Abstract
Oral candidiasis is an opportunistic infection caused by fungi of the genus Candida. Nystatin, fluconazole, and miconazole are the most widely used antifungal drugs in dentistry, but in recent years, they have been shown to be less effective due to the increase in the resistance to antifungal drugs. The growing challenge of antifungal resistance emphasizes the importance of exploring not only alternative strategies in the fight against Candida spp. infections but also supportive treatment for pharmacological treatment for oral candidiasis. This review aims to evaluate and compare the in vitro reports on antifungal efficacy against Candida spp. exhibited by mouthwashes distributed on the European market. The research question was elaborated through the PEO framework recommended by PRISMA 2020. A bibliographic search strategy was developed for the scientific online databases Pubmed and ScienceDirect. According to the eligibility criteria, 21 papers were included in this study over a 27-year period. Mouthwashes containing chlorhexidine digluconate, cetylpyridinium chloride, hexetidine, and fluorine compounds among others, and natural antimicrobials, such as menthol, thymol, eucalyptol, and Glycyrrhiza glabra extracts, have demonstrated antifungal effectiveness. Nonetheless, the methodological variance introduces ambiguity concerning the comparative efficacy of distinct molecules or mouthwash formulations and complicates the evaluation and the comparison of results between studies. Some mouthwashes commercially available in Europe have the potential to be used in anti-Candida therapy and prevention since they have shown antifungal effect.
1. Introduction
Oral care products are used daily by billions of people all around the world [1]. The market is extensively supplied with various types of mouthwashes, also called mouth rinse, with very different purposes. Since these products are classified as “cosmetic products” on the European market, their formulation, composition, and indications focussed on marketing messages that are subject to modest regulation and little, if any, scrutiny.
Among the products used “to treat” different “diseases” of the gums and oral mucous without a medical diagnosis, without a medical prescription, and without the benefit/risk of their use having been analysed are mouthwashes that can contain synthetic and/or natural antimicrobials, such as chlorhexidine digluconate (CHX), cetylpyridinium chloride (CPC), hexetidine (HX), fluoride compounds, menthol, thymol, eucalyptol, Glycyrrhiza glabra extracts, and Mentha piperita extracts [2,3]. CHX, developed in the early 1950s (UK), is a bisbiguanide compound used as a topical broad-spectrum antimicrobial in dental practice for the treatment of inflammatory oral conditions caused by Gram-positive and Gram-negative bacteria, yeasts, and viruses. Its antimicrobial activity is dose-dependent: at lower concentrations (0.02–0.06%), CHX is bacteriostatic, and at higher concentrations (≥0.12%), it is bactericidal [4]. CPC, developed in the 1930s (USA), is a cationic quaternary ammonium compound used as a topical antiseptic in some types of mouthwashes, toothpastes, and nasal sprays in varying concentrations (0.045–0.1%) [5]. CPC has broad-spectrum antimicrobial properties (viruses, fungus, and bacteria), which act through the disorganisation in its structure and the leakage of low-molecular components out of the cell [6]. HX is a cationic antiseptic with a wide spectrum of actions against Gram-positive and Gram-negative bacteria, as well as some fungi and parasites. It is used as a 0.1% mouthwash for local infections and oral hygiene [7]. Fluorine compounds found in mouthwashes consist mainly of sodium fluoride, but we can also find calcium fluoride, potassium fluoride, stannous fluoride, and monofluorophosphate among others [8]. Fluorine compounds are usually used in mouthwashes for their remineralizing effect, but they also show antibacterial effects through their interference with the uptake and degradation of polysaccharides by the bacterial cells, and also by reducing their ability to maintain pH homeostasis [9]. Oral candidiasis is an opportunistic infection caused by fungi of the genus Candida associated with poor oral hygiene with the use of a removable dental prosthesis, sexual practices, and is more prevalent in immunocompromised patients [10,11,12]. About 90% of Candida infections are caused by five species: Candida albicans, Nakaseomyces glabrataa (formerly classified as Candida glabrata), Candida tropicalis, Candida parapsilosis, and Pichia kudriavzevii (formerly classified as Candida krusei) [13]. Nystatin, fluconazole, and miconazole are the most widely used antifungal drugs in dentistry, but in recent years, they have been shown to be less effective [14].
Resistance to antifungal drugs is acquired by a learning process by microorganisms, which adapt through mutations after having been exposed to a drug, making it much more difficult to eliminate [15]. The World Health Organization published a recent list of priority pathogenic fungi to focus and drive research and political interventions to strengthen the global response to fungal infections and antifungal resistance in which C. albicans, C. glabrata, C. tropicalis, and C. parapsilosis are mentioned [16,17].
The growing challenge of antifungal resistance emphasises the importance of exploring alternative strategies in the fight against Candida spp. infections. The aim of this study was to carry out a systematic review to evaluate and compare the antifungal efficacy against Candida spp. mouthwashes available on the European Market.
2. Methods
This study question was created in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) that strongly recommends constructing the research question in accordance with the Population, Exposure, Outcome (PEO) policy format [18]. The resulting question was as follows: which mouthwashes available in Europe have an antifungal effect against Candida spp.?
The study variables were defined, namely, the Minimum Inhibitory Concentration (MIC), the Minimum Fungicidal Concentration (MFC), and the Minimum Biofilm Eradication Concentration (MBEC) of mouthwashes based on CHX and/or CPC against Candida spp., type of assay, methodology followed, media, and time points. Variables were compiled in Microsoft Excel version 16.0.15330.20196 (32-bit) spreadsheet software (Microsoft Corporation, Redmond, WA, USA).
A specific bibliographic search strategy was developed for the PubMed and Science Direct databases up to 30 April 2024. No time limit was applied. An advanced search strategy was carried out based on keywords and MeSH (Medical Subject Headings) terms mouthwashes, antifungal agents, and Candida using Boolean operators. The advance search expression used was: “Mouthwashes” [Mesh] AND “Antifungal Agents” [Mesh] AND “Candida” [Mesh]. The revised nomenclature published in 2021 by Borman et al. was considered [13].
Inclusion criteria included in vitro and in vivo studies that cumulatively evaluated the efficacy against Candida spp. in isolates from the oral cavity of patients and/or in standards from the oral cavity, studies with a complete description of the materials and methods, and MIC and MFC results against Candida spp. Exclusion criteria included studies that evaluated Candida spp. in isolates not belonging to the oral cavity, mouthwashes not sold on the European market, review studies, books, and book chapters.
The risk of bias was carefully assessed to evaluate the quality of in vitro studies. Our review encompassed in vitro studies, and to assess the risk of bias, we evaluated key aspects such as the type of in vitro methodologies and completeness of outcome data. Furthermore, the risk of bias assessment was conducted primarily at the study level, considering overall quality (e.g., use of guidelines as a base to drug testing assays) and internal validity of each included study (cause-and-effect relationship). Assessment results informed the interpretation and synthesis of study findings, particularly in discussing the strength of evidence and potential limitations affecting study outcomes. Two independent reviewers were involved in the quality assessment process to minimize bias/errors. Any discrepancies in reviewers’ judgments were resolved through discussion and consensus. When consensus was not reached, a third reviewer arbitrated to achieve agreement [19].
3. Results and Discussion
3.1. Bibliographic Research
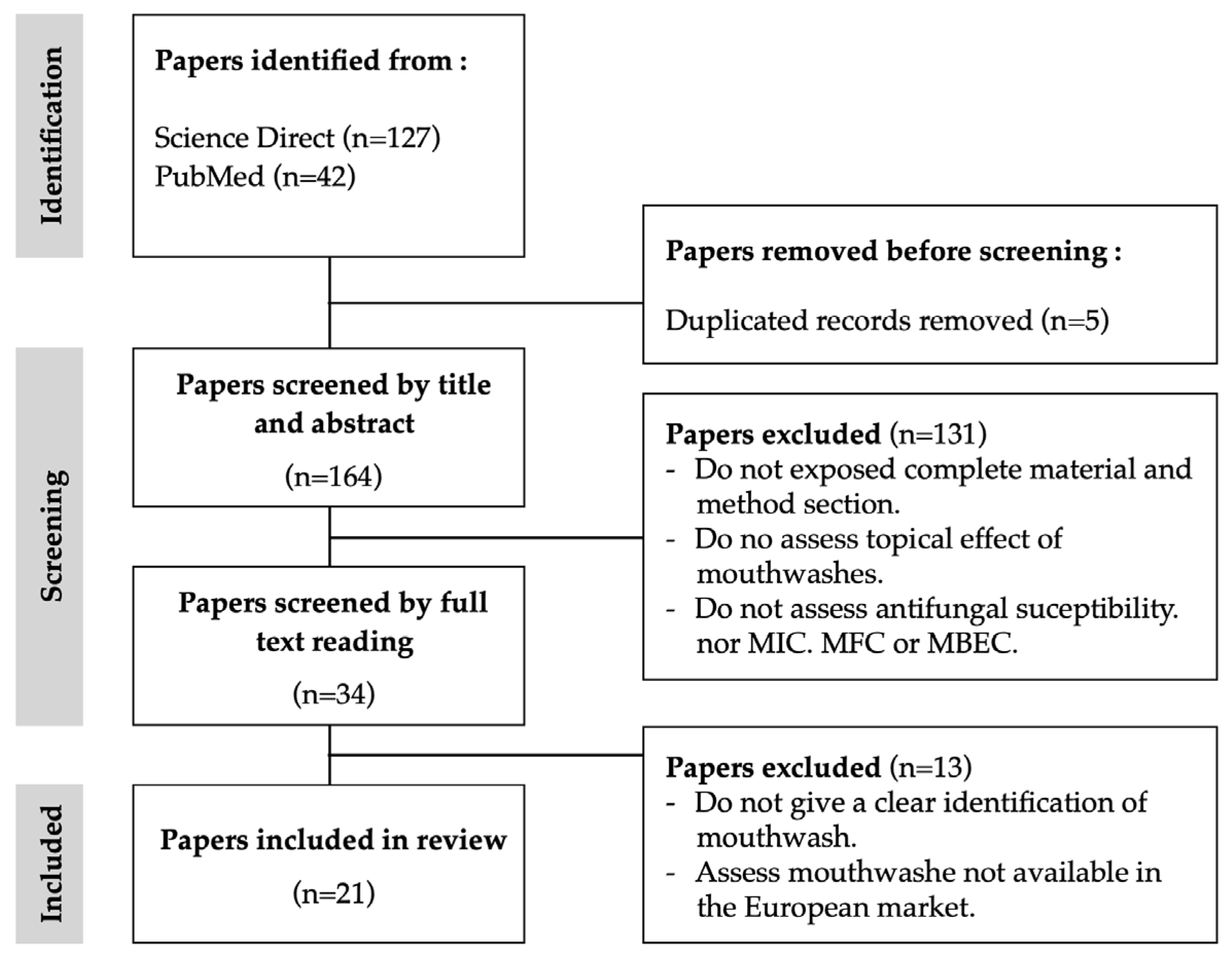
After removing duplicates, the search flow diagram selected a total of 169 potentially eligible studies (Figure 1). According to the eligibility criteria, 21 papers were selected to include in this study. The time frame was 27 years, from 1997 to 2024. Notably, the period spanning from 2011 to 2020 accounted for the highest publication frequency, constituting more than 50% of the total publications. According to the inclusion criteria, only studies on mouthwash products available on the European market were included. The majority of the studies included and analysed were performed in European countries, such as Italy [2,20,21,22,23], Poland [24,25,26], Sweden-Portugal [27], Norway [27], France [28] and Finland [29,30]. The remaining ones were performed by non-European countries, namely, Chile [31], China [32], India [33], Iran [34], Malaysia [35], South Africa [36], Turkey [37], and the USA [38]. The cumulative results, as per the eligibility criteria, indicated that only two studies were in vivo studies [29,36], while the remaining encompassed in vitro experimental protocols.
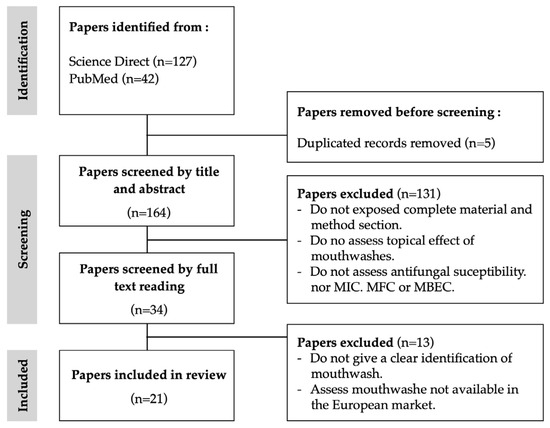
Figure 1.
Flowchart for the bibliographic research.
3.2. Antifungal Activity of Active Ingredients in Mouthwashes
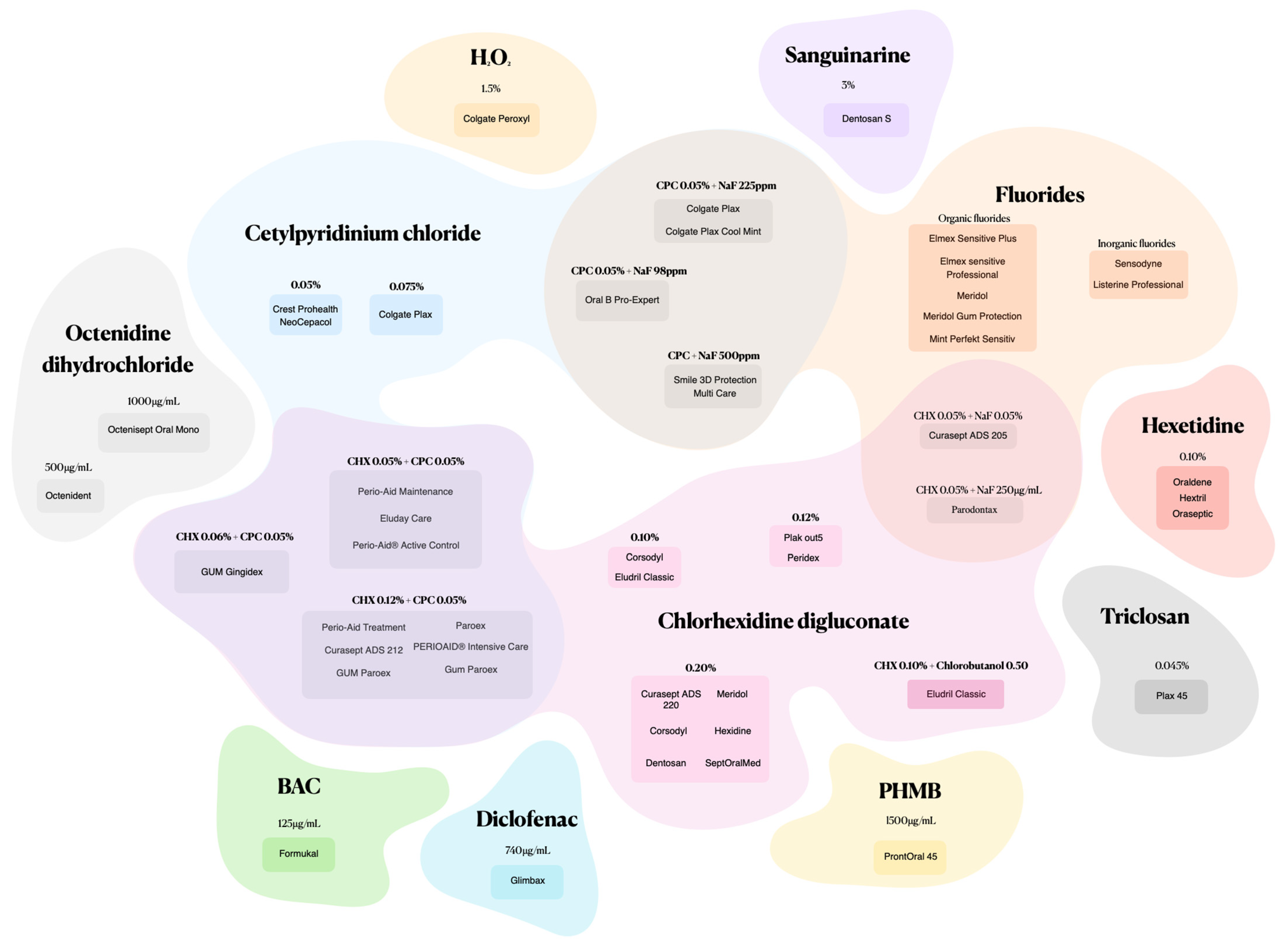
In the literature, there is a substantial number of mouthwashes which have been tested against Candida strains. In our analysis, mouthwashes were grouped according to the active substances, synthetic or natural compounds. From the analysis, it was found that most pharmaceutical laboratories or manufacturers accurately described the composition of mouthwashes; however, the opposite was also found. From the analysis, it was also noticeable that changes and mergers of pharmaceutical laboratories over time had an impact on the composition of products that maintained the same commercial identity, such as Corsodyl [27] (Table 1).

Table 1.
Mouthwashes analysed in previous studies (by alphabetic order).
3.2.1. Synthetic Active Compounds
Mouthwashes formulated with synthetic active ingredients and various combinations offer a targeted approach to candidiasis fight (Figure 2).
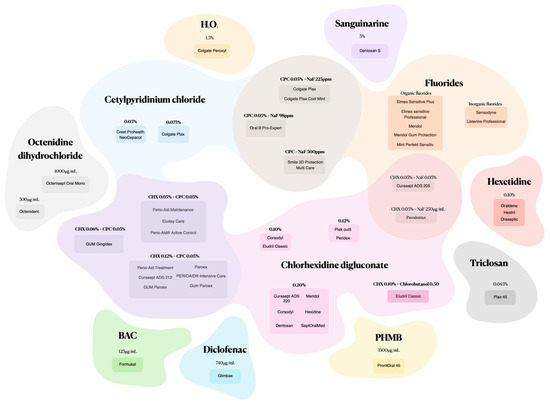
Figure 2.
Mouthwashes composed of synthetic active ingredients and combinations.
Chlorhexidine Digluconate (CHX)
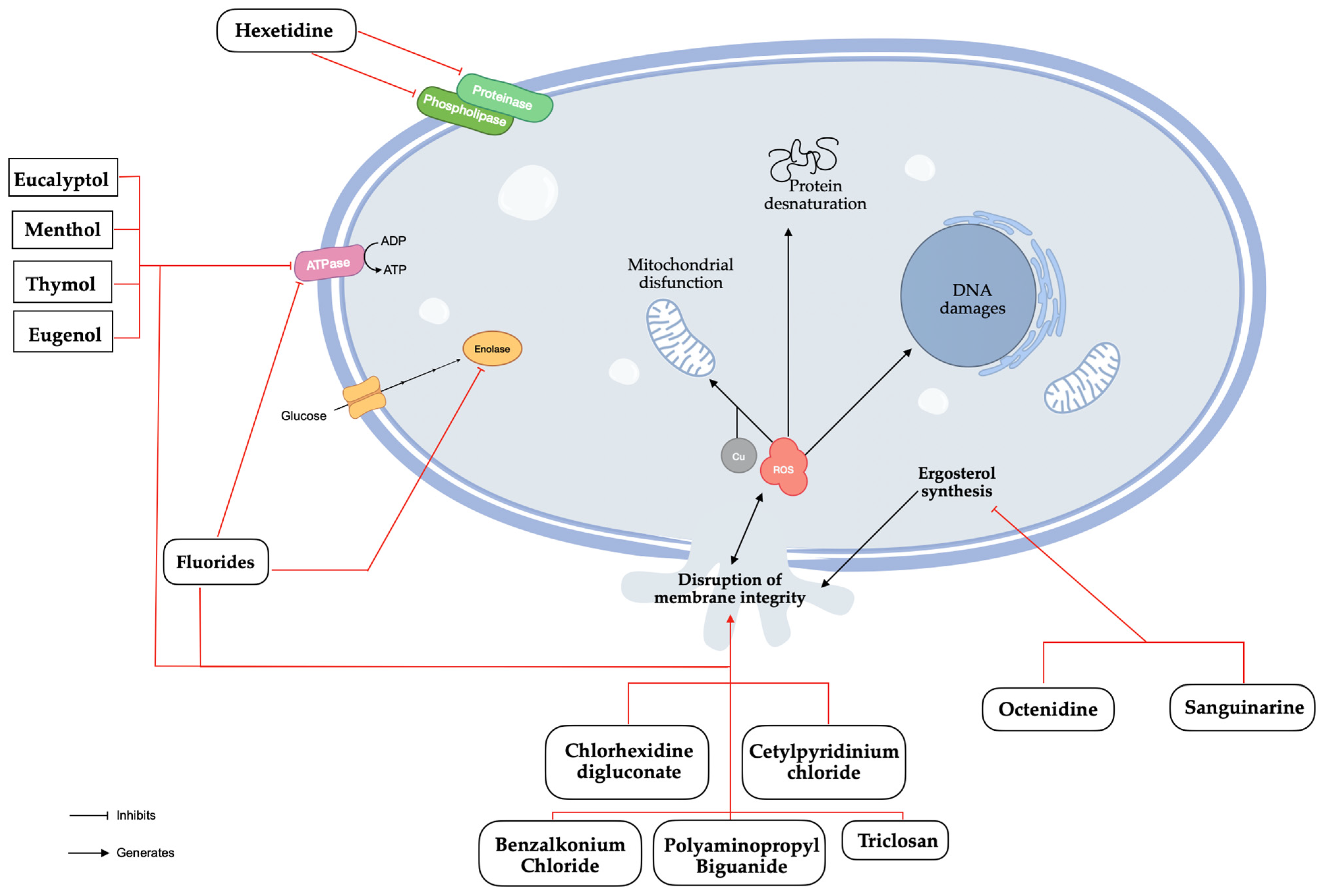
CHX is probably the most common antimicrobial compound present in mouthwashes with therapeutic aim in the literature [8,38]. As a positively charged di-cationic compound, CHX initially interacts with the negatively charged cell wall, subsequently binding to phospholipids in the cell membrane, leading to a leakage of potassium ions and progressive irreversible damage to the membrane and cytoplasm, ultimately resulting in bactericidal effects [41,42]. Novel studies found that CHX could induce apoptosis of C. albicans cells through the disruption of metal ion and ROS homeostasis, which may help to identify new targets for fungal infections (Figure 3) [43]. Potential adverse effects include tooth staining, taste changes, mouth irritation, allergic reactions, and rarely, severe irritation or chemical burns in children [6]. From our analysis, it can be stated that the concentrations of CHX found in mouthwashes were in the range of 0.1% to 0.2%, and that in the same range, the results showed similar fungistatic and fungicidal effects. C. albicans species showed better MIC results than non-C. albicans Candida species, particularly for CK and CP [24,40]. However, the result for CG was substantially better than for CA (Table 2) [24].
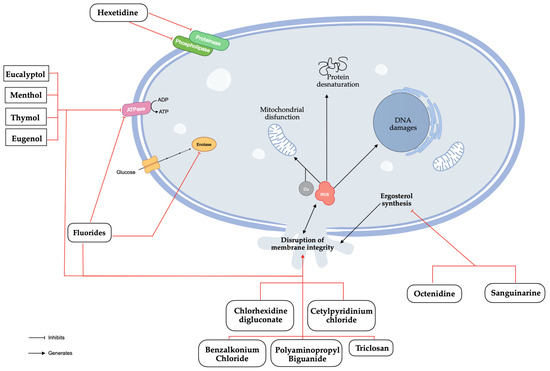
Figure 3.
Antifungal mechanisms of natural and synthetic substances found in mouthwashes.

Table 2.
Antifungal effect results of chlorhexidine digluconate (CHX)-based mouthwashes.
Cetylpyridinium Chloride (CPC)
CPC, a cationic quaternary ammonium compound, operates similarly to CHX by disrupting cell membrane [44]. Its antibacterial potential is well described by a disruption of the fungal membrane integrity causing a leakage of bacterial cytoplasmic components, interference with cellular metabolism, and the inhibition of cell growth [45]. The potential adverse effects include tooth staining, taste changes, gastric upset, allergic reactions, and mouth/tongue irritation [46]. CA obtained weaker MFC results than non-C. albicans Candida species, namely, CP and CK [21], results that were contradicted in more recent studies whose MIC, MFC, and disc diffusion assay results were worse in CA than in non-albicans species, particularly CK (Table 3) [32].

Table 3.
Antifungal effect results of cetylpyridinium chloride (CPC)-based mouthwashes.
Hexetidine (HX)
HX belongs to the group of pyrimidine derivatives, is only found at a 0.1% concentration, and acts as an inhibitor of the phospholipase and proteinase production of C. albicans [37]. However, oral retention appears to be limited so that the antimicrobial activity does not last long [47]. CA showed better results in MFC compared to non-C. albicans Candida species in clinical isolates [21]. However, recent studies indicate that HX achieves similar inhibition percentages in CA, CG, and CK and performs better than in CP and CT in ATCC species (Table 4) [28].

Table 4.
Antifungal effect results of hexetidine (HX)-based mouthwashes.
Fluoride Compounds
While fluoride is known to inhibit bacterial metabolism, its exact mechanism in combating Candida species remains to be fully elucidated. Research suggests potential effects on enzyme activity and cell wall integrity. Bearing in mind that fluoride is an extremely small chemical entity and considering that when it is present in high concentrations inside cells, it displays fungicidal activity, it has led the international scientific community to test synergy models. This is the case of the association of different compounds with fungicidal activity based on mechanisms of action that disrupts the integrity of the cell membrane and of the cell [46,48]. The results between CA and non-C. albicans Candida species are inconsistent. In the disc diffusion assay, the inhibition halos for the ELMEX mouthwash were smaller for CA than for CG, CK, and CP standard species. However, the Mint Perfect Sensitive mouthwash showed better results in CA compared to non-Candida albicans Candida species (Table 5) [26].

Table 5.
Antifungal effect results of fluorine-based mouthwashes.
Combinations of Substances
These developments explain the emergence of the combination of active substances in mouthwashes, such as the combination of CHX and CPC, or CHX and fluoride, a strategy that has been shown to increase fungicidal activity and reduce toxicity [26]. Results highlighted that the association of CHX with CPC were moderately dependent on the concentration of CHX; in fact, the antifungal effect between CHX 0.05% + CPC 0.05% and CHX 0.12% + CPC 0.05% were very similar [31]. According to the literature, CHX is often associated with CPC to improve antifungal results [44] but in the papers studied, CHX 0.10% undoubtedly obtained better results than CHX 0.12% + CPC 0.05% due to excipients [25].
The antifungal effect of the combination of CPC and NaF is more effective on non-C. albicans Candida standard species than on CA regardless of the NaF concentration (Table 6) [26,32].

Table 6.
Antifungal effect results of pharmacological combinations.
Other Substances
Octenidine (OCT), sanguinarine (SNG), benzalkonium chloride (BAC), polyaminopropyl biguanide (PHMB), and triclosan (TCS) are occasionally found in other mouthwashes. OCT and SNG have inhibitory effects on ergosterol’s biosynthesis which lead to the generation of ROS and the disruption of the membrane integrity [49,50]. BAC is known to denature proteins and disrupt the membrane integrity through penetration of the hydrophobic bilayer, compromising cellular permeability controls and causing cell leakage and lysis [51]. PHMB interacts with the cell membrane through electrostatic interactions and disrupt it, followed by an accumulation within the cytosol where it disrupts the nuclear membrane and fragments DNA [52]. TCS disorganizes the microbial cytoplasmic membrane inducing microorganisms’ lysis [53]. In January 2009, the Scientific Committee on Consumer Safety (SCCS) adopted an opinion on its human health safety, considering that TCS should only be used as a preservative in mouthwashes at a maximum concentration of 0.2% [54]. Given the current data, it is important to consider that the observed results may be attributed, at least in part, to the presence of TCS. In clinical isolates, TCS has a similar effect on CA, CK, and CP, but MFC is worse for CK and CP (Table 7) [21].

Table 7.
Antifungal effect results of mouthwashes with other pharmacologic active substances.
3.2.2. Natural Active Compounds
Menthol, eucalyptol, thymol, and eugenol are the main natural active ingredients found in mouthwashes. Menthol, thymol, eugenol, and eucalyptol have similar mechanisms of action. They disrupt biosynthetic and signalling pathways, causing mitochondrial dysfunction by inhibiting the electron transport system, reducing mitochondrial transmembrane potential, blocking respiratory proton pumps, decreasing ATP production, and increasing ROS generation, which ultimately triggers apoptosis [55,56,57,58]. The MFC results were similar across all species, but the MIC results were better for CA, CP, and CD compared to CG, CK, and CT [40]. However, recent studies have not confirmed these findings for C. albicans species, which show lower MIC, MFC, and disc diffusion results (Table 8) [29,32,33].

Table 8.
Antifungal effect results of mouthwashes with natural active compounds.
3.2.3. Excipients
Regrettably, the literature does not directly address the potential role of excipients in the antifungal activity of mouthwashes. However, excipients can influence the bioavailability of active antifungal compounds by enhancing absorption or altering release kinetics. This can lead to higher concentrations of active ingredients at the target site, boosting antifungal efficacy. Consequently, mouthwashes with the same active ingredients but different excipients may exhibit varying antimicrobial effectiveness.
Nonetheless, some excipients used in mouthwashes, such as preservatives, surfactants, or solvents may have inherent antimicrobial properties that could contribute to the overall antifungal activity of the formulation. For instance, sodium benzoate, methylparaben, and ethylparaben prevent microbial growth in mouthwashes [8,59]. Others, such as ethanol, 2-propanol, dichlorobenzyl alcohol, and phenethyl alcohol have also shown antimicrobial efficacy [60,61].
Potential adverse effects of the chronical use of alcohol-based mouthwashes may further increase the risk of developing an oral cancer where other risk factors for oral cancer are present [62].
3.3. Bias and Studies’ Limits
Most authors assume that mouthwashes only have one active substance in their composition, and that this substance is the only one that may or may not have fungicidal activity. Studies that have tested the antifungal effect of mouthwashes against microorganisms in the biofilm phase are scarce, as most focus on planktonic phases [25,29] However, it is acknowledged that Candida spp. can form biofilms to increase its survival by secreting an extracellular matrix, increasing its adhesion to oral mucous membranes, and to the gingiva in particular, increasing colonization through its ability to create hyphae, pseudohyphae, and polyspecies biofilms, as confirmed in several reports [63,64]. Thus, the published results regarding the effectiveness of mouthwashes against Candida spp. may be overestimated, since the methodological scenarios do not include colonization in the biofilm phase, where the resistance is clearly greater to antifungal agents, which in turn is a treat to global health. Naturally, this highlights the importance of the biofilm phase in the research plans for the development of antifungal therapies for oral infections, as stated in the literature [65,66].
The risk of bias in a study is the key point for many researchers; however, the importance and analysis of the transposition of in vitro models to in vivo models cannot be neglected. Hence, we risk saying that the analysis in many cases must undergo additional scrutiny and verify whether models are realistic or not. From our study, it is clear that there is a great diversity of methods, especially of co-culturing time points. The method that most closely mimics in vivo conditions under which mouthwashes are administered may be considered the most realistic: rinsing for 30–60 s (time recommended by most manufacturers) [2,20,22,23,29,32,36]. Nevertheless, the substantivity of antiseptics in saliva is around 6 h and reaches 11 h in the oral dental film, the interdental zone, the anterior labial mucosa, and the posterior buccal mucosa [67]. Therefore, more articles become relevant because they test longer time scales [23,35,39,40]. More than analysing the potential bias of a study, the diversity of methods poses great difficulty when comparing results and in the consistency of the analysis of the results, which in itself can even be an interpretation bias when comparing results (Table 9).

Table 9.
Contact time between mouthwashes and Candida strains by assay type.
Thus, the lack of standardized methodologies (gold standard protocols) is the first major difficulty for comparing results. It complicates the evaluation, the comparison of results from diverse studies, and does not allow scientific rigor when stating which mouthwash has the best antifungal effect. On the other hand, the real in vivo conditions in which oral mouthwashes are used are very far from the conditions in which oral mouthwashes are tested, to the point that the tested study models may be considered, within the context of a constructive scientific analysis, unrealistic. This is clear for no other reason than the contradiction among manufacturers’ recommendations regarding the contact time of mouthwashes [2,20,22,23,29,32,36] and the contact time of the different study models tested. Even when considering the substantivity of oral mouthwashes in saliva, this contradiction is not solvable and calls for more accurate models, as has already been raised in the literature [23,35,39,40]. Once there, with standardized and realistic methods, it will be necessary to invest in a bias analysis, which at this moment seems to us to be of little relevance from a scientific point of view.
4. Conclusions
While conducting this review, significant methodological variabilities across the studies included in the work were revealed. Several key factors contributing to this issue, such as differences in study design, variation in Candida strains, and the use of different methodologies were identified. This variability presents a challenge in drawing definitive conclusions at an absolute level, as it can result in a significant impact on the outcomes and limit the comparability of the results. For example, different strains may respond differently to the same mouthwash, making it difficult to generalize the findings across all studies. Also, the methodologies used to measure the anti-Candida spp.’s effectiveness varied, including disparities in the type of assays, endpoints, and criteria for determining success, which, per se, can lead to different interpretations of the effectiveness of the mouthwashes. Also, given these methodological differences, this study focused on identifying trends and general observations rather than attempting to establish definitive rankings or absolute conclusions. Indeed, with the available data, it is not possible to state that the oral mouthwashes available on the European market, regardless of their composition, are generally effective against Candida spp. It can only be admitted that oral mouthwashes made from CHX, CPC, HX, fluorinated compounds, and natural compounds demonstrated antifungal efficacy in in vitro study models; critically, no consideration was given to the excipients that are part of the pharmaceutical preparation.
We also underscore the importance of developing standardized protocols for future research to enable more accurate and reliable comparisons. Until such standardization is achieved, our conclusions remain tentative and should be interpreted within the context of the noted methodological variability. Future investigations are suggested in standardized studies using realistic in vivo models that can assess the effectiveness of oral mouthwashes in oral candidiasis associated with traditional pharmacological treatment to define more effective clinical protocols.
Author Contributions
Conceptualization, M.M. and P.R.; methodology, M.M. and P.R.; validation, C.F.R., P.R. and J.C.A.; formal analysis, C.F.R., P.R. and J.C.A.; data curation, M.M.; writing—original draft preparation, M.M.; writing—review and editing, C.F.R., P.R., and J.C.A.; visualization, M.M.; supervision, C.F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aspinall, S.R.; Parker, J.K.; Khutoryanskiy, V.V. Oral Care Product Formulations, Properties and Challenges. Colloids Surf. B Biointerfaces 2021, 200, 111567. [Google Scholar] [CrossRef] [PubMed]
- Di Lodovico, S.; Dotta, T.C.; Cellini, L.; Iezzi, G.; D’Ercole, S.; Petrini, M. The Antibacterial and Antifungal Capacity of Eight Commercially Available Types of Mouthwash against Oral Microorganisms: An In Vitro Study. Antibiotics 2023, 12, 675. [Google Scholar] [CrossRef]
- Aoun, G.; Cassia, A.; Berberi, A. Effectiveness of a Chlorhexidine Digluconate 0.12% and Cetylpyridinium Chloride 0.05% Solution in Eliminating Candida albicans Colonizing Dentures: A Randomized Clinical In Vivo Study. J. Contemp. Dent. Pract. 2015, 16, 433–436. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information PubChem Compound Summary for CID 9552079, Chlorhexidine. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chlorhexidine (accessed on 23 April 2024).
- National Center for Biotechnology Information PubChem Compound Summary for CID 31239, Cetylpyridinium Chloride. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cetylpyridinium-Chloride (accessed on 23 April 2024).
- Brookes, Z.; McGrath, C.; McCullough, M. Antimicrobial Mouthwashes: An Overview of Mechanisms—What Do We Still Need to Know? Int. Dent. J. 2023, 73, S64–S68. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information PubChem Compound Summary for CID 3607, Hexetidine. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hexetidine (accessed on 23 April 2024).
- Radzki, D.; Wilhelm-Węglarz, M.; Pruska, K.; Kusiak, A.; Ordyniec-Kwaśnica, I. A Fresh Look at Mouthwashes—What Is Inside and What Is It For? Int. J. Environ. Res. Public. Health 2022, 19, 3926. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, I.R. Biochemical Effects of Fluoride on Oral Bacteria. J. Dent. Res. 1990, 69, 660–667. [Google Scholar] [CrossRef] [PubMed]
- AlEraky, D.M.; Abuohashish, H.M.; Gad, M.M.; Alshuyukh, M.H.; Bugshan, A.S.; Almulhim, K.S.; Mahmoud, M.M. The Antifungal and Antibiofilm Activities of Caffeine against Candida albicans on Polymethyl Methacrylate Denture Base Material. Biomedicines 2022, 10, 2078. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, B.C.; Epifanio, R.N. Update on Oral Fungal Infections. Dent. Clin. N. Am. 2013, 57, 561–581. [Google Scholar] [CrossRef]
- Borman, A.M.; Johnson, E.M. Name Changes for Fungi of Medical Importance, 2018 to 2019. J. Clin. Microbiol 2021, 59. [Google Scholar] [CrossRef]
- Andrade, J.C.; Kumar, S.; Kumar, A.; Černáková, L.; Rodrigues, C.F. Application of Probiotics in Candidiasis Management. Crit. Rev. Food Sci. Nutr. 2022, 62, 8249–8264. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Nobile, C.J. Antifungal Drug-Resistance Mechanisms in Candida Biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 24 April 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and Validation of a Risk-of-Bias Tool for Assessing in Vitro Studies Conducted in Dentistry: The QUIN. J. Prosthet. Dent. 2024, 131, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoni, A.; Pericolini, E.; Paulone, S.; Orsi, C.F.; Castagnoli, A.; Oliva, I.; Strozzi, E.; Blasi, E. In Vitro Effects of Commercial Mouthwashes on Several Virulence Traits of Candida albicans, Viridans Streptococci and Enterococcus Faecalis Colonizing the Oral Cavity. PLoS ONE 2018, 13, e0207262. [Google Scholar] [CrossRef]
- Giuliana, G.; Pizzo, G.; Milici, M.E.; Musotto, G.C.; Giangrecó, R. Antifungal Properties of Mouthrinses Containing Antimicrobial Agents. J. Periodontol. 1997, 68, 729–733. [Google Scholar] [CrossRef]
- Giuliana, G.; Pizzo, G.; Milici, E.M.; Giangreco, R. In Vitro Activities of Antimicrobial Agents against Candida Species. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 87, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Paulone, S.; Malavasi, G.; Ardizzoni, A.; Orsi, C.F.; Peppoloni, S.; Neglia, R.G.; Blasi, E. Candida albicans Survival, Growth and Biofilm Formation Are Differently Affected by Mouthwashes: An In Vitro Study. New Microbiol. 2017, 40, 1121–7138. [Google Scholar]
- Moroz, J.; Kurnatowska, A.J.; Kurnatowski, P. The In Vitro Activity of Selected Mouthrinses on Candida Strains Isolated from the Oral Cavity. Ann. Parasitol. 2020, 66, 539–545. [Google Scholar] [CrossRef]
- Korbecka-Paczkowska, M.; Karpiński, T.M. In Vitro Assessment of Antifungal and Antibiofilm Efficacy of Commercial Mouthwashes against Candida albicans. Antibiotics 2024, 13, 117. [Google Scholar] [CrossRef]
- Olejnik, E.; Biernasiuk, A.; Malm, A.; Szymanska, J. Evaluation of Antibacterial and Antifungal Properties of Selected Mouthwashes: In Vitro Studies. Curr. Issues Pharm. Med. Sci. 2021, 34, 164–168. [Google Scholar] [CrossRef]
- Černáková, L.; Jordao, L.; Bujdáková, H. Impact of Farnesol and Corsodyl® on Candida albicans Forming Dual Biofilm with Streptococcus mutans. Oral Dis. 2018, 24, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Jolivot, P.A.; Dunyach-Remy, C.; Roussey, A.; Jalabert, A.; Mallié, M.; Hansel-Esteller, S. Étude d’activité in Vitro et de Stabilité de Suspensions Antifongiques Pour Bain de Bouche: Vers Une Remise En Question de Pratiques Empiriques? Pathol. Biol. 2012, 60, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Jose, A.; Coco, B.; Rajendran, R.; Rautemaa, R.; Murray, C.; Lappin, D.F.; Bagg, J. Commercial Mouthwashes Are More Effective than Azole Antifungals against Candida albicans Biofilms In Vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Flisfisch, S.; Meyer, J.; Meurman, J.H.; Waltimo, T. Effects of Fluorides on Candida albicans. Oral Dis. 2008, 14, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Handschuh Briones, R.A.; Silva Arcos, E.N.; Urrutia, M.; Godoy-Martínez, P. Antifungal Activity of Mouthwashes against Candida albicans and Rhodotorula Mucilaginosa: An in Vitro Study. Rev. Iberoam. Micol. 2020, 37, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wei, P.; Zhao, C.; He, C.; Yan, Z.; Hua, H. In Vitro Antifungal Effect and Inhibitory Activity on Biofilm Formation of Seven Commercial Mouthwashes. Oral Dis. 2014, 20, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Rimal, J.; Rao, A.; Sequeira, P.S.; Doshi, D.; Bhat, G.K. In Vitro Antifungal Effect of Mouth Rinses Containing Chlorhexidine and Thymol. J. Dent. Sci. 2011, 6, 1–5. [Google Scholar] [CrossRef]
- Talebi, S.; Sabokbar, A.; Riazipour, M.; Saffari, M. Comparison of the in Vitro Effect of Chemical and Herbal Mouthwashes on Candida albicans. Jundishapur J. Microbiol. 2014, 7, e12563. [Google Scholar] [CrossRef]
- Fathilah, A.R.; Himratul-Aznita, W.H.; Fatheen, A.R.N.; Suriani, K.R. The Antifungal Properties of Chlorhexidine Digluconate and Cetylpyrinidinium Chloride on Oral Candida. J. Dent. 2012, 40, 609–615. [Google Scholar] [CrossRef]
- Patel, M.; Shackleton, J.A.; Coogan, M.M.; Galpin, J. Antifungal Effect of Mouth Rinses on Oral Candida Counts and Salivary Flow in Treatment-Naïve HIV-Infected Patients. AIDS Patient Care STDS 2008, 22, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Uygun-Can, B.; Kadir, T.; Gumru, B. Effect of Oral Antiseptic Agents on Phospholipase and Proteinase Enzymes of Candida albicans. Arch. Oral Biol. 2016, 62, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Yazicioglu, O.; Ucuncu, M.K.; Guven, K. Ingredients in Commercially Available Mouthwashes: A Review. Int. Dent. J. 2023, 74, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.; Giertsen, E.; Guggenheim, B.; Guggenheim, B. An In Vitro Oral Biofilm Model for Comparing the Efficacy of Antimicrobial Mouthrinses. Caries Res. 2002, 36, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Meiller, T.F.; Kelley, J.I.; Jabra-Rizk, M.A.; DePaola, L.G.; Baqui, A.A.M.A.; Falkler, W.A. In Vitro Studies of the Efficacy of Antimicrobials against Fungi. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Kalesinskas, P.; Kačergius, T.; Ambrozaitis, A.; Pečiulienė, V.; Ericson, D. Reducing Dental Plaque Formation and Caries Development. A Review of Current Methods and Implications for Novel Pharmaceuticals. Balt. Dent. Maxillofac. J. 2014, 16, 44–52. [Google Scholar]
- Scheibler, E.; Garcia, M.C.R.; Medina da Silva, R.; Figueiredo, M.A.; Salum, F.G.; Cherubini, K. Use of Nystatin and Chlorhexidine in Oral Medicine: Properties, Indications and Pitfalls with Focus on Geriatric Patients. Gerodontology 2017, 34, 291–298. [Google Scholar] [CrossRef]
- Jiang, Q.; Deng, Y.; Li, S.; Yang, D.; Tao, L. Sub-Lethal Concentrations of Chlorhexidine Inhibit Candida albicans Growth by Disrupting ROS and Metal Ion Homeostasis. J. Oral Microbiol. 2023, 15, 2278937. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Zhang, X.; Zhang, P.; Tian, Q.; Ma, C.; Shi, C. Combination of Cetylpyridinium Chloride and Chlorhexidine Acetate: A Promising Candidate for Rapid Killing of Gram-Positive/Gram-Negative Bacteria and Fungi. Curr. Microbiol. 2023, 80, 97. [Google Scholar] [CrossRef]
- Arce-Toribio, J.P.; Arbildo Vega, H.I.; López-Flores, A.I. Antimicrobial Efficacy of the Use of Mouthwash with Cetylpiridinium Chloride for Aerosol Producing Procedures. A Systematic Review. Odovtos-Int. J. Dent. Sci. 2023, 25, 10–23. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Tadakamadla, S.K.; Connelly, S.T.; Sforza, C.; Martín, C. Adverse Events Associated with Home Use of Mouthrinses: A Systematic Review. Ther. Adv. Drug Saf. 2019, 10, 204209861985488. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, F.A.; Van der Sluijs, E.; Ciancio, S.G.; Slot, D.E. Can Chemical Mouthwash Agents Achieve Plaque/Gingivitis Control? Dent. Clin. N. Am. 2015, 59, 799–829. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Breaker, R.R. Fluoride Enhances the Activity of Fungicides That Destabilize Cell Membranes. Bioorg. Med. Chem. Lett. 2012, 22, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Xiong, J.; Wang, L.; Feng, Z.; Hang, S.; Yu, J.; Li, W.; Feng, Y.; Lu, H.; Jiang, Y. Unexpected Inhibitory Effect of Octenidine Dihydrochloride on Candida albicans Filamentation by Impairing Ergosterol Biosynthesis and Disrupting Cell Membrane Integrity. Antibiotics 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Hu, H.; Hu, Z.; Zhong, X.; Guan, Y.; Zhao, Y.; Wang, L.; Ye, L.; Ming, L.; Riaz Rajoka, M.S.; et al. Sanguinarine, Isolated From Macleaya Cordata, Exhibits Potent Antifungal Efficacy Against Candida albicans Through Inhibiting Ergosterol Synthesis. Front. Microbiol. 2022, 13, 908461. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.E.; Henriques, M.; Silva, S. Disinfectants to Fight Oral Candida Biofilms. In Fungal Biofilms and Related Infections. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; pp. 83–93. [Google Scholar]
- Ntow-Boahene, W.; Papandronicou, I.; Miculob, J.; Good, L. Fungal Cell Barriers and Organelles Are Disrupted by Polyhexamethylene Biguanide (PHMB). Sci. Rep. 2023, 13, 2790. [Google Scholar] [CrossRef] [PubMed]
- Vranic, E.; Lacevic, A.; Mehmedagic, A.; Uzunovic, A. Formulation Ingredients for Toothpastes and Mouthwashes. Bosinan J. Basic Med. Sci. 2004, 4, 51. [Google Scholar] [CrossRef]
- European Parliament and of the Council on Cosmetic Products Commission Regulation (EU). N°358/2014 Amending Annexes II and V to Regulation (EC) N°1223/2009. Off. J. Eur. Union 2009, 342, 59. [Google Scholar]
- Saharkhiz, M.J.; Motamedi, M.; Zomorodian, K.; Pakshir, K.; Miri, R.; Hemyari, K. Chemical Composition, Antifungal and Antibiofilm Activities of the Essential Oil of Mentha piperita L. ISRN Pharm. 2012, 2012, 718645. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.; Feder-Kubis, J.; Krasowska, A. Antiadhesive Properties of Imidazolium Ionic Liquids Based on Menthol against Candida spp. Int. J. Mol. Sci. 2021, 22, 7543. [Google Scholar] [CrossRef]
- Müller-Sepúlveda, A.; Chevecich, C.C.; Jara, J.A.; Belmar, C.; Sandoval, P.; Meyer, R.S.; Quijada, R.; Moura, S.; López-Muñoz, R.; Díaz-Dosque, M.; et al. Chemical Characterization of Lavandula Dentata Essential Oil Cultivated in Chile and Its Antibiofilm Effect against Candida albicans. Planta Med. 2020, 86, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Chatrath, A.; Gangwar, R.; Kumari, P.; Prasad, R. In Vitro Anti-Biofilm Activities of Citral and Thymol Against Candida Tropicalis. J. Fungi 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Kamath, S.; Ariaee, A.; Prestidge, C.; Joyce, P. The Impact of Common Pharmaceutical Excipients on the Gut Microbiota. Expert. Opin. Drug Deliv. 2023, 20, 1297–1314. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, E. Evaluation of the Antimicrobial Effects of Sodium Benzoate and Dichlorobenzyl Alcohol against Dental Plaque Microorganisms: An in Vitro Study. Acta Odontol. Scand. 1994, 52, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Kornum, F.; Knudsen, M.; Lau, E.F. Antimicrobial Efficiency of Ethanol and 2-Propanol Alcohols Used on Contaminated Storage Phosphor Plates and Impact on Durability of the Plate. Dentomaxillofacial Radiol. 2013, 42, 20120353. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.; Aslam-Pervez, B. Does the Use of Alcohol Mouthwash Increase the Risk of Developing Oral Cancer? Evid. Based Dent. 2022, 23, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.M.C.V.; Lopes, B.O.; Leite, A.C.R.d.M.; Cruz, G.S.; Brito, É.H.S.d.; Lima, L.F.d.; Černáková, L.; Azevedo, N.F.; Rodrigues, C.F. Characterization of Oral Candida spp. Biofilms in Children and Adults Carriers from Eastern Europe and South America. Antibiotics 2023, 12, 797. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Alves, D.; Henriques, M. Combination of Posaconazole and Amphotericin B in the Treatment of Candida Glabrata Biofilms. Microorganisms 2018, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Maziere, M.; Andrade, J.C.; Rompante, P.; Rodrigues, C.F. Evaluation of the Antifungal Effect of Plant Extracts on Oral Candida spp.—A Critical Methodological Analysis of the Last Decade. Crit. Rev. Microbiol. 2024, 1–11. [Google Scholar] [CrossRef]
- Reda, B.; Hollemeyer, K.; Trautmann, S.; Hannig, M.; Volmer, D.A. Determination of Chlorhexidine Retention in Different Oral Sites Using Matrix-Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry. Arch. Oral Biol. 2020, 110, 104623. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).