Molecular and Morphological Identification of Sarocladium Species Causing Sheath Rot of Rice in Thailand and Their Division into Physiological Races
Abstract
1. Introduction
2. Materials and Methods
2.1. Disease Survey and Morphological Identification
2.2. DNA Extraction, PCR and Phylogenetic Analysis
2.3. Physiological Race Identification
| Grade | Symptom |
| 0 | No symptoms |
| 1 | Spot lesions < 1% on flag leaf sheath area, and panicle emergence normal |
| 2 | Spot lesions 1–5% on flag leaf sheath area, and panicle emergence normal |
| 3 | Spot lesions 6–25% on flag leaf sheath area, and 75% of the panicle emerged |
| 4 | Spot lesions 26–50% on flag leaf sheath area, and 50% of the panicle emerged |
| 5 | Spot lesions 51–100% on flag leaf sheath area, and 25% of the panicle emerged |
3. Results
3.1. Morphology
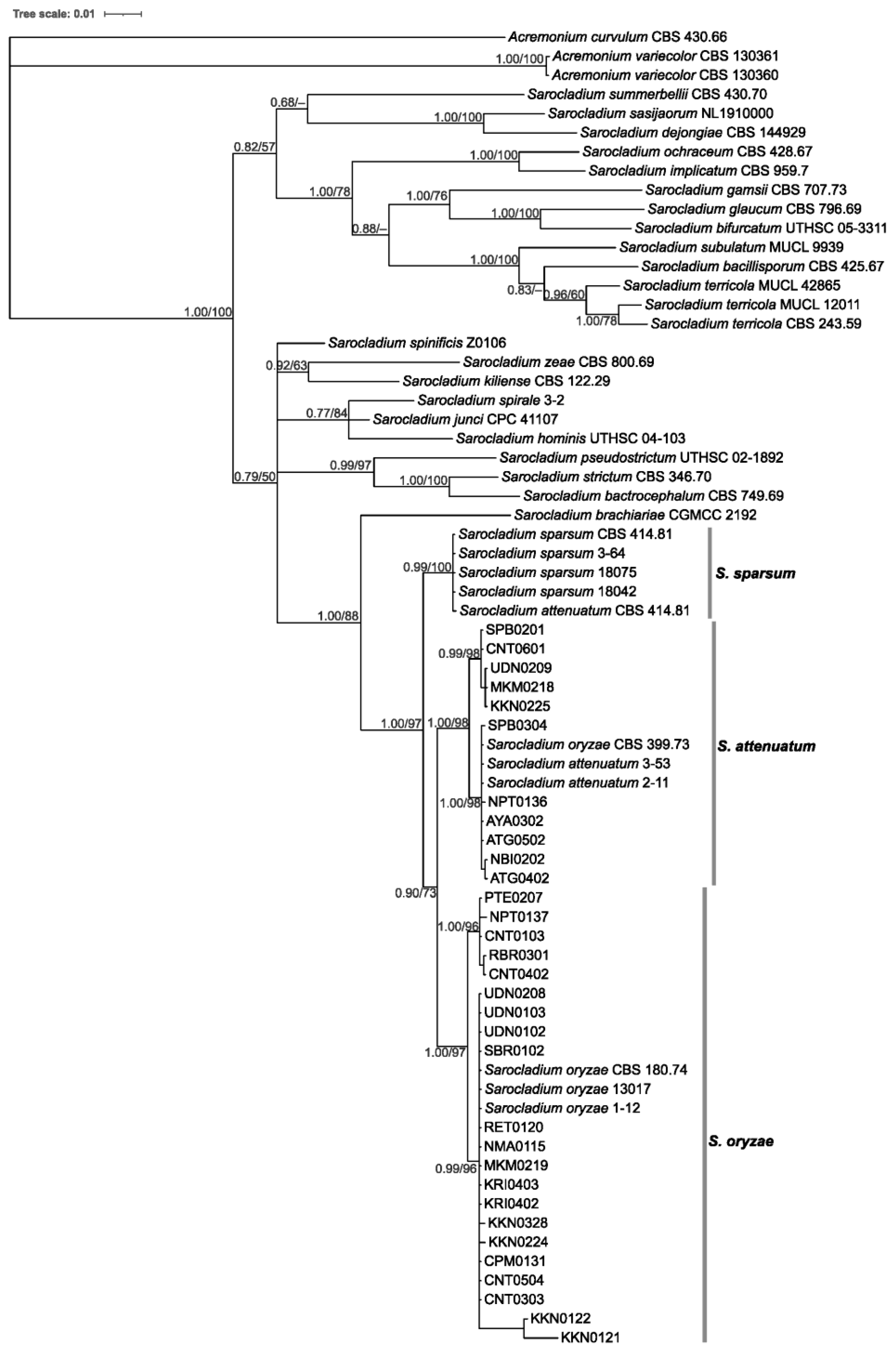
3.2. Phylogeny
3.3. Physiological Race Identification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afolabi, O.O.; Bigirimana, V.d.P.; Hua, G.K.H.; Oni, F.E.; Bertier, L.; Onwughalu, J.; Oyetunji, O.E.; Ogunbayo, A.; Van De Velde, M.; Nyamangyoku, O.I.; et al. Fusarium and Sarocladium species associated with rice sheath rot disease in Sub-Saharan Africa. Diversity 2023, 15, 1090. [Google Scholar] [CrossRef]
- Unartngam, J.; Naunnet, T.; Sangsuk, S.; Chountragoon, O.; Kerdkhong, C.; Tantirungkij, M. Effectiveness of bacteria isolated from peat swamp forests to control rice dirty panicle fungi in Thailand. AGRIVITA J. Agric. Sci. 2021, 43, 11. [Google Scholar] [CrossRef]
- Riangwong, K.; Aesomnuk, W.; Sonsom, Y.; Siangliw, M.; Unartngam, J.; Toojinda, T.; Wanchana, S.; Arikit, S. QTL-seq identifies genomic regions associated with resistance to dirty panicle disease in rice. Agronomy 2023, 13, 1905. [Google Scholar] [CrossRef]
- CABI. Sarocladium oryzae (rice sheath rot). CABI Compendium. 2022. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.48393 (accessed on 27 July 2024).
- Arunyanart, P.; Surin, A.; Sirisanta, W.; Napeerong, N.; Phutthasamai, K. Studies on Rice Seed Discoloration Disease; Plant Pathology and Microbiology Division: Bangkok, Thailand, 1979. [Google Scholar]
- Bigirimana, V.D.; Hua, G.K.H.; Nyamangyoku, O.I.; Höfte, M. Rice sheath rot: An emerging ubiquitous destructive disease complex. Front. Plant Sci. 2015, 6, 1066. [Google Scholar] [CrossRef]
- Chakravarty, D.K.; Biswas, S. Estimation of yield loss in rice affected by sheath rot. Plant Dis. Rep. 1978, 62, 226–227. [Google Scholar]
- Kongcharoen, N.; Kaewsalong, N.; Dethoup, T. Efficacy of fungicides in controlling rice blast and dirty panicle diseases in Thailand. Sci. Rep. 2020, 10, 16233. [Google Scholar] [CrossRef]
- Mvuyekure, S.M.; Sibiya, J.; Derera, J.; Nzungize, J.; Nkima, G. Genetic analysis of mechanisms associated with inheritance of resistance to sheath rot of rice. Plant Breed. 2017, 136, 509–515. [Google Scholar] [CrossRef]
- IRRI. Standard Evaluation System (SES) for Rice, 5th ed.; International Rice Research Institute: Manila, Philippines, 2013. [Google Scholar]
- Giraldo, A.; Gene, J.; Sutton, D.A.; Madrid, H.; de Hoog, G.S.; Cano, J.; Decock, C.; Crous, P.W.; Guarro, J. Phylogeny of Sarocladium (Hypocreales). Persoonia 2015, 34, 10–24. [Google Scholar] [CrossRef]
- Ou, J.-H.; Lin, G.-C.; Chen, C.-Y. Sarocladium species associated with rice in Taiwan. Mycol. Prog. 2020, 19, 67–80. [Google Scholar] [CrossRef]
- Cortes, M.V.D.B.; Guimaraes, R.A.; Freire, D.M.G.; Prabhu, A.S.; da Silva-Lobo, V.L. An overview of the virulence factors and the biocontrol potential of Sarocladium oryzae. Fungal Biol. Rev. 2021, 37, 1–7. [Google Scholar] [CrossRef]
- Peeters, K.J.; Haeck, A.; Harinck, L.; Afolabi, O.O.; Demeestere, K.; Audenaert, K.; Höfte, M. Morphological, pathogenic and toxigenic variability in the rice sheath rot pathogen Sarocladium oryzae. Toxins 2020, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Pramunadipta, S.; Widiastuti, A.; Wibowo, A.; Suga, H.; Priyatmojo, A. Sarocladium oryzae associated with sheath rot disease of rice in Indonesia. Biodiversitas 2020, 21, 1243–1249. [Google Scholar] [CrossRef]
- Ayyadurai, N.; Kirubakaran, S.; Srisha, S.; Sakthivel, N. Biological and molecular variability of Sarocladium oryzae, the sheath rot pathogen of rice (Oryza sativa L.). Curr. Microbiol. 2005, 50, 319–323. [Google Scholar] [CrossRef]
- Browder, L.E.; Lyon, F.L.; Eversmeyer, M.G. Races, pathogenicity phenotypes, and type cultures of plant-pathogens. Phytopathology 1980, 70, 581–583. [Google Scholar] [CrossRef]
- Stakman, E.C. Plant diseases are shifty enemies. Am. Sci. 1947, 35, 321–350. [Google Scholar] [PubMed]
- Habgood, R.M. Designation of physiological races of plant pathogens. Nature 1970, 227, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S. Introduction to Principles of Plant Pathology; CBS Publishers & Distributors: New Delhi, India, 2017. [Google Scholar]
- Jørgensen, H.J.L.; Smedegaard-Petersen, V. Pathogenic variation of Rhynchosporium secalis in Denmark and sources of resistance in barley. Plant Dis. 1995, 79, 297–301. [Google Scholar]
- Zimand, G.; Valinsky, L.; Elad, Y.; Chet, I.; Manulis, S. Use of the RAPD procedure for the identification of Trichoderma strains. Mycol. Res. 1994, 98, 531–534. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997, 35, 1216–1223. [Google Scholar] [CrossRef]
- Voigt, K.; Wöstemeyer, J. Reliable amplification of actin genes facilitates deep-level phylogeny. Microbiol. Res. 2000, 155, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LO, USA, 14 November 2010. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- McMaugh, T. Guidelines for Surveillance for Plant Pests in Asia and the Pacific; Australian Centre for International Agricultural Research: Canberra, Australian, 2005; Volume 119, p. 192. [Google Scholar]
- Lanoiselet, V.; You, M.P.; Li, Y.P.; Wang, C.P.; Shivas, R.G.; Barbetti, M.J. First report of Sarocladium oryzae causing sheath rot on rice (Oryza sativa) in Western Australia. Plant Dis. 2012, 96, 1382. [Google Scholar] [CrossRef] [PubMed]
- Gams, W.; Hawksworth, D.L. The idendity of Acrocylindrium oryzae Sawada and a similar fungus causing sheath rot of rice. Kawaka 1975, 3, 57–61. [Google Scholar]
- Bills, G.F.; Platas, G.; Gams, W. Conspecificity of the cerulenin and helvolic acid producing ’Cephalosporium caerulens’, and the hypocrealean fungus Sarocladium oryzae. Mycol. Res. 2004, 108, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Bridge, P.D.; Hawksworth, D.L.; Kavishe, D.F.; Farnell, P.A. A revision of the species concept in Sarocladium, the causal agent of sheath-rot in rice and bamboo blight, based on biochemical and morphometric analyses. Plant Pathol. 1989, 38, 239–245. [Google Scholar] [CrossRef]
- Eckardt, N.A.; Cutler, S.; Juenger, T.E.; Marshall-Colon, A.; Udvardi, M.; Verslues, P.E. Focus on climate change and plant abiotic stress biology. Plant Cell 2023, 35, 1–3. [Google Scholar] [CrossRef]
- Al, W.; Orking, G.; Clima, O. Climate Change and Food Security: A Framework Document; FAO: Rome, Italy, 2008. [Google Scholar]
- Fetene, D.Y.; Birhan, M.; Zeleke, T. Screening of rice germplasms for their resistance against sheath rot disease (Sarocladium oryzae) at Fogera, Ethiopia. J. Plant Pathol. Microbiol. 2020, 11, 10–24. [Google Scholar] [CrossRef]
- Manzoor, T.; Ahanger, M.A.; Altaf, H. Variability of Sarocladium oryzae [(Sawada) Games & Hawksworth] and identification of novel donors for sheath rot resistance among temperate germplasm lines of rice. Plant Genet. Resour. 2023, 21, 349–356. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Amudha, R.; Jayachandran, S.; Sakthivel, N. Detection and quantification of phytotoxic metabolites of in sheath rot-infected grains of rice. Lett. Appl. Microbiol. 2002, 34, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, N.; Amudha, R.; Muthukrishnan, S. Production of phytotoxic metabolites by Sarocladium oryzae. Mycol. Res. 2002, 106, 609–614. [Google Scholar] [CrossRef]
- Rice Department, Ministry of Agriculture. Rice Diseases and Control; Rice Department, Ministry of Agriculture: Bangkok, Thailand, 2007; p. 58. (In Thai)


| Isolate Code (TBRC No.) | Symptom | Location | Rice Growing Region | Accession No. ITS/28S/ACT |
|---|---|---|---|---|
| NPT0137 (TBRC18772) | Sheath rot | Nakhon Pathom | Central | LC582677/LC652592/LC652753 |
| KKN0122 (TBRC18773) | Dirty panicle | Khon Kaen | Northeastern | LC582679/LC652594/LC652755 |
| KKN0224 (TBRC18774) | Dirty panicle | Khon Kaen | Northeastern | LC582681/LC652596/LC652757 |
| KKN0328 (TBRC10919) | Dirty panicle | Khon Kaen | Northeastern | LC582683/LC652598/LC652759 |
| UDN0102 (TBRC18775) | Dirty panicle | Udon Thani | Northeastern | LC582685/LC652600/LC652761 |
| UDN0103 (TBRC18776) | Dirty panicle | Udon Thani | Northeastern | LC582686/LC652601/LC652762 |
| UDN0208 (TBRC18777) | Dirty panicle | Udon Thani | Northeastern | LC582688/LC652603/LC652764 |
| NMA0115 (TBRC18772) | Sheath rot | Nakhon Ratchasima | Northeastern | LC582691/LC652606/LC652767 |
| MKM0219 (TBRC18779) | Dirty panicle | Maha Sarakham | Northeastern | LC582693/LC652608/LC652769 |
| CPM0131 (TBRC10575) | Dirty panicle | Chaiyaphum | Northeastern | LC582694/LC652609/LC652770 |
| RET0120 (TBRC10635) | Dirty panicle | Roi Et | Northeastern | LC582715/LC652610/LC652771 |
| KRI0402 (TBRC18781) | Dirty panicle | Kanchanaburi | Western | LC582696/LC652612/LC652773 |
| KRI0403 (TBRC18782) | Sheath rot | Kanchanaburi | Western | LC582697/LC652613/LC652774 |
| CNT0103 (TBRC18783) | Sheath rot | Chai Nat | Central | LC582698/LC652614/LC652775 |
| CNT0303 (TBRC18784) | Dirty panicle | Chai Nat | Central | LC582699/LC652615/LC652776 |
| CNT0504 (TBRC18785) | Sheath rot | Chai Nat | Central | LC582700/LC652616/LC652777 |
| CNT0402 (TBRC18786) | Dirty panicle | Chai Nat | Central | LC582701/LC652617/LC652778 |
| PTE0207 (TBRC18791) | Dirty panicle | Pathum Thani | Central | LC582705/LC652621/LC652782 |
| RBR0301 (TBRC18788) | Sheath rot | Ratchaburi | Western | LC582707/LC652623/LC652784 |
| SBR0102 (TBRC18789) | Dirty panicle | Sing Buri | Central | LC582709/LC652624/LC652785 |
| KKN0121 (TBRC18800) | Dirty panicle | Khon Kaen | Northeastern | LC582680/LC652595/LC652756 |
| NPT0136 (TBRC10636) | Dirty panicle | Nakhon Pathom | Northeastern | LC582676/LC652591/LC652752 |
| KKN0225 (TBRC18792) | Dirty panicle | Khon Kaen | Northeastern | LC582682/LC652597/LC652758 |
| UDN0209 (TBRC10918) | Sheath rot | Udon Thani | Northeastern | LC582689/LC652604/LC652765 |
| MKM0218 (TBRC10574) | Dirty panicle | Maha Sarakham | Northeastern | LC582692/LC652607/LC652768 |
| CNT0601 (TBRC18793) | Dirty panicle | Chai Nat | Central | LC582702/LC652618/LC652779 |
| NBI0202 (TBRC18794) | Sheath rot | Nonthaburi | Central | LC582703/LC652619/LC652780 |
| AYA0302 (TBRC18795) | Dirty panicle | Ayutthaya | Central | LC582706/LC652622/LC652783 |
| SPB0201 (TBRC18796) | Sheath rot | Suphan Buri | Central | LC582710/LC652625/LC652786 |
| ATG0402 (TBRC18797) | Dirty panicle | Ang Thong | Central | LC582713/LC652627/LC652788 |
| ATG0502 (TBRC18798) | Dirty panicle | Ang Thong | Central | LC582714/LC652628/LC652789 |
| SPB0304 (TBRC18790) | Sheath rot | Suphan Buri | Central | LC582711/LC652626/LC652787 |
| Race | Strain | Source 1 | Rice Cultivar 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| S. oryzae | ||||||||||||
| 1 | NPT0137, MKM0219, RET0120 | SR, DP, DP | S | S | S | S | S | R | R | S | S | S |
| 2 | KKN0122, CNT0504 | DP, SR | S | R | S | S | R | S | R | S | S | S |
| 3 | KKN0224 | DP | S | S | S | S | R | S | R | S | S | S |
| 4 | KKN0328 | DP | S | S | S | S | R | S | S | S | S | S |
| 5 | UDN0102 | DP | S | S | S | S | R | S | R | S | R | S |
| 6 | UDN0103, KRI0402, SBR0102 | DP, DP, DP | S | S | S | S | S | S | R | S | R | S |
| 7 | UDN0208 | DP | S | S | R | S | S | R | S | S | S | S |
| 8 | NMA0115 | DP | S | R | S | S | S | R | S | S | S | S |
| 9 | CPM0131 | DP | S | R | S | S | S | R | R | S | S | S |
| 10 | KRI0403 | SR | S | R | S | S | S | S | R | S | R | S |
| 11 | CNT0103 | SR | S | S | R | S | S | S | R | S | S | S |
| 12 | CNT0303 | DP | S | R | R | S | S | S | R | R | R | S |
| 13 | CNT0402 | DP | S | S | S | S | S | S | R | R | R | S |
| 14 | PTE0207 | DP | S | R | R | S | S | S | S | S | S | S |
| 15 | RBR0301 | SR | S | R | S | S | R | S | R | R | S | S |
| 16 | KKN0121 | DP | S | S | S | S | S | R | R | S | R | R |
| S. attenuatum | ||||||||||||
| 1 | NPT0136 | DP | S | S | S | S | R | S | R | S | S | S |
| 2 | KKN0225 | DP | S | S | S | S | S | R | R | S | S | S |
| 3 | UDN0209 | SR | S | S | S | S | S | S | S | S | S | S |
| 4 | MKM0218 | DP | S | S | S | S | S | R | S | S | S | S |
| 5 | CNT0601 | DP | S | R | S | S | R | S | R | R | R | S |
| 6 | AYA0302 | DP | S | R | R | S | S | S | R | S | S | S |
| 7 | NBI0202 | SR | S | R | S | S | R | S | S | S | S | S |
| 8 | SPB0201 | SR | S | R | S | S | S | S | R | S | R | S |
| 9 | ATG0402 | DP | S | S | S | S | S | S | R | S | R | S |
| 10 | ATG0502 | DP | S | S | S | S | R | S | R | S | R | S |
| 11 | SPB0304 | SR | S | R | S | S | R | S | R | S | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unartngam, J.; Kopmoo, N.; Pinruan, U.; Kosawang, C.; Jørgensen, H.J.L. Molecular and Morphological Identification of Sarocladium Species Causing Sheath Rot of Rice in Thailand and Their Division into Physiological Races. J. Fungi 2024, 10, 535. https://doi.org/10.3390/jof10080535
Unartngam J, Kopmoo N, Pinruan U, Kosawang C, Jørgensen HJL. Molecular and Morphological Identification of Sarocladium Species Causing Sheath Rot of Rice in Thailand and Their Division into Physiological Races. Journal of Fungi. 2024; 10(8):535. https://doi.org/10.3390/jof10080535
Chicago/Turabian StyleUnartngam, Jintana, Noppol Kopmoo, Umpawa Pinruan, Chatchai Kosawang, and Hans Jørgen Lyngs Jørgensen. 2024. "Molecular and Morphological Identification of Sarocladium Species Causing Sheath Rot of Rice in Thailand and Their Division into Physiological Races" Journal of Fungi 10, no. 8: 535. https://doi.org/10.3390/jof10080535
APA StyleUnartngam, J., Kopmoo, N., Pinruan, U., Kosawang, C., & Jørgensen, H. J. L. (2024). Molecular and Morphological Identification of Sarocladium Species Causing Sheath Rot of Rice in Thailand and Their Division into Physiological Races. Journal of Fungi, 10(8), 535. https://doi.org/10.3390/jof10080535






