Inhibitory Effects and Mechanisms of Perilla Essential Oil and Perillaldehyde against Chestnut Pathogen Botryosphaeria dothidea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Characterization of PEO by GC/MS Analysis
2.3. Antifungal Assays In Vitro
2.3.1. Agar Dilution Method
2.3.2. Fumigation Method
2.3.3. Fungal Biomass Determination
2.3.4. Scanning Electron Microscopy (SEM) Observation of the Effect of PEO and PAE on Mycelial Morphology
2.4. Study on the Antifungal Mechanism of PEO and PAE against B. dothidea
2.4.1. Effects of PEO and PAE on B. dothidea Cell Wall
2.4.2. Effects of PEO and PAE on B. dothidea Cell Membrane
2.4.3. Determination of Ergosterol Content
2.4.4. Effects of PEO and PAE on Cellular Content Leakage
2.5. Antifungal Activity In Vivo
2.5.1. Antifungal Effects of PEO and PAE on Chestnut Kernels
2.5.2. Preventative Effects of PEO and Perilla Leaves on Chestnut Fruit Rot
2.6. Statistical Analysis
3. Results
3.1. GC/MS Analysis of Composition of PEO
3.2. In Vitro Antifungal Activity Results
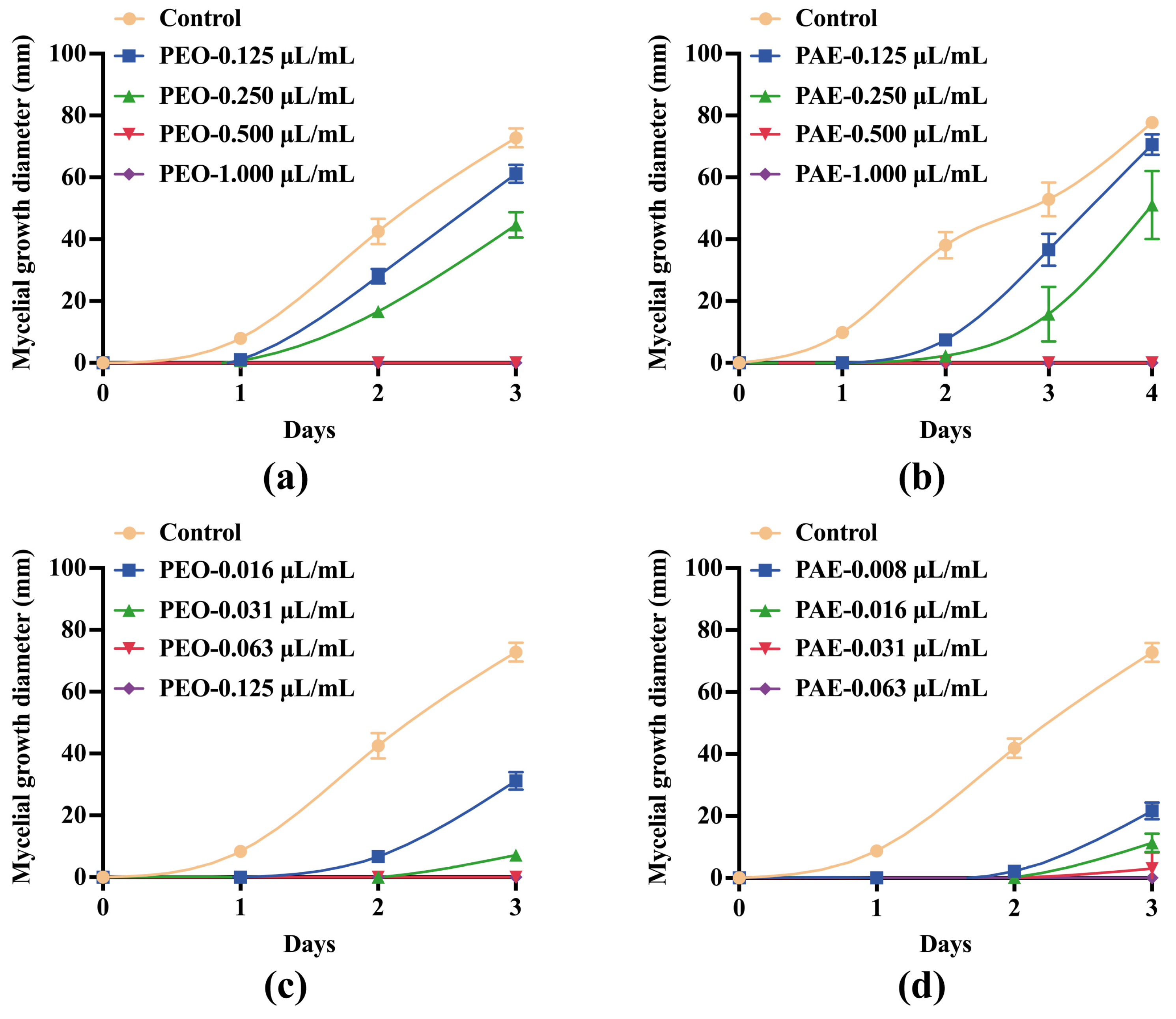
3.2.1. Effects of PEO and PAE on Mycelial Growth
3.2.2. Effects of PEO and PAE on Fungal Biomass
3.2.3. SEM Observation of the Effects of PEO and PAE on Fungal Morphology
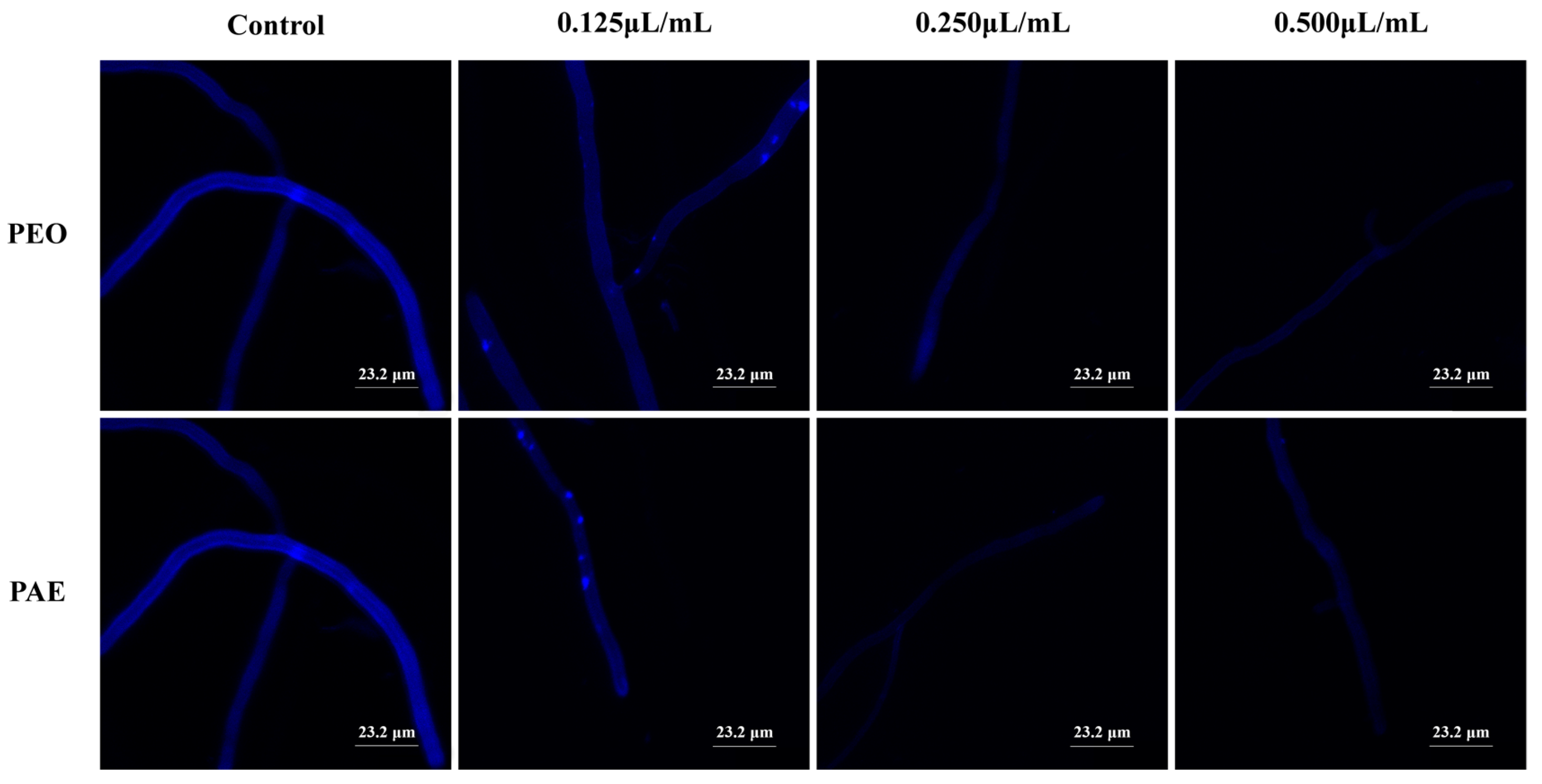
3.2.4. Effects of PEO and PAE on B. dothidea Cell Walls
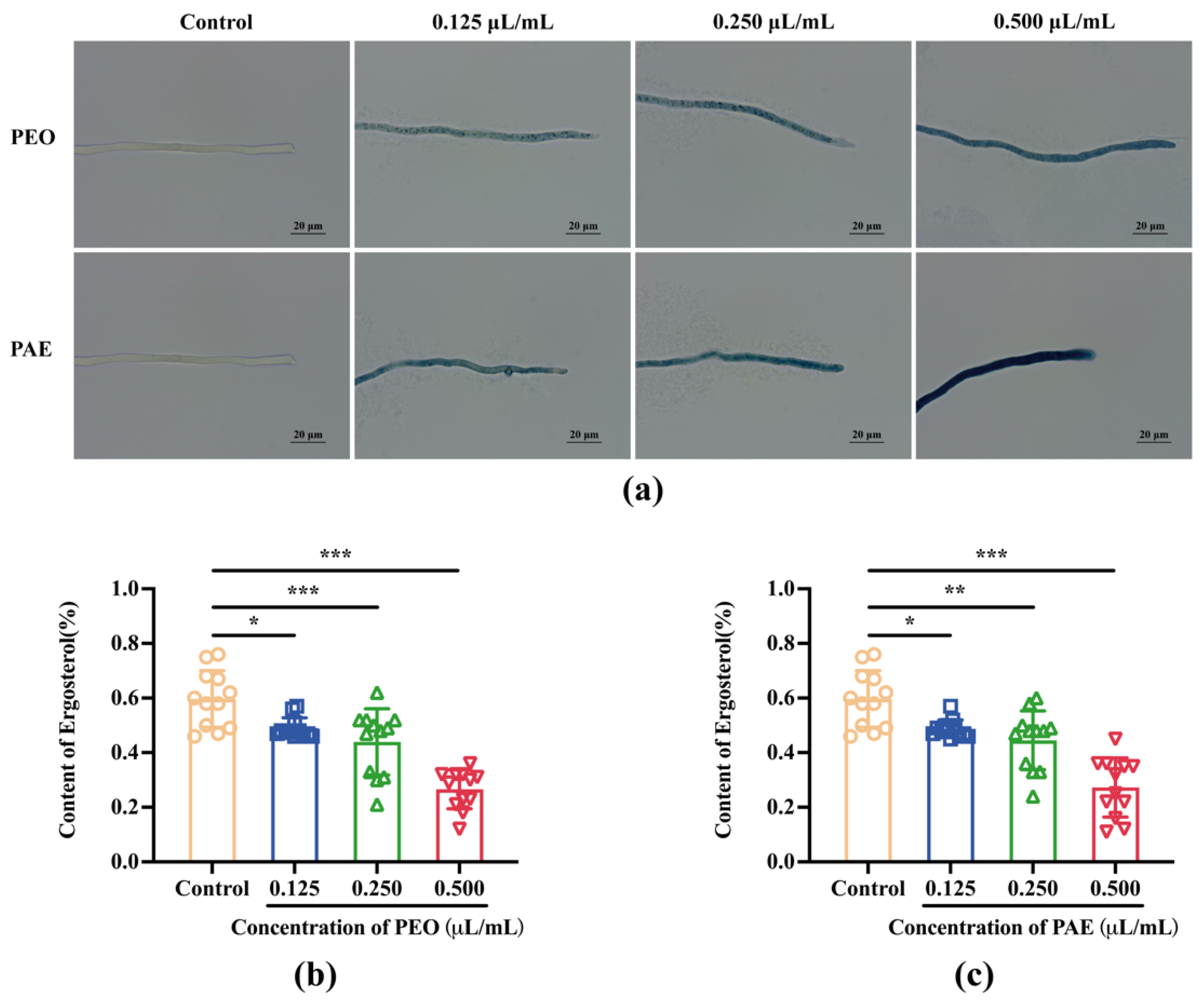
3.2.5. Effects of PEO and PAE on B. dothidea Cell Membranes
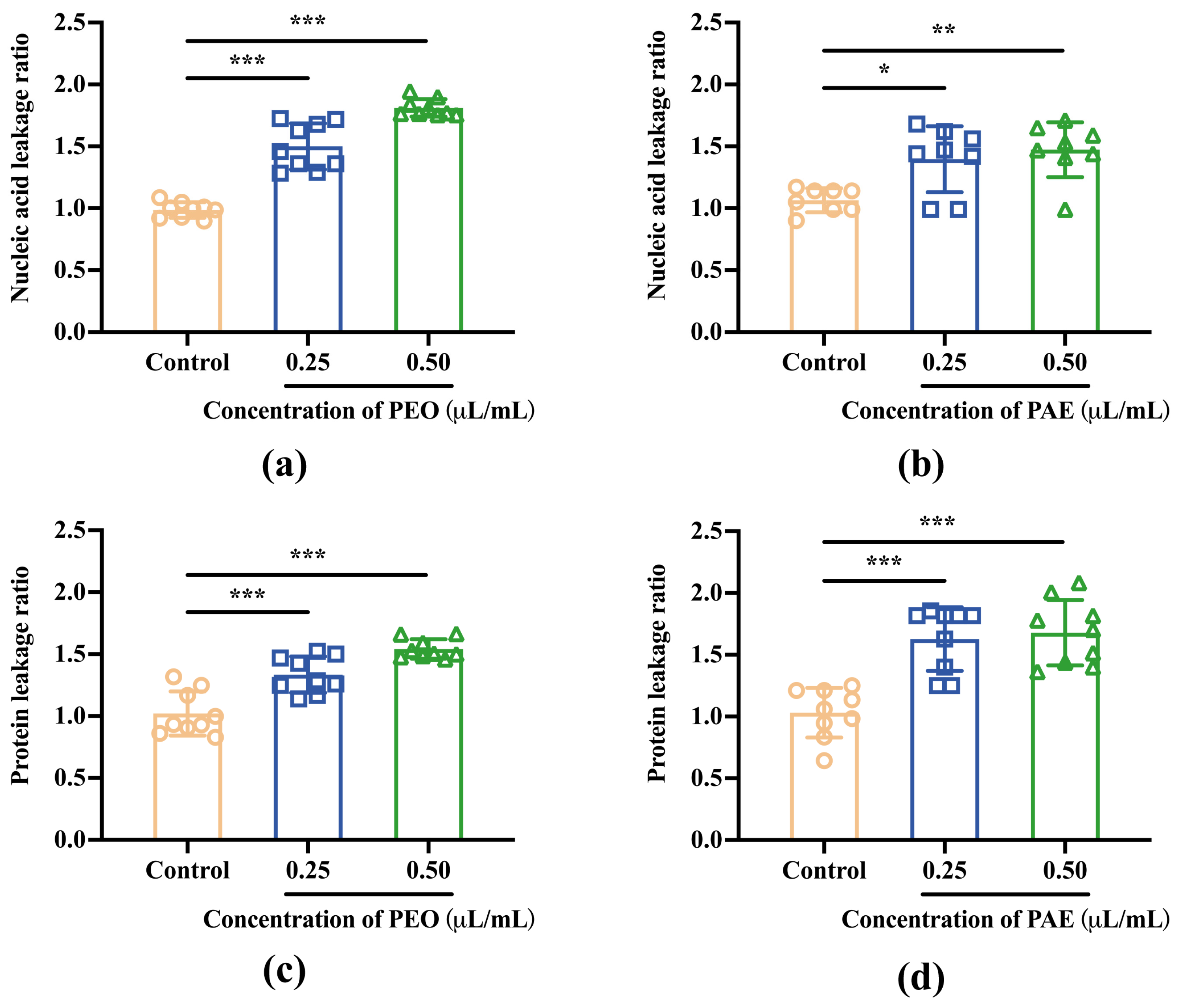
3.2.6. Nucleic Acids and Proteins Released from Damaged Membranes
3.3. Antifungal Activity In Vivo
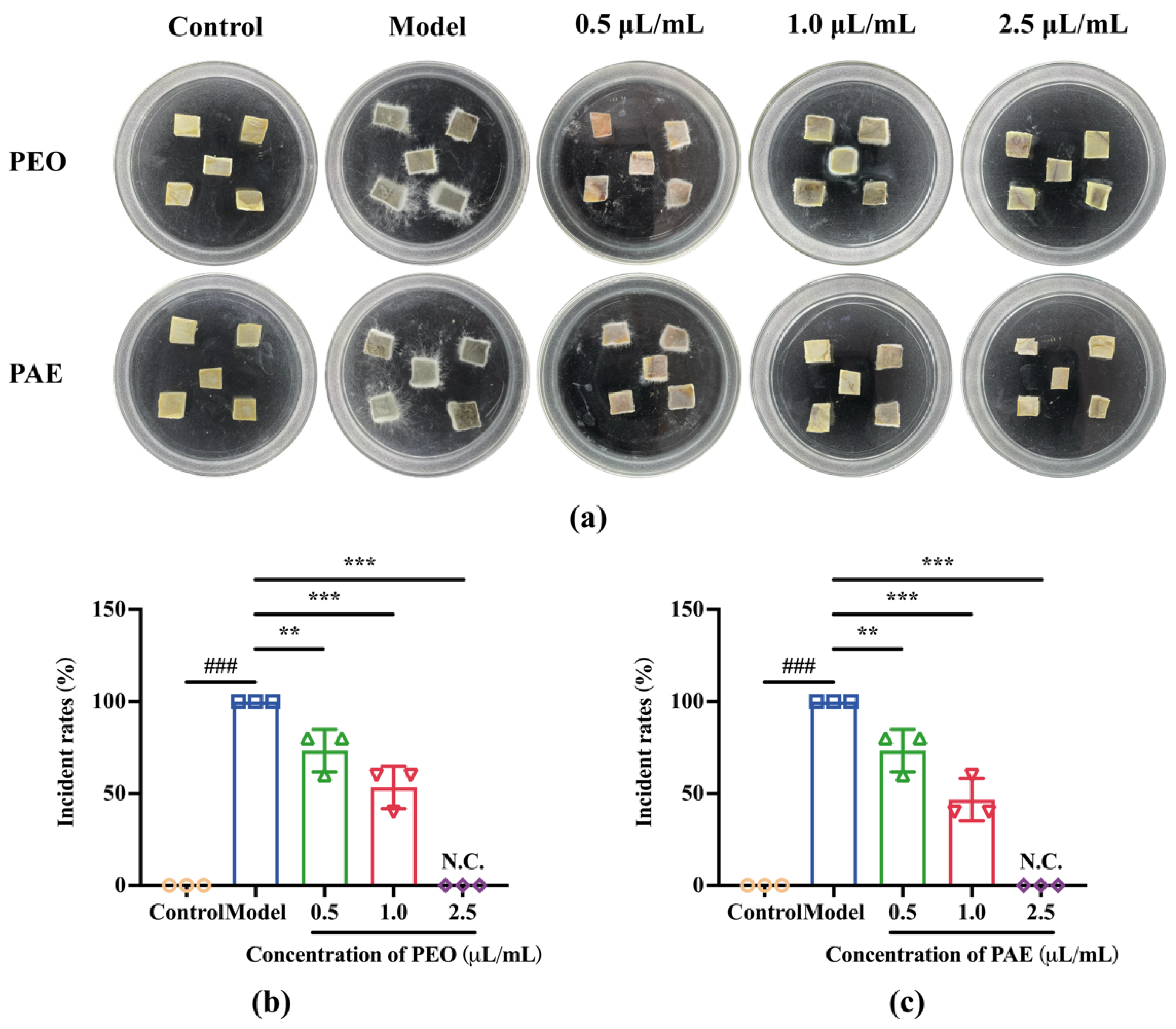
3.3.1. Antifungal Effects of PEO and PAE on Chestnut Kernel Pieces
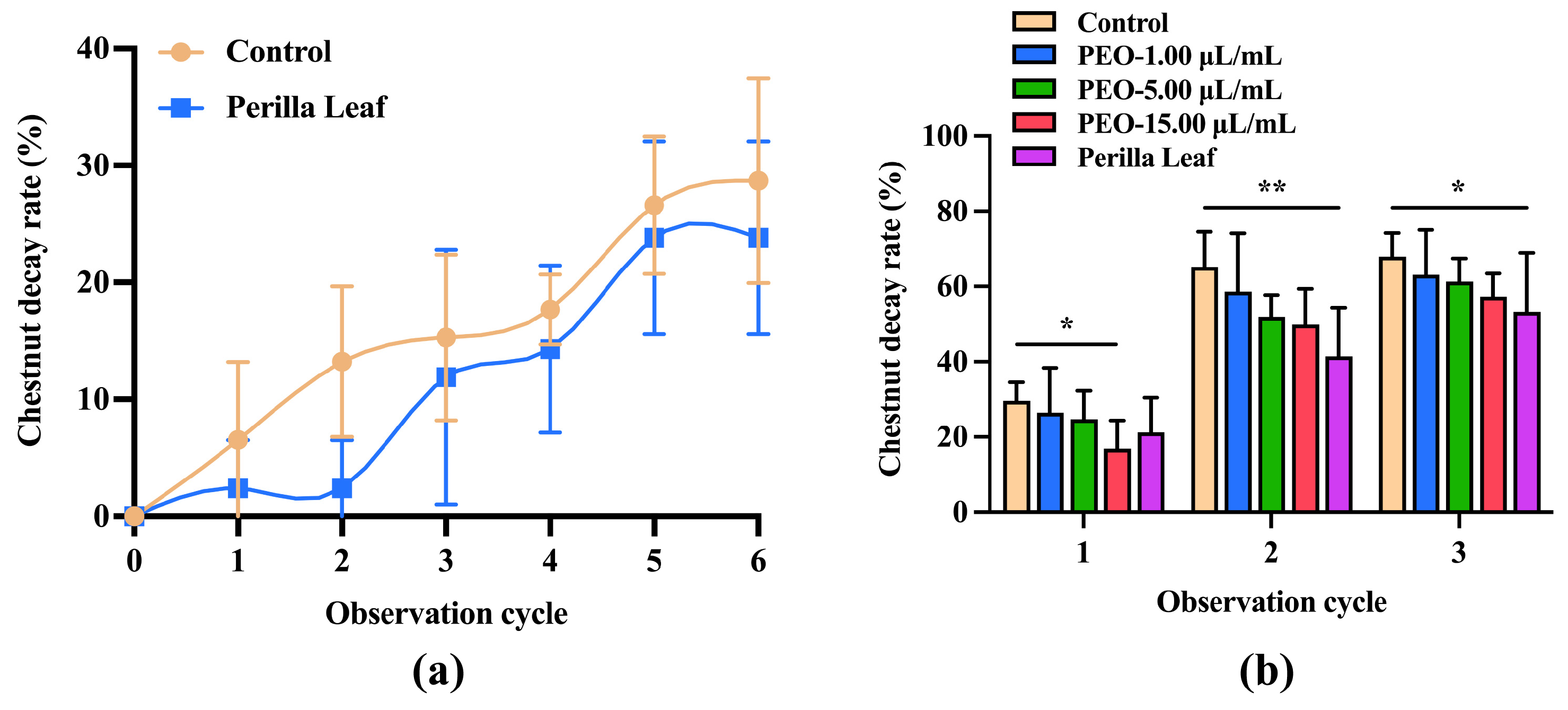
3.3.2. Preventative Effects of PEO and Perilla Leaves on Intact Chestnut Fruit Rot
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fraga, H. Influence of Climate Change on Chestnut Trees: A Review. Plants 2021, 10, 1463. [Google Scholar] [CrossRef]
- Massantini, R.; Moscetti, R.; Frangipane, M.T. Evaluating progress of chestnut quality: A review of recent developments. Trends Food Sci. Technol. 2021, 113, 245–254. [Google Scholar] [CrossRef]
- Lee, E.-S.; Song, E.-J.; Nam, Y.-D.; Nam, T.G.; Kim, H.-J.; Lee, B.-H.; Seo, M.-J.; Seo, D.-H. Effects of enzymatically modified chestnut starch on the gut microbiome, microbial metabolome, and transcriptome of diet-induced obese mice. Int. J. Biol. Macromol. 2020, 145, 235–243. [Google Scholar] [CrossRef]
- Enrico, S.; Stefano, P.; Urska, V.; Marco, F.; Saba, K.; Domenico, M.; Chiara, D.L.; Luca, C.; Fulvio, M.; Emma, D.F. A bio-guided approach for the development of a chestnut-based proanthocyanidin-enriched nutraceutical with potential anti-gastritis properties. Pharmacol. Res. 2018, 134, 145–155. [Google Scholar]
- Sverguzova, S.V.; Shaikhiev, I.G.; Fomina, E.V.; Galimova, R.Z. Use of chestnut sheel (Castánea) as adsorption material for removing pollutants from natural and sewage waters: A review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 945, 012072. [Google Scholar] [CrossRef]
- Seddaiu, S.; Mello, A.; Sechi, C.; Cerboneschi, A.; Linaldeddu, B.T. First Report of Neofusicoccum parvum Associated with Chestnut Nut Rot in Italy. Plant Dis. 2021, 105, 3743. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Y.; Gou, L.; Zhou, G. Antifungal Effect of Natamycin Emulsion and Application of Natamycin Emulsion in Preventing and Treating Chestnut Spoilage. CN116473069A, 25 July 2023. [Google Scholar]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef]
- Nicoletti, R.; Beccaro, G.L.; Sekara, A.; Cirillo, C.; Di Vaio, C. Endophytic Fungi and Ecological Fitness of Chestnuts. Plants 2021, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-L.; Cheng, Z.-Z.; Leng, W.-F.; Li, B.-H.; Xu, X.-M.; Lian, S.; Wang, C.-X. Progression of symptoms caused by Botryosphaeria dothidea on apple branches. Phytopathology® 2021, 111, 1551–1559. [Google Scholar] [CrossRef]
- Ha, Y.J.; Sa, K.J.; Lee, J.K. Identifying SSR markers associated with seed characteristics in Perilla (Perilla frutescens L.). Physiol. Mol. Biol. Plants 2021, 27, 93–105. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yuan, K.; Zhou, Q.; Lu, C.; Du, L.; Liu, F. Mechanism of antifungal activity of Perilla frutescens essential oil against Aspergillus flavus by transcriptomic analysis. Food Control 2021, 123, 107703. [Google Scholar] [CrossRef]
- Wang, H.; Guo, L.; Liu, L.; Han, B.; Niu, X. Composite chitosan films prepared using nisin and Perilla frutescense essential oil and their use to extend strawberry shelf life. Food Biosci. 2021, 41, 101037. [Google Scholar] [CrossRef]
- Erhunmwunsee, F.; Pan, C.; Yang, K.; Li, Y.; Liu, M.; Tian, J. Recent development in biological activities and safety concerns of perillaldehyde from perilla plants: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6328–6340. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, J.; Li, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/zein nanofiber films loaded with perillaldehyde, thymol, or ɛ-polylysine and evaluation of their effects on the preservation of chilled chicken breast. Food Chem. 2022, 373, 131439. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-T.; Gou, L.-J.; Zeng, H.; Zhou, G.; Dong, W.-R.; Cui, Y.; Cai, Q.; Chen, Y.-X. Inhibitory Effect and Mechanism of Dill Seed Essential Oil on Neofusicoccum parvum in Chinese Chestnut. Separations 2022, 9, 296. [Google Scholar] [CrossRef]
- Khruengsai, S.; Pripdeevech, P.; Tanapichatsakul, C.; Srisuwannapa, C.; D’Souza, P.E.; Panuwet, P. Antifungal properties of volatile organic compounds produced by Daldinia eschscholtzii MFLUCC 19-0493 isolated from Barleria prionitis leaves against Colletotrichum acutatum and its post-harvest infections on strawberry fruits. PeerJ 2021, 9, e11242. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wei, M.; Peng, S.; Mo, H.; Huang, L.; Yao, L.; Hu, L. Cuminaldehyde in cumin essential oils prevents the growth and aflatoxin B1 biosynthesis of Aspergillus flavus in peanuts. Food Control 2021, 125, 107985. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Sun, Y.; Chen, Q.; Zhang, L.; Le, T. The antifungal effects of cinnamaldehyde against Aspergillus niger and its application in bread preservation. Food Chem. 2020, 317, 126405. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Zhang, R.; Yao, W. Major components in Lilac and Litsea cubeba essential oils kill Penicillium roqueforti through mitochondrial apoptosis pathway. Ind. Crops Prod. 2020, 149, 112349. [Google Scholar] [CrossRef]
- Contreras Martínez, O.I.; Angulo Ortíz, A.; Santafé Patiño, G.; Peñata-Taborda, A.; Berrio Soto, R. Isoespintanol Antifungal Activity Involves Mitochondrial Dysfunction, Inhibition of Biofilm Formation, and Damage to Cell Wall Integrity in Candida tropicalis. Int. J. Mol. Sci. 2023, 24, 10187. [Google Scholar] [CrossRef]
- Donadu, M.G.; Peralta-Ruiz, Y.; Usai, D.; Maggio, F.; Molina-Hernandez, J.B.; Rizzo, D.; Bussu, F.; Rubino, S.; Zanetti, S.; Paparella, A. Colombian essential oil of Ruta graveolens against nosocomial antifungal resistant Candida strains. J. Fungi 2021, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, X.; Zhao, Y.; Xie, Y. The antifungal activity of o-vanillin against Aspergillus flavus via disrupting ergosterol biosynthesis and promoting oxidative stress, and an RNA-seq analysis thereof. Lwt 2022, 164, 113635. [Google Scholar] [CrossRef]
- Kosalec, I.; Jembrek, M.J.; Vlainić, J. The Spectrum of Berberine Antibacterial and Antifungal Activities. In Promising Antimicrobials from Natural Products; Springer: Cham, Switzerland, 2022; pp. 119–132. [Google Scholar]
- Gou, L.-J.; Liu, T.-T.; Zeng, Q.; Dong, W.-R.; Wang, L.; Long, S.; Su, J.-T.; Chen, Y.-X.; Zhou, G. Natamycin Has an Inhibitory Effect on Neofusicoccum parvum, the Pathogen of Chestnuts. Molecules 2023, 28, 3707. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Antifungal activity, mycotoxin inhibitory efficacy, and mode of action of hop essential oil nanoemulsion against Fusarium graminearum. Food Chem. 2023, 400, 134016. [Google Scholar] [CrossRef]
- Jordá, T.; Puig, S. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Hardy, G.E.S.; Burgess, T.I. Endophytes as potential pathogens of the baobab species Adansonia gregorii: A focus on the Botryosphaeriaceae. Fungal Ecol. 2011, 4, 1–14. [Google Scholar] [CrossRef]
- Sturrock, R.; Frankel, S.; Brown, A.; Hennon, P.; Kliejunas, J.; Lewis, K.; Worrall, J.; Woods, A. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.-L.; Marçais, B.; Nageleisen, L.-M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- He, W.; Shi, C.; Long, X.; Liu, X.; Zhao, X. Antimicrobial activity and mechanism of action of Perilla essential oil against Staphylococcus aureus. E3S Web Conf. 2020, 145, 01015. [Google Scholar] [CrossRef]
- Wu, X.; Dong, S.; Chen, H.; Guo, M.; Sun, Z.; Luo, H. Perilla frutescens: A traditional medicine and food homologous plant. Chin. Herb. Med. 2023, 15, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Valente, V.M.M.; Jham, G.N.; Jardim, C.M.; Dhingra, O.D.; Ghiviriga, I. Major antifungals in nutmeg essential oil against Aspergillus flavus and A. ochraceus. J. Food Res. 2015, 4, 51. [Google Scholar] [CrossRef]
- Jassal, K.; Kaushal, S.; Rashmi; Rani, R. Antifungal potential of guava (Psidium guajava) leaves essential oil, major compounds: Beta-caryophyllene and caryophyllene oxide. Arch. Phytopathol. Plant Prot. 2021, 54, 2034–2050. [Google Scholar] [CrossRef]
- Gogoi, R.; Begum, T.; Sarma, N.; Pandey, S.K.; Bhandari, S.; Saikia, S.; Tamang, R.; Saikia, R.J.; Lal, M. Elemicin-rich Cymbopogon khasianus (Hack) Stapf (ex Bor) essential oil: Pharmacological effects, toxicological investigation, and compositional analysis. Curr. Anal. Chem. 2022, 18, 1092–1107. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Dubey, N.K. Exploration of some potential bioactive essential oil components as green food preservative. LWT 2021, 137, 110498. [Google Scholar] [CrossRef]
- Seneme, E.F.; dos Santos, D.C.; Silva, E.M.R.; Franco, Y.E.M.; Longato, G.B. Pharmacological and Therapeutic Potential of Myristicin: A Literature Review. Molecules 2021, 26, 5914. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.J.P.; Souza, P.F.N.; Malveira, E.A.; Neto, N.A.S.; Silva, R.R.S.; Melo, G.L.C.; Silva, A.F.B.; Lima, L.B.; de Albuquerque, C.C.; Bastos, R.W.; et al. Antimicrobial Activity the Essential Oil from Croton pluriglandulosus Carn. Leaves against Microorganisms of Clinical Interest. J. Fungi 2023, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Swee-Hua-Erin, L.; Lai, K.-S. Antibacterial Activity and Mode of Action of β-caryophyllene on. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Li, J.; Fan, L. Study on the antifungal effect and mechanism of oregano essential oil fumigation against Aspergillus flavus. J. Food Process. Preserv. 2022, 46, e17026. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.-H.; Ye, M.; Wang, K.-B.; Fan, L.-M.; Su, F.-W. Chemical composition and antifungal activity of essential oil from Origanum vulgare against Botrytis cinerea. Food Chem. 2021, 365, 130506. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, L.; Jiang, D.; Wang, M.; Liu, H.; Yu, H.; Yao, W. Transcriptomic analysis of inhibition by eugenol of ochratoxin A biosynthesis and growth of Aspergillus carbonarius. Food Control 2022, 135, 108788. [Google Scholar] [CrossRef]
- Xu, T.; Cao, L.; Zeng, J.; Franco, C.M.; Yang, Y.; Hu, X.; Liu, Y.; Wang, X.; Gao, Y.; Bu, Z. The antifungal action mode of the rice endophyte Streptomyces hygroscopicus OsiSh-2 as a potential biocontrol agent against the rice blast pathogen. Pestic. Biochem. Physiol. 2019, 160, 58–69. [Google Scholar] [CrossRef]
- Lima, S.L.; Colombo, A.L.; de Almeida Junior, J.N. Fungal cell wall: Emerging antifungals and drug resistance. Front. Microbiol. 2019, 10, 2573. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, X.; Wang, N.; Mo, P.; Shen, J.; Liu, M.; Zhang, H.; Wang, P.; Zhang, Z. Membrane component ergosterol builds a platform for promoting effector secretion and virulence in Magnaporthe oryzae. New Phytol. 2023, 237, 930–943. [Google Scholar] [CrossRef]
- Chtioui, W.; Heleno, S.; Migheli, Q.; Rodrigues, P. Plant extracts as biocontrol agents against Aspergillus carbonarius growth and ochratoxin A production in grapes. Int. J. Food Microbiol. 2023, 407, 110425. [Google Scholar] [CrossRef]
- Qiao, L.; Jiao, Y.; Li, X.; Zhang, Y.; Lu, L.; Zhang, X.; Liu, X. Herbal smoke fumigation for controlling Penicillium crustosum in fresh walnuts. Food Res. Int. 2023, 167, 112709. [Google Scholar] [CrossRef]
- Wang, H.; Fu, L.; Li, C.; Zhang, X.; Narcisse, K.E.; Qi, H.; Han, C.; Wang, X.; Ma, H.; Zhu, C.; et al. Tannic acid exerts antifungal activity in vitro and in vivo against Alternaria alternata causing postharvest rot on apple fruit. Physiol. Mol. Plant Pathol. 2023, 125, 102012. [Google Scholar] [CrossRef]
- Kan, L.; Li, Q.; Xie, S.; Hu, J.; Wu, Y.; Ouyang, J. Effect of thermal processing on the physicochemical properties of chestnut starch and textural profile of chestnut kernel. Carbohydr. Polym. 2016, 151, 614–623. [Google Scholar] [CrossRef]
- Yang, Q.; Kan, L.; Wu, Y.; Liu, Y.; Ouyang, J. Influence of nutritional components on the texture characteristics and sensory properties of cooked chestnut kernel. J. Food Process. Preserv. 2019, 43, e14112. [Google Scholar] [CrossRef]


| Peak No. | Retention Time (min) | Component | Molecular Weight | Relative Content (%) |
|---|---|---|---|---|
| 1 | 9.670 | Perilla ketone | 166.2170 | 7.42 |
| 2 | 10.461 | 1-(furan-2-yl)-4-Methylpentan-1-one | 166.2170 | 0.24 |
| 3 | 11.187 | Isoegomaketone | 164.2011 | 3.93 |
| 4 | 12.961 | β-Elemene | 204.3511 | 0.20 |
| 5 | 13.698 | Caryophyllene | 204.3511 | 10.92 |
| 6 | 14.478 | (Z)-β-Farnesene | 204.3511 | 11.43 |
| 7 | 15.140 | Germacrene D | 204.3511 | 0.95 |
| 8 | 15.407 | (Z, E)-α-Farnesene | 204.3511 | 0.46 |
| 9 | 16.027 | δ-Cadinene | 204.3511 | 0.14 |
| 10 | 16.155 | Myristicin | 192.2100 | 10.85 |
| 11 | 16.849 | Elemicin | 208.2500 | 48.31 |
| 12 | 17.266 | Caryophyllene oxide | 220.3505 | 3.02 |
| 13 | 17.715 | Benzene,1,2,3-trimethoxy-5-(1E)-1-propen-1-yl- | 208.2500 | 0.36 |
| All | 98.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Wang, L.; Long, S.; Dong, W.; Li, Y.; Chen, Y.; Zhou, G. Inhibitory Effects and Mechanisms of Perilla Essential Oil and Perillaldehyde against Chestnut Pathogen Botryosphaeria dothidea. J. Fungi 2024, 10, 526. https://doi.org/10.3390/jof10080526
Zeng Q, Wang L, Long S, Dong W, Li Y, Chen Y, Zhou G. Inhibitory Effects and Mechanisms of Perilla Essential Oil and Perillaldehyde against Chestnut Pathogen Botryosphaeria dothidea. Journal of Fungi. 2024; 10(8):526. https://doi.org/10.3390/jof10080526
Chicago/Turabian StyleZeng, Qi, Lu Wang, Sha Long, Wanrong Dong, Yaoyao Li, Yuxin Chen, and Gao Zhou. 2024. "Inhibitory Effects and Mechanisms of Perilla Essential Oil and Perillaldehyde against Chestnut Pathogen Botryosphaeria dothidea" Journal of Fungi 10, no. 8: 526. https://doi.org/10.3390/jof10080526





