Research Progress on the Mechanism and Function of Histone Acetylation Regulating the Interaction between Pathogenic Fungi and Plant Hosts
Abstract
:1. Introduction
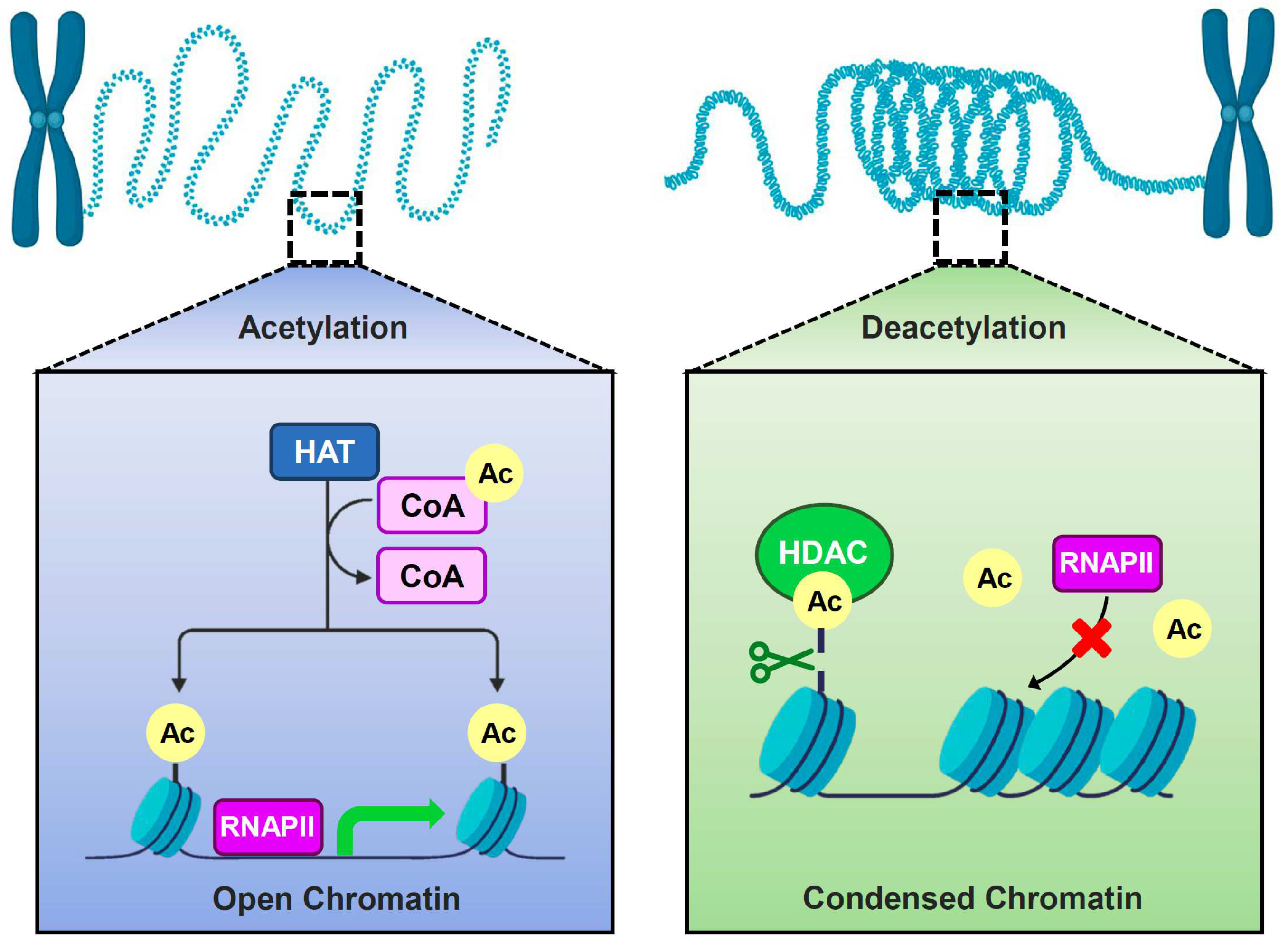
2. Enzymes for Histone Acetylation Modification
2.1. The Biological Function of Histone Acetyltransferases in Yeast
2.2. The Biological Function of Histone Deacetyltransferases in Yeast
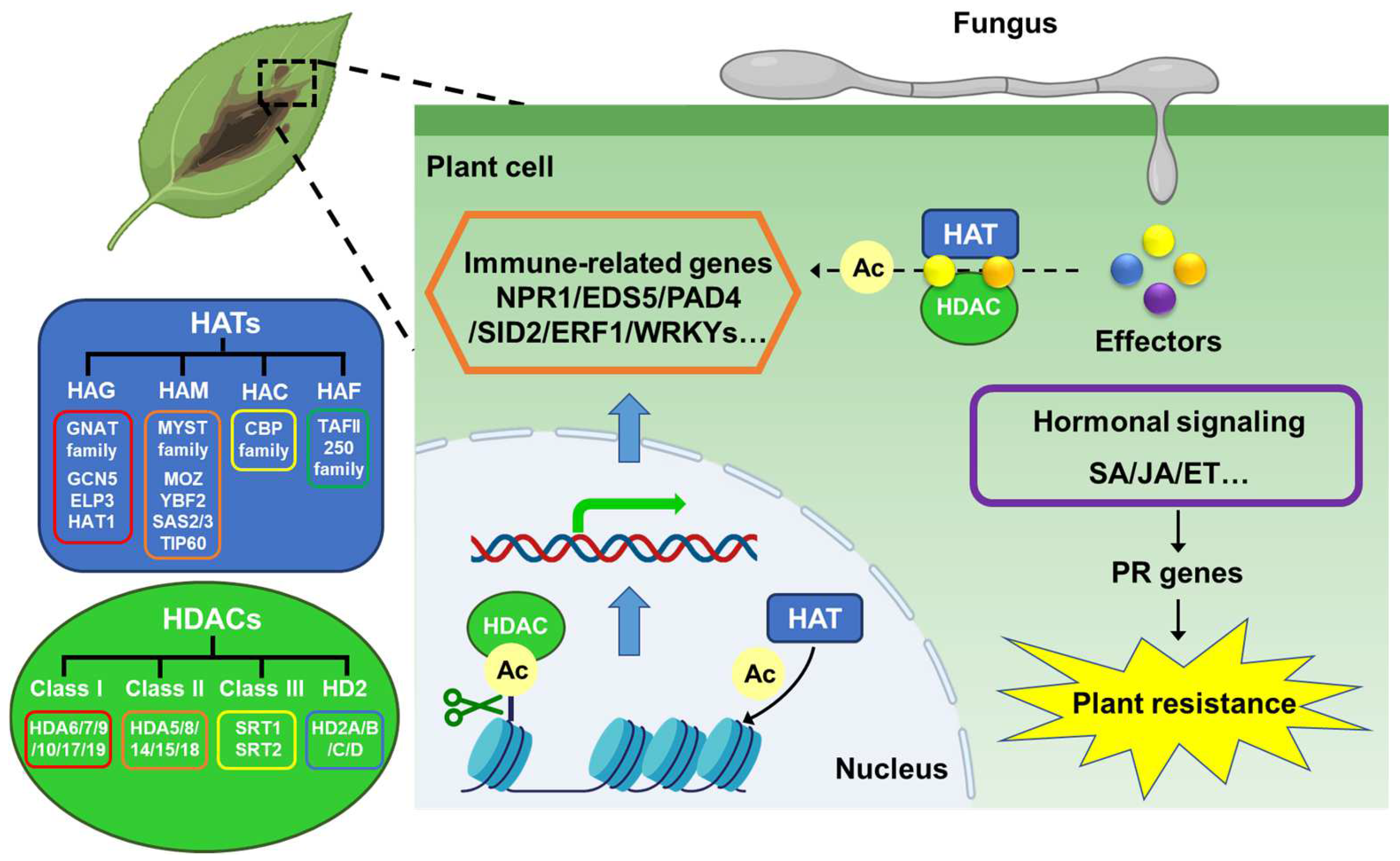
3. Effect of Histone Acetylation on Fungal Pathogenicity
3.1. Histone Acetyltransferase
3.2. Histone Deacetylase
4. Effect of Histone Acetylation on Plant Disease Resistance
4.1. Histone Acetyltransferase
4.2. Histone Deacetylase
5. Crosstalk between Histone Acetylation and Other Epigenetic Modifications
5.1. DNA Methylation
5.2. Histone Methylation
5.3. Other Epigenetic Modifications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.J.; Chen, D.; Dekker, F.J.; Quax, W.J. Improving TRAIL-induced apoptosis in cancers by interfering with histone modifications. Cancer Drug Resist. 2020, 3, 791–803. [Google Scholar] [CrossRef]
- Tzelepis, K.; Rausch, O.; Kouzarides, T. RNA-modifying enzymes and their function in a chromatin context. Nat. Struct. Mol. Biol. 2019, 26, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, M.D.; Grunstein, M. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007, 76, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Wakamori, M.; Okabe, K.; Ura, K.; Funatsu, T.; Takinoue, M.; Umehara, T. Quantification of the effect of site-specific histone acetylation on chromatin transcription rate. Nucleic Acids Res. 2020, 48, 12648–12659. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, R.; Zhou, M.-M. Writers and readers of histone acetylation: Structure, mechanism, and inhibition. Cold Spring Harbor Perspect. Biol. 2014, 6, a018762. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.O.; Fujimori, D.G. Functional coupling between writers, erasers and readers of histone and DNA methylation. Curr. Opin. Struct. Biol. 2015, 35, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Duan, C. Epigenetic regulation of plant immunity: From chromatin codes to plant disease resistance. aBIOTECH 2023, 4, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lang, Z. The mechanism and function of active DNA demethylation in plants. J. Integr. Plant Biol. 2019, 62, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Kong, X.; Song, H.; Han, Q.; Zhang, S. Advances in proteome-wide analysis of plant lysine acetylation. Plant Commun. 2021, 3, 100266. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Jeon, J. Epigenetic regulation of development and pathogenesis in fungal plant pathogens. Mol. Plant Pathol. 2017, 18, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kwon, S.; Lee, Y.-H. Histone acetylation in fungal pathogens of plants. Plant Pathol. J. 2014, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sterner, D.E.; Berger, S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001, 70, 81–120. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Gao, L.; Li, T.; Chen, L. Phylogenetic and expression analysis of histone acetyltransferases in Brachypodium distachyon. Genomics 2019, 111, 1966–1976. [Google Scholar] [CrossRef]
- Hinnebusch, A.G.; Fink, G.R. Positive regulation in the general amino acid control of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1983, 80, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, T.; Thireos, G. Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription. EMBO J. 1992, 11, 4145–4152. [Google Scholar] [CrossRef]
- Brownell, J.E.; Zhou, J.; Ranalli, T.; Kobayashi, R.; Edmondson, D.G.; Roth, S.Y.; Allis, C.D. Tetrahymena histone acetyltransferase A: A Homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 1996, 84, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.A.; Eberharter, A.; John, S.; Cook, R.G.; Turner, B.M.; Workman, J.L. Expanded lysine acetylation specificity of Gcn5 in native complexes. J. Biol. Chem. 1999, 274, 5895–5900. [Google Scholar] [CrossRef] [PubMed]
- Soffers, J.H.M.; Workman, J.L. The SAGA chromatin-modifying complex: The sum of its parts is greater than the whole. Genes Dev. 2020, 34, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.; Brown, C.E.; Lechner, T.; Workman, J.L. Histone acetyltransferase complexes and their link to transcription. Crit. Rev. Eukaryot. Gene. Expr. 1999, 9, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Suka, N.; Suka, Y.; Carmen, A.A.; Wu, J.; Grunstein, M. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol. Cell 2001, 8, 473–479. [Google Scholar] [CrossRef]
- Zhang, W.; Bone, J.R.; Edmondson, D.G.; Turner, B.M.; Roth, S.Y. Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. EMBO J. 1998, 17, 3155–3167. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Pray-Grant, M.G.; Selleck, W.; Grant, P.A.; Tan, S. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J. Biol. Chem. 2002, 277, 7989–7995. [Google Scholar] [CrossRef]
- Boyer, L.A.; Langer, M.R.; Crowley, K.A.; Tan, S.; Denu, J.M.; Peterson, C.L. Essential role for the SANT domain in the functioning of multiple chromatin remodeling enzymes. Mol. Cell 2002, 10, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Candau, R.; Berger, S.L. Structural and Functional Analysis of Yeast Putative Adaptors. J. Biol. Chem. 1996, 271, 5237–5245. [Google Scholar] [CrossRef] [PubMed]
- Candau, R.; Zhou, J.X.; Allis, C.D.; Berger, S.L. Histone acetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo. EMBO J. 1997, 16, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Sterner, D.E.; Wang, X.; Bloom, M.H.; Simon, G.M.; Berger, S.L. The SANT domain of Ada2 is required for normal acetylation of histones by the yeast SAGA complex. J. Biol. Chem. 2002, 277, 8178–8186. [Google Scholar] [CrossRef]
- Sapountzi, V.; Côté, J. MYST-family histone acetyltransferases: Beyond chromatin. Cell. Mol. Life Sci. 2011, 68, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Eisen, A.; Gu, W.; Sattah, M.; Pannuti, A.; Zhou, J.; Cook, R.G.; Lucchesi, J.C.; Allis, C.D. ESA1 is a histone acetyltransferase that is essential for growth in yeast. Proc. Natl. Acad. Sci. USA 1998, 95, 3561–3565. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Lu, J.-Y.; Zhang, J.; Walter, W.; Dang, W.; Wan, J.; Tao, S.-C.; Qian, J.; Zhao, Y.; Boeke, J.D.; et al. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell 2009, 136, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Heise, F.; Chung, H.-R.; Weber, J.M.; Xu, Z.; Klein-Hitpass, L.; Steinmetz, L.M.; Vingron, M.; Ehrenhofer-Murray, A.E. Genome-wide H4 K16 acetylation by SAS-I is deposited independently of transcription and histone exchange. Nucleic Acids Res. 2011, 40, 65–74. [Google Scholar] [CrossRef]
- Sutton, A.; Shia, W.-J.; Band, D.; Kaufman, P.D.; Osada, S.; Workman, J.L.; Sternglanz, R. Sas4 and Sas5 are required for the histone acetyltransferase activity of Sas2 in the SAS complex. J. Biol. Chem. 2003, 278, 16887–16892. [Google Scholar] [CrossRef] [PubMed]
- Meijsing, S.H.; Ehrenhofer-Murray, A.E. The silencing complex SAS-I links histone acetylation to the assembly of repressed chromatin by CAF-I and Asf1 in Saccharomyces cerevisiae. Genes Dev. 2001, 15, 3169–3182. [Google Scholar] [CrossRef] [PubMed]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, K.; Son, H.; Park, A.R.; Kim, J.-C.; Choi, G.J.; Lee, Y.-W. ELP3 is involved in sexual and asexual development, virulence, and the oxidative stress response in Fusarium graminearum. Mol. Plant-Microbe Interact. 2014, 27, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Suka, N.; Luo, K.; Grunstein, M. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat. Genet. 2002, 32, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Goómez, E.B.; Nugent, R.L.; Laria, S.N.; Forsburg, S.L. Schizosaccharomyces pombe Histone Acetyltransferase Mst1 (KAT5) Is an Essential Protein Required for Damage Response and Chromosome Segregation. Genetics 2008, 179, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.L.; Johnsson, A.; Fleharty, B.; Gogol, M.; Xue-Franzén, Y.; Seidel, C.; Wright, A.P.H.; Forsburg, S.L. Expression profiling of S. pombe acetyltransferase mutants identifies redundant pathways of gene regulation. BMC Genom. 2010, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Church, M.; Smith, K.C.; Alhussain, M.M.; Pennings, S.; Fleming, A.B. Sas3 and Ada2(Gcn5)-dependent histone H3 acetylation is required for transcription elongation at the de-repressed FLO1 gene. Nucleic Acids Res. 2017, 45, 4413–4430. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Howe, L.; Tafrov, S.T.; Grant, P.A.; Sternglanz, R.; Workman, J.L. The something about silencing protein, Sas3, is the catalytic subunit of NuA3, a yTAF(II)30-containing HAT complex that interacts with the Spt16 subunit of the yeast CP (Cdc68/Pob3)-FACT complex. Genes Dev. 2000, 14, 1196–1208. [Google Scholar] [CrossRef]
- Howe, L.; Auston, D.; Grant, P.; John, S.; Cook, R.G.; Workman, J.L.; Pillus, L. Histone H3 specific acetyltransferases are essential for cell cycle progression. Genes Dev. 2001, 15, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Saka, K.; Kobayashi, T. Rtt109 prevents hyper-amplification of ribosomal RNA genes through histone modification in budding yeast. PLoS Genet. 2013, 9, e1003410. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Garai, P.; Sethi, S.C.; Naqvi, N.; Yadav, B.; Kumar, P.; Singh, S.L.; Yadav, U.; Bhatnagar, S.; Rahul; et al. Modulation of azole sensitivity and filamentation by GPI15, encoding a subunit of the first GPI biosynthetic enzyme, in Candida albicans. Sci. Rep. 2019, 9, 8508. [Google Scholar] [CrossRef] [PubMed]
- Lercher, L.; Danilenko, N.; Kirkpatrick, J.; Carlomagno, T. Structural characterization of the Asf1–Rtt109 interaction and its role in histone acetylation. Nucleic Acids Res. 2017, 46, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Cote, J.M.; Kuo, Y.-M.; Henry, R.A.; Scherman, H.; Krzizike, D.D.; Andrews, A.J. Two factor authentication: Asf1 mediates crosstalk between H3 K14 and K56 acetylation. Nucleic Acids Res. 2019, 47, 7380–7391. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, J.; Recht, J.; Silva, A.C.; Suter, B.; Emili, A.; Stagljar, I.; Krogan, N.J.; Allis, C.D.; Keogh, M.-C.; Greenblatt, J.F. Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109. Mol. Cell. Biol. 2008, 28, 4342–4353. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [PubMed]
- Rundlett, S.E.; Carmen, A.A.; Kobayashi, R.; Bavykin, S.; Turner, B.M.; Grunstein, M. HDA1 and RPD3 are members of distinct yeast histone deacetylase complexes that regulate silencing and transcription. Proc. Natl. Acad. Sci. USA 1996, 93, 14503–14508. [Google Scholar] [CrossRef] [PubMed]
- Alejandro-Osorio, A.L.; Huebert, D.J.; Porcaro, D.T.; Sonntag, M.E.; Nillasithanukroh, S.; Will, J.L.; Gasch, A.P. The histone deacetylase Rpd3p is required for transient changes in genomic expression in response to stress. Genome Biol. 2009, 10, R57. [Google Scholar] [CrossRef] [PubMed]
- Carrozza, M.J.; Li, B.; Florens, L.; Suganuma, T.; Swanson, S.K.; Lee, K.K.; Shia, W.-J.; Anderson, S.; Yates, J.; Washburn, M.P.; et al. Histone H3 methylation by set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 2005, 123, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Keogh, M.-C.; Kurdistani, S.K.; Morris, S.A.; Ahn, S.H.; Podolny, V.; Collins, S.R.; Schuldiner, M.; Chin, K.; Punna, T.; Thompson, N.J.; et al. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell 2005, 123, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gogol, M.; Carey, M.; Lee, D.; Seidel, C.; Workman, J.L. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science 2007, 316, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Wu, J.; Li, B. Chromatin remodelers fine-tune H3K36me-directed deacetylation of neighbor nucleosomes by Rpd3S. Mol. Cell 2013, 52, 255–263. [Google Scholar] [CrossRef]
- Huh, J.W.; Wu, J.; Lee, C.H.; Yun, M.; Gilada, D.; Brautigam, C.A.; Li, B. Multivalent di-nucleosome recognition enables the Rpd3S histone deacetylase complex to tolerate decreased H3K36 methylation levels. EMBO J. 2012, 31, 3564–3574. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Lee, C.-H.; Cui, H.; Li, S.; Li, B. Nucleosome contact triggers conformational changes of Rpd3S driving high-affinity H3K36me nucleosome engagement. Cell Rep. 2015, 10, 204–215. [Google Scholar] [CrossRef]
- Kadosh, D.; Struhl, K. Targeted recruitment of the Sin3-Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin In vivo. Mol. Cell. Biol. 1998, 18, 5121–5127. [Google Scholar] [CrossRef] [PubMed]
- Carrozza, M.J.; Florens, L.; Swanson, S.K.; Shia, W.-J.; Anderson, S.; Yates, J.; Washburn, M.P.; Workman, J.L. Stable incorporation of sequence specific repressors Ash1 and Ume6 into the Rpd3L complex. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2005, 1731, 77–87. [Google Scholar] [CrossRef]
- Pijnappel, W.W.; Schaft, D.; Roguev, A.; Shevchenko, A.; Tekotte, H.; Wilm, M.; Rigaut, G.; Séraphin, B.; Aasland, R.; Stewart, A.F. The S. cerevisiae SET3 complex includes two histone deacetylases, Hos2 and Hst1, and is a meiotic-specific repressor of the sporulation gene program. Genes Dev. 2001, 15, 2991–3004. [Google Scholar] [CrossRef]
- Torres-Machorro, A.L.; Clark, L.G.; Chang, C.S.; Pillus, L. The Set3 complex antagonizes the MYST acetyltransferase Esa1 in the DNA damage response. Mol. Cell. Biol. 2015, 35, 3714–3725. [Google Scholar] [CrossRef]
- Wang, A.; Kurdistani, S.K.; Grunstein, M. Requirement of Hos2 histone deacetylase for gene activity in yeast. Science 2002, 298, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Suka, N.; Carlson, M.; Grunstein, M. TUP1 utilizes histone H3/H2B–specific HDA1 deacetylase to repress gene activity in yeast. Mol. Cell 2001, 7, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Turner, E.L.; Menzel, J.; Malo, M.E.; Harkness, T.A.A. Antagonistic Gcn5-Hda1 interactions revealed by mutations to the Anaphase promoting complex in yeast. Cell Div. 2011, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Cao, X.; Sun, L.; Zhu, J.-Y.; Wasko, B.M.; Liu, W.; Crutcher, E.; Liu, H.; Jo, M.C.; Qin, L.; et al. Inactivating histone deacetylase HDA promotes longevity by mobilizing trehalose metabolism. Nat. Commun. 2021, 12, 1981. [Google Scholar] [CrossRef] [PubMed]
- Tanner, K.G.; Landry, J.; Sternglanz, R.; Denu, J.M. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc. Natl. Acad. Sci. USA 2000, 97, 14178–14182. [Google Scholar] [CrossRef] [PubMed]
- Tanny, J.C.; Moazed, D. Coupling of histone deacetylation to NAD breakdown by the yeast silencing protein Sir2: Evidence for acetyl transfer from substrate to an NAD breakdown product. Proc. Natl. Acad. Sci. USA 2001, 98, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.D.; Denu, J.M. Structural Identification of 2′- and 3′-O-Acetyl-ADP-ribose as novel metabolites derived from the Sir2 family of β-NAD+-dependent histone/protein deacetylases. J. Biol. Chem. 2002, 277, 18535–18544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Rusche, L.N. Sirtuins in epigenetic silencing and control of gene expression in model and pathogenic fungi. Annu. Rev. Microbiol. 2022, 76, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Robyr, D.; Suka, Y.; Xenarios, I.; Kurdistani, S.K.; Wang, A.; Suka, N.; Grunstein, M. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 2002, 109, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Álvarez, Y.; Suelves, M. HDAC11: A multifaceted histone deacetylase with proficient fatty deacylase activity and its roles in physiological processes. FEBS J. 2022, 289, 2771–2792. [Google Scholar] [CrossRef]
- Chang, P.; Fan, X.; Chen, J. Function and subcellular localization of Gcn5, a histone acetyltransferase in Candida albicans. Fungal Genet. Biol. 2015, 81, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Shivarathri, R.; Tscherner, M.; Zwolanek, F.; Singh, N.K.; Chauhan, N.; Kuchler, K. The fungal histone acetyl transferase Gcn5 controls virulence of the human pathogen Candida albicans through multiple pathways. Sci. Rep. 2019, 9, 9445. [Google Scholar] [CrossRef]
- Lan, H.; Sun, R.; Fan, K.; Yang, K.; Zhang, F.; Nie, X.Y.; Wang, X.; Zhuang, Z.; Wang, S. The Aspergillus flavus histone acetyltransferase AflGcnE regulates morphogenesis, aflatoxin biosynthesis, and pathogenicity. Front. Microbiol. 2016, 7, 1324. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, J.-J.; Fu, B.; Ying, S.-H.; Feng, M.-G. Gcn5-dependent histone H3 acetylation and gene activity is required for the asexual development and virulence of Beauveria bassiana. Environ. Microbiol. 2018, 20, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Workman, J.L. Histone acetyltransferase complexes: One size doesn’t fit all. Nat. Rev. Mol. Cell Biol. 2007, 8, 284–295. [Google Scholar] [CrossRef]
- Wang, X.; Chang, P.; Ding, J.; Chen, J. Distinct and redundant roles of the two MYST histone acetyltransferases Esa1 and Sas2 in cell growth and morphogenesis of Candida albicans. Eukaryot. Cell 2013, 12, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Akhberdi, O.; Wei, D.; Chen, L.; Liu, H.; Wang, D.; Hao, X.; Zhu, X. A MYST histone acetyltransferase modulates conidia development and secondary metabolism in Pestalotiopsis microspora, a taxol producer. Sci. Rep. 2018, 8, 8199. [Google Scholar] [CrossRef] [PubMed]
- Soukup, A.A.; Chiang, Y.-M.; Bok, J.W.; Reyes-Dominguez, Y.; Oakley, B.R.; Wang, C.C.C.; Strauss, J.; Keller, N.P. Overexpression of the Aspergillus nidulans histone 4 acetyltransferase EsaA increases activation of secondary metabolite production. Mol. Microbiol. 2012, 86, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Mi, W.; Liu, Z.; Zeng, G.; Zhang, P.; Hu, Y.; Fang, W.; Yin, W.-B. Deletion of a histone acetyltransferase leads to the pleiotropic activation of natural products in Metarhizium robertsii. Org. Lett. 2017, 19, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Song, L.M.; Bai, T.T.; Liang, W.X. BcSas2-mediated histone H4K16 acetylation is critical for virulence and oxidative stress response of Botrytis cinerea. Mol. Plant-Microbe Interact. 2020, 33, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wen, M.; Wu, L.; Lan, H.; Yuan, J.; Wang, S. The Fungi-specific histone acetyltransferase Rtt109 mediates morphogenesis, aflatoxin synthesis and pathogenicity in Aspergillus flavus by acetylating H3K9. IMA Fungus 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, J.; Ye, J.; Lu, L. The fungal-specific histone acetyltransferase Rtt109 regulates development, DNA damage response, and virulence in Aspergillus fumigatus. Mol. Microbiol. 2021, 115, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Gong, P.; Luo, Q.; Chen, W.; Wang, C. Histone acetyltransferase Rtt109 regulates development, morphogenesis, and citrinin biosynthesis in Monascus purpureus. J. Fungi 2023, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, Z.-K.; Shao, W.; Ying, S.-H.; Feng, M.-G. Essential role of Rpd3-dependent lysine modification in the growth, development and virulence of Beauveria bassiana. Environ. Microbiol. 2018, 20, 1590–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Liang, S.; Zhang, P.; Kang, R.; Zhang, M.; Wang, M.; Chen, L.; Yuan, H.; Ding, S.; et al. FpDep1, a component of Rpd3L histone deacetylase complex, is important for vegetative development, ROS accumulation, and pathogenesis in Fusarium pseudograminearum. Fungal Genet. Biol. 2020, 135, 103299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, Z.; Zhang, Z.; Liang, W. BcRPD3-mediated histone deacetylation is involved in growth and pathogenicity of Botrytis cinerea. Front. Microbiol. 2020, 11, 1832. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Cao, X.; Qu, Z.; Zhang, S.; Naqvi, N.I.; Deng, Y.Z.; Mitchell, A.P. The histone deacetylases MoRpd3 and MoHst4 regulate growth, conidiation, and pathogenicity in the rice blast fungus Magnaporthe oryzae. mSphere 2021, 6, e0011821. [Google Scholar] [CrossRef] [PubMed]
- Baidyaroy, D.; Brosch, G.; Ahn, J.-H.; Graessle, S.; Wegener, S.; Tonukari, N.J.; Caballero, O.; Loidl, P.; Walton, J.D. A gene related to yeast HOS2 histone deacetylase affects extracellular depolymerase expression and virulence in a plant pathogenic fungus. Plant Cell 2001, 13, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Liu, W.; Wang, G.; Kang, Z.; Kistler, H.C.; Xu, J.-R. The HDF1 histone deacetylase gene is important for conidiation, sexual reproduction, and pathogenesis in Fusarium graminearum. Mol. Plant-Microbe Interact. 2011, 24, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Majer, O.; Frohner, I.E.; Komnenovic, V.; Kuchler, K. The Set3/Hos2 histone deacetylase complex attenuates cAMP/PKA signaling to regulate morphogenesis and virulence of Candida albicans. PLoS Pathog. 2010, 6, e1000889. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Schwarzmüller, T.; Kuchler, K. Transcriptional loops meet chromatin: A dual-layer network controls white–opaque switching in Candida albicans. Mol. Microbiol. 2009, 74, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Elías-Villalobos, A.; Fernández-Álvarez, A.; Moreno-Sánchez, I.; Helmlinger, D.; Ibeas, J.I. The Hos2 histone deacetylase controls Ustilago maydis virulence through direct regulation of mating-type genes. PLoS Pathog. 2015, 11, e1005134. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Liu, W.; Iliuk, A.; Ribot, C.; Vallet, J.; Tao, A.; Wang, Y.; Lebrun, M.H.; Xu, J.R. The tig1 histone deacetylase complex regulates infectious growth in the rice blast fungus Magnaporthe oryzae. Plant Cell 2010, 22, 2495–2508. [Google Scholar] [CrossRef] [PubMed]
- Brandão, F.; Esher, S.K.; Ost, K.S.; Pianalto, K.; Nichols, C.B.; Fernandes, L.; Bocca, A.L.; Poças-Fonseca, M.J.; Alspaugh, J.A. HDAC genes play distinct and redundant roles in Cryptococcus neoformans virulence. Sci. Rep. 2018, 8, 5209. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Oh, J.-H.; Keats Shwab, E.; Dagenais, T.R.T.; Andes, D.; Keller, N.P. HdaA, a class 2 histone deacetylase of Aspergillus fumigatus, affects germination and secondary metabolite production. Fungal Genet. Biol. 2009, 46, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Studt, L.; Schmidt, F.J.; Jahn, L.; Sieber, C.M.K.; Connolly, L.R.; Niehaus, E.-M.; Freitag, M.; Humpf, H.-U.; Tudzynski, B. Two histone deacetylases, FfHda1 and FfHda2, are important for Fusarium fujikuroi secondary metabolism and virulence. Appl. Environ. Microbiol. 2013, 79, 7719–7734. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Izawa, M.; Nakajima, Y.; Jin, Q.; Hirose, T.; Nakamura, T.; Koshino, H.; Kanamaru, K.; Ohsato, S.; Kamakura, T.; et al. Increased metabolite production by deletion of an HDA1-type histone deacetylase in the phytopathogenic fungi, Magnaporthe oryzae (Pyricularia oryzae) and Fusarium asiaticum. Lett. Appl. Microbiol. 2017, 65, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, L.; Wang, B.; Pan, L. The histone deacetylases HosA and HdaA affect the phenotype and transcriptomic and metabolic profiles of Aspergillus niger. Toxins 2019, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, M.; Jamnischek, A.; Weinzierl, G.; Ladendorf, O.; Huber, S.; Kahmann, R.; Kämper, J. The histone deacetylase Hda1 from Ustilago maydis is essential for teliospore development. Mol. Microbiol. 2002, 46, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Torreblanca, J.; Stumpferl, S.; Basse, C.W. Histone deacetylase Hda1 acts as repressor of the Ustilago maydis biotrophic marker gene mig1. Fungal Genet. Biol. 2003, 38, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Ma, M.; Ran, L.; Zheng, J.; Tong, J.; Zhu, J.; Ma, C.; Sun, Y.; Zhang, S.; Feng, W.; et al. Function and molecular mechanism of acetylation in autophagy regulation. Science 2012, 336, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Leach, M.D.; Cowen, L.E. Lysine deacetylases Hda1 and Rpd3 regulate Hsp90 function thereby governing fungal drug resistance. Cell Rep. 2012, 2, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, W.; Chang, P.; Wu, H.; Liu, H.; Chen, J. Merge and separation of NuA4 and SWR1 complexes control cell fate plasticity in Candida albicans. Cell Discov. 2018, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Kothe, G.O.; Matsen, C.B.; Khlafallah, T.K.; Adhvaryu, K.K.; Hemphill, M.; Freitag, M.; Motamedi, M.R.; Selker, E.U. The fungus Neurospora crassa displays telomeric silencing mediated by multiple sirtuins and by methylation of histone H3 lysine 9. Epigenet. Chromatin 2008, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Masuo, S.; Fujita, T.; Doi, Y.; Kamimura, Y.; Takaya, N. Hydrolase controls cellular NAD, sirtuin, and secondary metabolites. Mol. Cell. Biol. 2012, 32, 3743–3755. [Google Scholar] [CrossRef] [PubMed]
- Itoh, E.; Shigemoto, R.; Oinuma, K.-I.; Shimizu, M.; Masuo, S.; Takaya, N. Sirtuin A regulates secondary metabolite production by Aspergillus nidulans. J. Gen. Appl. Microbiol. 2017, 63, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, C.B.; Sherman, J.M.; Devine, S.E.; Cameron, E.E.; Pillus, L.; Boeke, J.D. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995, 9, 2888–2902. [Google Scholar] [CrossRef]
- Freeman-Cook, L.L.; Sherman, J.M.; Brachmann, C.B.; Allshire, R.C.; Boeke, J.D.; Pillus, L. The Schizosaccharomyces pombe hst4+ gene is a SIR2 homologue with silencing and centromeric functions. Mol. Cell. Biol. 1999, 10, 3171–3186. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, B.J.; Rall, N.A.; Ostwal, Y.; Kruitwagen, T.; Hiragami-Hamada, K.; Winkler, M.; Barral, Y.; Fischle, W.; Neumann, H. A cascade of histone modifications induces chromatin condensation in mitosis. Science 2014, 343, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Domergue, R.; Castaño, I.; De Las Peñas, A.; Zupancic, M.; Lockatell, V.; Hebel, J.R.; Johnson, D.; Cormack, B.P. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science 2005, 308, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Arras, S.D.M.; Chitty, J.L.; Wizrah, M.S.I.; Erpf, P.E.; Schulz, B.L.; Tanurdzic, M.; Fraser, J.A. Sirtuins in the phylum Basidiomycota: A role in virulence in Cryptococcus neoformans. Sci. Rep. 2017, 7, 46567. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Marroquin-Guzman, M.; Nandakumar, R.; Shijo, S.; Cornwell, K.M.; Li, G.; Wilson, R.A. Plant defence suppression is mediated by a fungal sirtuin during rice infection by Magnaporthe oryzae. Mol. Microbiol. 2014, 94, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, Y.; Yu, Y.; Zhang, Y.; Wei, F.; Zhu, Q.H.; Zhou, J.; Zhao, L.; Zhang, Y.; Feng, Z.; et al. Acetylation of GhCaM7 enhances cotton resistance to Verticillium dahliae. Plant J. 2023, 114, 1405–1424. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Müller, A.; Napoli, C.A.; Selinger, D.A.; Pikaard, C.S.; Richards, E.J.; Bender, J.; Mount, D.W.; Jorgensen, R.A. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 2002, 30, 5036–5055. [Google Scholar] [CrossRef] [PubMed]
- Perrella, G.; Consiglio, M.F.; Aiese-Cigliano, R.; Cremona, G.; Sanchez-Moran, E.; Barra, L.; Errico, A.; Bressan, R.A.; Franklin, F.C.H.; Conicella, C. Histone hyperacetylation affects meiotic recombination and chromosome segregation in Arabidopsis. Plant J. 2010, 62, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, S.; Yu, C.-W.; Chen, C.-Y.; Wu, K. Histone acetylation and plant development. Enzymes 2016, 40, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Tran, G.-B.; Nguyen, N.H. Homeostasis of histone acetylation is critical for auxin signaling and root morphogenesis. Plant Mol. Biol. 2020, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cao, H.; Sun, Y.; Li, X.; Chen, F.; Carles, A.; Li, Y.; Ding, M.; Zhang, C.; Deng, X.; et al. Arabidopsis paired amphipathic helix proteins SNL1 and SNL2 redundantly regulate primary seed dormancy via abscisic acid–ethylene antagonism mediated by histone deacetylation. Plant Cell 2013, 25, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, D.; Lin, Z.; Guan, B.; Liu, D.; Yang, L.; Deng, X.; Mei, F.; Zhou, Z. Histone acetylation modification affects cell wall degradation and aerenchyma formation in wheat seminal roots under waterlogging. Plant Growth Regul. 2019, 87, 149–163. [Google Scholar] [CrossRef]
- Liu, X.; Luo, M.; Zhang, W.; Zhao, J.; Zhang, J.; Wu, K.; Tian, L.; Duan, J. Histone acetyltransferases in rice (Oryza sativa L.): Phylogenetic analysis, subcellular localization and expression. BMC Plant Biol. 2012, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Aiese Cigliano, R.; Sanseverino, W.; Cremona, G.; Ercolano, M.R.; Conicella, C.; Consiglio, F.M. Genome-wide analysis of histone modifiers in tomato: Gaining an insight into their developmental roles. BMC Genom. 2013, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, X.; Luo, M.; Yang, S.; Wu, K. Involvement of histone modifications in plant abiotic stress responses. J. Integr. Plant Biol. 2013, 55, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Song, N.; Zheng, M.; Liu, X.; Liu, Z.; Xing, J.; Ma, J.; Guo, W.; Yao, Y.; Peng, H.; et al. Histone acetyltransferase GCN5 is essential for heat stress-responsive gene activation and thermotolerance in Arabidopsis. Plant J. 2015, 84, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Piquerez, S.J.M.; Ramirez-Prado, J.S.; Mastorakis, E.; Veluchamy, A.; Latrasse, D.; Manza-Mianza, D.; Brik-Chaouche, R.; Huang, Y.; Rodriguez-Granados, N.Y.; et al. GCN5 modulates salicylic acid homeostasis by regulating H3K14ac levels at the 5’ and 3’ ends of its target genes. Nucleic Acids Res. 2020, 48, 5953–5966. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Qiu, X.; Kang, J.; Wang, Y.; Chen, H.; Huang, J.; Qiu, M.; Zhao, Y.; Kong, G.; Ma, Z.; et al. A Phytophthora effector manipulates host histone acetylation and reprograms defense gene expression to promote infection. Curr. Biol. 2017, 24, 981–991. [Google Scholar] [CrossRef] [PubMed]
- DeFraia, C.T.; Wang, Y.; Yao, J.; Mou, Z. Elongator subunit 3 positively regulates plant immunity through its histone acetyltransferase and radical S-adenosylmethionine domains. BMC Plant Biol. 2013, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Lin, J.; Liu, X.; Liu, Z.; Liu, D.; Chu, W.; Li, J.; Chen, Y.; Chang, S.; Yang, Q.; et al. Histone acetyltransferase TaHAG1 interacts with TaPLATZ5 to activate TaPAD4 expression and positively contributes to powdery mildew resistance in wheat. New Phytol. 2022, 236, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Xie, H.; Zhang, K.; Li, H.; Gao, Y.; Zhang, J.; Xu, B.; Peng, L.; Yang, G.; Wang, G.L.; et al. Nuclear EPL-HAM complex is essential for the development of chloroplasts. J. Genet. Genom. 2022, 49, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ge, Z.; Zhou, M.; Bai, R.; Zeng, Q.; Wei, Y.; He, C.; Shi, H. Histone acetyltransferase HAM1 interacts with molecular chaperone DNAJA2 and confers immune responses through salicylic acid biosynthetic genes in cassava. Plant Cell Environ. 2023, 46, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Choi, S.-M.; Kang, M.-J.; Yun, S.-H.; Kwon, D.-J.; Noh, Y.-S.; Noh, B. Salicylic acid-induced transcriptional reprogramming by the HAC–NPR1–TGA histone acetyltransferase complex in Arabidopsis. Nucleic Acids Res. 2018, 46, 11712–11725. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, A.B.; Zhong, X. Functions and mechanisms of plant histone deacetylases. Sci. China Life Sci. 2020, 63, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Wan, D.; Li, R.; Han, X.; Li, G.; Wang, R. Calmodulin-binding protein CBP60g functions as a negative regulator in Arabidopsis anthocyanin accumulation. PLoS ONE 2017, 12, e0173129. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, L.; Jin, Y.; Chen, J.; Shi, H.; Wang, Y.; Yang, W. Histone deacetylase 6 suppresses salicylic acid biosynthesis to repress autoimmunity. Plant Physiol. 2021, 187, 2592–2607. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhi, P.; Wang, X.; Fan, Q.; Chang, C. Wheat WD40-repeat protein TaHOS15 functions in a histone deacetylase complex to fine-tune defense responses to Blumeria graminis f.sp. tritici. J. Exp. Bot. 2019, 70, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, X.; Wang, Z.; Sun, Q.; Hong, A.; Zhang, A.; Zhong, X.; Hua, J. HOS15 and HDA9 negatively regulate immunity through histone deacetylation of intracellular immune receptor NLR genes in Arabidopsis. New Phytol. 2020, 226, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Bellizzi, M.D.R.; Ning, Y.; Meyers, B.C.; Wang, G.-L. HDT701, a Histone H4 deacetylase, negatively regulates plant innate immunity by modulating histone H4 acetylation of defense-related genes in rice. Plant Cell 2012, 24, 3783–3794. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Duan, Y.; Qiao, F.; Liu, H.; Huang, J.; Luo, C.; Chen, X.; Li, G.; Xie, K.; Hsiang, T.; et al. A secreted fungal effector suppresses rice immunity through host histone hypoacetylation. New Phytol. 2022, 235, 1977–1994. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Q.; Duan, Y.; Liu, H.; Chen, X.; Huang, J.; Luo, C.; Zhou, D.-X.; Zheng, L. Ustilaginoidea virens modulates lysine 2-hydroxyisobutyrylation in rice flowers during infection. J. Integr. Plant Biol. 2021, 63, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Gao, F.; Wu, J.G.; Dai, J.L.; Wei, C.H.; Li, Y. Arabidopsis putative deacetylase AtSRT2 regulates basal defense by suppressing PAD4, EDS5 and SID2 expression. Plant Cell Physiol. 2010, 51, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Walley, J.W.; Shen, Z.; McReynolds, M.R.; Schmelz, E.A.; Briggs, S.P. Fungal-induced protein hyperacetylation in maize identified by acetylome profiling. Proc. Natl. Acad. Sci. USA 2018, 115, 210–215. [Google Scholar] [CrossRef]
- Choi, S.-M.; Song, H.-R.; Han, S.-K.; Han, M.; Kim, C.-Y.; Park, J.; Lee, Y.-H.; Jeon, J.-S.; Noh, Y.-S.; Noh, B. HDA19 is required for the repression of salicylic acid biosynthesis and salicylic acid-mediated defense responses in Arabidopsis. Plant J. 2012, 71, 135–146. [Google Scholar] [CrossRef]
- Kim, K.C.; Lai, Z.; Fan, B.; Chen, Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell 2008, 20, 2357–2371. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, L.; Duan, J.; Miki, B.; Wu, K. Histone deacetylase19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell 2005, 17, 1196–1204. [Google Scholar] [CrossRef]
- Latrasse, D.; Jégu, T.; Li, H.; de Zelicourt, A.; Raynaud, C.; Legras, S.; Gust, A.; Samajova, O.; Veluchamy, A.; Rayapuram, N.; et al. MAPK-triggered chromatin reprogramming by histone deacetylase in plant innate immunity. Genome Biol. 2017, 18, 131. [Google Scholar] [CrossRef]
- Li, S.; Lyu, S.; Liu, Y.; Luo, M.; Shi, S.; Deng, S. Cauliflower mosaic virus P6 dysfunctions histone deacetylase HD2C to promote virus infection. Cells 2021, 10, 2278. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, X.; Chen, S.; Zhong, H.; Liu, Y.; Zhang, L.; Xia, Y.; Kragler, F.; Luo, M.; Li, X.D.; et al. AtHDA6 functions as an H3K18ac eraser to maintain pericentromeric CHG methylation in Arabidopsis thaliana. Nucleic Acids Res. 2021, 49, 9755–9767. [Google Scholar] [CrossRef]
- Gong, Z.; Morales-Ruiz, T.; Ariza, R.R.; Roldán-Arjona, T.; David, L.; Zhu, J.K. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 2002, 111, 803–814. [Google Scholar] [CrossRef]
- Yu, A.; Lepère, G.; Jay, F.; Wang, J.; Bapaume, L.; Wang, Y.; Abraham, A.L.; Penterman, J.; Fischer, R.L.; Voinnet, O.; et al. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc. Natl. Acad. Sci. USA 2013, 110, 2389–2394. [Google Scholar] [CrossRef]
- Sánchez, A.L.; Stassen, J.H.; Furci, L.; Smith, L.M.; Ton, J. The role of DNA (de)methylation in immune responsiveness of Arabidopsis. Plant J. 2016, 88, 361–374. [Google Scholar] [CrossRef]
- Qian, W.; Miki, D.; Zhang, H.; Liu, Y.; Zhang, X.; Tang, K.; Kan, Y.; La, H.; Li, X.; Li, S.; et al. A histone acetyltransferase regulates active DNA demethylation in Arabidopsis. Science 2012, 336, 1445–1448. [Google Scholar] [CrossRef]
- Song, Y.; Wu, K.; Dhaubhadel, S.; An, L.; Tian, L. Arabidopsis DNA methyltransferase AtDNMT2 associates with histone deacetylase AtHD2s activity. Biochem. Biophys. Res. Commun. 2010, 396, 187–192. [Google Scholar] [CrossRef]
- Smith, K.M.; Dobosy, J.R.; Reifsnyder, J.E.; Rountree, M.R.; Anderson, D.C.; Green, G.R.; Selker, E.U. H2B- and H3-specific histone deacetylases are required for DNA methylation in Neurospora crassa. Genetics 2010, 186, 1207–1216. [Google Scholar] [CrossRef]
- Lawrence, R.J.; Earley, K.; Pontes, O.; Silva, M.; Chen, Z.J.; Neves, N.; Viegas, W.; Pikaard, C.S. A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol. Cell 2004, 13, 599–609. [Google Scholar] [CrossRef]
- Li, H.; Luan, S. AtFKBP53 is a histone chaperone required for repression of ribosomal RNA gene expression in Arabidopsis. Cell Res. 2010, 20, 357–366. [Google Scholar] [CrossRef]
- Vijayapalani, P.; Hewezi, T.; Pontvianne, F.; Baum, T.J. An effector from the cyst nematode Heterodera schachtii derepresses host rRNA genes by altering histone acetylation. Plant Cell 2018, 30, 2795–2812. [Google Scholar] [CrossRef]
- Jaskiewicz, M.; Conrath, U.; Peterhänsel, C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011, 12, 50–55. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Wang, C.; Liu, S.; Yu, G.; Gao, M.; Qian, H.; Liu, M.; Luisi, B.F.; Gabriel, D.W.; et al. Acetylation of a fungal effector that translocates host PR1 facilitates virulence. ELife 2022, 11, e82628. [Google Scholar] [CrossRef]
| Kingdom | Species | HATs | HDACs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HAG | HAM | HAC | HAF | Class I | Class II | Class III | HD2 | ||
| Fungi | Aspergillus nidulans | 3 | 6 | 1 | 1 | 2 | 2 | 6 | 0 |
| Botrytis cinerea | 3 | 5 | 1 | 1 | 2 | 0 | 2 | 0 | |
| Colletotrichum graminicola | 3 | 5 | 2 | 1 | 2 | 2 | 5 | 0 | |
| Fusarium graminearum | 3 | 4 | 1 | 1 | 2 | 2 | 6 | 0 | |
| Fusarium oxysporum | 5 | 3 | 2 | 1 | 2 | 2 | 7 | 0 | |
| Magnaporthe oryzae | 4 | 6 | 0 | 2 | 2 | 2 | 5 | 0 | |
| Mycosphaerella graminicola | 3 | 4 | 0 | 1 | 2 | 2 | 9 | 0 | |
| Puccinia graminis | 4 | 4 | 1 | 1 | 5 | 2 | 3 | 0 | |
| Ustilago maydis | 3 | 5 | 0 | 1 | 4 | 2 | 4 | 0 | |
| Plants | Arabidopsis thaliana | 2 | 18 | 16 | 2 | 7 | 7 | 8 | 2 |
| Oryza sativa | 3 | 15 | 30 | 1 | 14 | 8 | 5 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhou, Y.; Liu, Y.; Li, B.; Tian, S.; Zhang, Z. Research Progress on the Mechanism and Function of Histone Acetylation Regulating the Interaction between Pathogenic Fungi and Plant Hosts. J. Fungi 2024, 10, 522. https://doi.org/10.3390/jof10080522
Zhang X, Zhou Y, Liu Y, Li B, Tian S, Zhang Z. Research Progress on the Mechanism and Function of Histone Acetylation Regulating the Interaction between Pathogenic Fungi and Plant Hosts. Journal of Fungi. 2024; 10(8):522. https://doi.org/10.3390/jof10080522
Chicago/Turabian StyleZhang, Xiaokang, Yuzhu Zhou, Yangzhi Liu, Boqiang Li, Shiping Tian, and Zhanquan Zhang. 2024. "Research Progress on the Mechanism and Function of Histone Acetylation Regulating the Interaction between Pathogenic Fungi and Plant Hosts" Journal of Fungi 10, no. 8: 522. https://doi.org/10.3390/jof10080522





