines or Commercial Cultivars | IS-RS-5′; IS-NS3′ | IS-tub2w-5′; IS-tub2w-3′ |
| L161 | + | + |
| L162 | + | + |
| L163 | + | + |
| L164 | + | + |
| L165 | + | + |
| L166 | + | + |
| L167 | + | + |
| JUMBO (E−) | − | − |
| ALTO AR1 (E+) | + | + |
| Experimental lines and Cultivars | Peramine in Leaves (µg/g) | Pupal Development Time (days) | Length (cm) | Weight (g) | Wing Length ♀ (cm) | Wing Length ♂ (cm) | Peramine in Carcasses (µg/g) | Peramine in Feces (µg/g) | Peramine in Guts (µg/g) |
|---|---|---|---|---|---|---|---|---|---|
| L161 | 179.3 ± 24.5 a | 28 ± 1.1 a | 1.7 ± 0.1 e | 0.5 ± 0.0 b | 0.17 ± 0.0 b | 0.63 ± 0.1 e | 12.1 ± 1.0 a | 31.7 ± 4.2 a | 0.0 ± 0.0 e |
| L162 | 99.8 ± 17.3 d | 25 ± 1.2 b | 1.8 ± 0.1 d | 0.6 ± 0.1 b | 0.14 ± 0.0 d | 0.99 ± 0.1 c | 8.2 ± 0.9 c | 13.7 ± 2.7 d | 0.8 ± 0.1 c |
| L163 | 15.2 ± 4.9 e | 24 ± 1.0 c | 2.9 ± 0.2 b | 0.7 ± 0.0 a | 0.13 ± 0.0 d | 1.32 ± 0.2 a | 1.6 ± 0.8 d | 1.1 ± 0.3 e | 0.0 ± 0.0 e |
| L164 | 160.8 ± 39.9 b | 26 ± 2.1 b | 1.9 ± 0.2 d | 0.5 ± 0.0 b | 0.15 ± 0.0 c | 0.70 ± 0.1 d | 11.3 ± 2.1 a | 27.9 ± 4.2 b | 0.2 ± 0.0 d |
| L165 | 127.0 ± 34.2 c | 25 ± 2.0 b | 2.4 ± 0.1 c | 0.6 ± 0.0 b | 0.16 ± 0.0 c | 1.19 ± 0.2 b | 9.5 ± 0.9 b | 19.7 ± 2.3 c | 1.5 ± 0.1 b |
| L166 | 146.4 ± 21.2 c | 26 ± 1.5 b | 2.0 ± 0.1 d | 0.5 ± 0.1 b | 0.12 ± 0.0 e | 0.80 ± 0.1 d | 10.1 ± 0.1 b | 24.0 ± 2.1 b | 1.7 ± 0.1 a |
| L167 | 17.1 ± 6.1 e | 24 ± 1.1 c | 3.1 ± 0.1 a | 0.8 ± 0.1 a | 0.15 ± 0.0 c | 1.20 ± 0.2 b | 2.2 ± 0.2 d | 1.1 ± 0.4 e | 0.0 ± 0.0 e |
| JUMBO (E−) | 0.0 ± 0.0 f | 23 ± 1.4 c | 3.2 ± 0.3 a | 0.7 ± 0.1 a | 0.20 ± 0.0 a | 1.25 ± 0.2 b | 0.0 ± 0.0 e | 0.0 ± 0.0 f | 0.0 ± 0.0 e |
| ALTO AR1 (E+) | 184.1 ± 29.8 a | 28 ± 1.2 a | 1.4 ± 0.1 f | 0.3 ± 0.0 c | 0.17 ± 0.0 b | 0.43 ± 0.1 f | 10.5 ± 1.1 a | 32.8 ± 4.4 a | 1.3 ± 0.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón-Fuentes, M.; Martínez-Cisterna, D.; Lizama, M.; Asencio-Cancino, V.; Matamala, I.; Bardehle, L. A Countermeasure Strategy against Peramine Developed by Chilesia rudis in the Endophyte–Ryegrass–Herbivore Model. J. Fungi 2024, 10, 512. https://doi.org/10.3390/jof10080512
Chacón-Fuentes M, Martínez-Cisterna D, Lizama M, Asencio-Cancino V, Matamala I, Bardehle L. A Countermeasure Strategy against Peramine Developed by Chilesia rudis in the Endophyte–Ryegrass–Herbivore Model. Journal of Fungi. 2024; 10(8):512. https://doi.org/10.3390/jof10080512
Chicago/Turabian StyleChacón-Fuentes, Manuel, Daniel Martínez-Cisterna, Marcelo Lizama, Valeria Asencio-Cancino, Ignacio Matamala, and Leonardo Bardehle. 2024. "A Countermeasure Strategy against Peramine Developed by Chilesia rudis in the Endophyte–Ryegrass–Herbivore Model" Journal of Fungi 10, no. 8: 512. https://doi.org/10.3390/jof10080512





