Aspergillus in the Indoor Air of Critical Areas of a Tertiary Hospital in Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection Procedure
2.2. Fungal Identification
2.3. Statistical Analyses
3. Results
3.1. Number of Colony-Forming Units and Identification of Aspergillus Sections and Species
3.1.1. Number of Filamentous Fungi Colony-Forming Units (CFU)
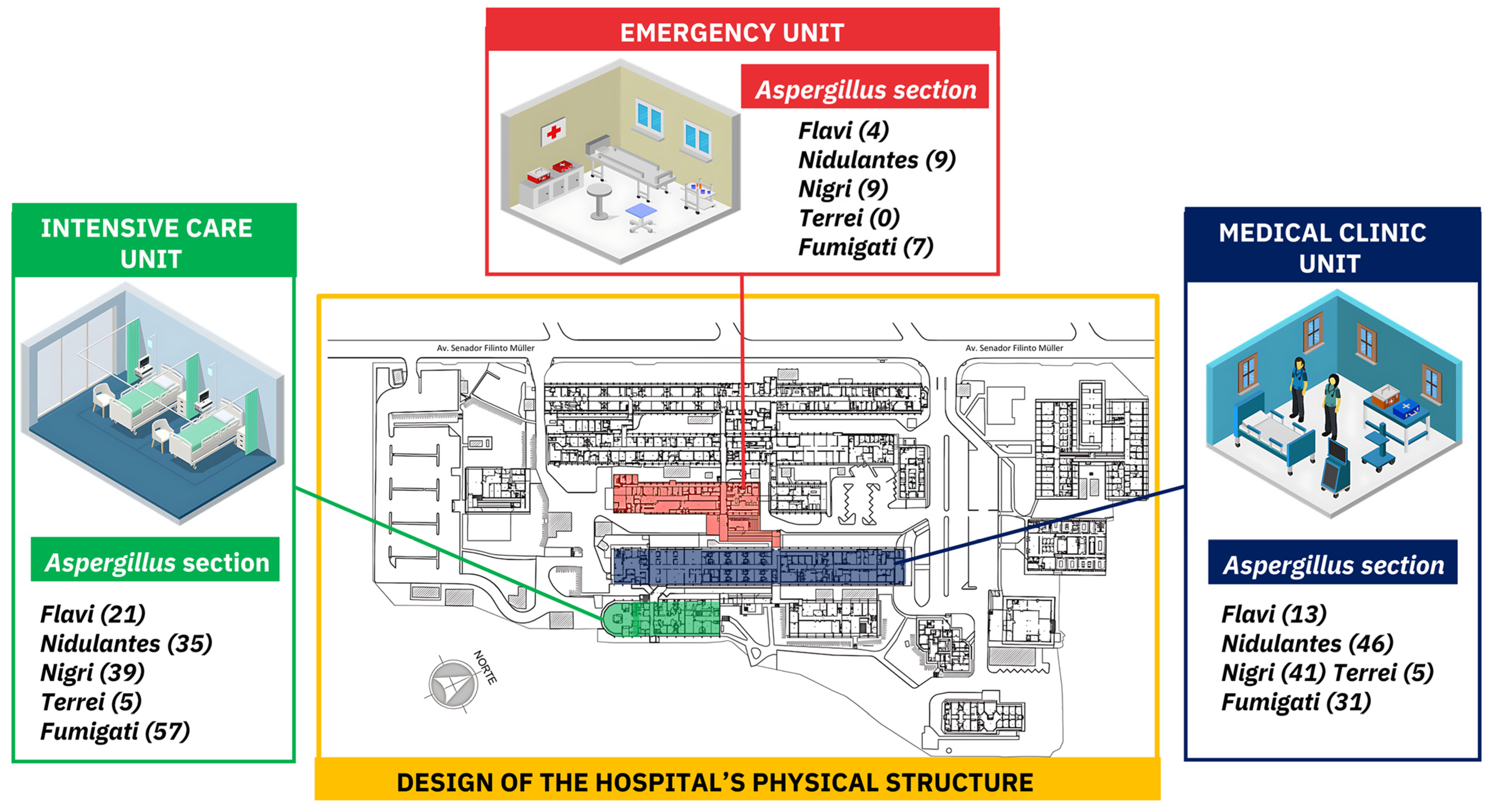
3.1.2. Aspergillus Section Per Hospitalization Unit
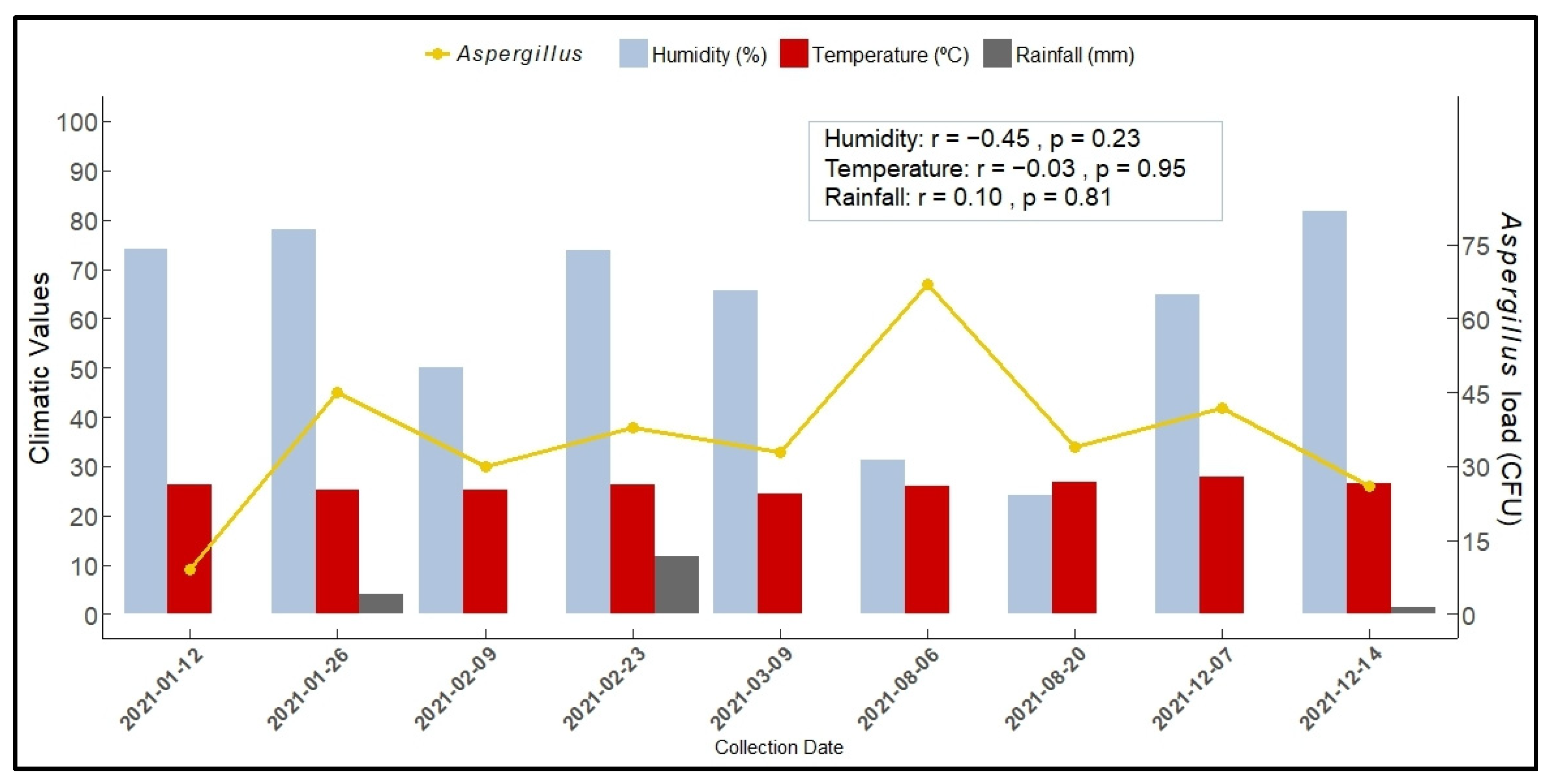
3.1.3. Aspergillus Load According to Section and Season
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boff, C.; Zoppas, B.C.D.A.; Aquino, V.R.; Kuplich, N.M.; Miron, D.; Pasqualotto, A.C. The indoor air as a potential determinant of the frequency of invasive aspergillosis in intensive care. Mycoses 2013, 56, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lee, D.G.; Choi, J.K.; Lee, H.J.; Kim, S.H.; Park, S.H.; Choi, S.M.; Choi, J.H.; Yoo, J.H.; Park, Y.J.; et al. Characteristics of culture-positive invasive pulmonary aspergillosis in patients with hematologic diseases. Medicine 2017, 96, e8841. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lee, D.G.; Kim, W.B.; Chun, H.S.; Park, C.; Myong, J.-P.; Park, Y.-J.; Choi, J.-K.; Lee, H.-J.; Kim, S.-H.; et al. Epidemiology and antifungal susceptibility profile of Aspergillus species: Comparison between environmental and clinical isolates from patients with hematologic malignancies. J. Clin. Microbiol. 2019, 57, e02023-18. [Google Scholar] [CrossRef] [PubMed]
- Loeffert, S.T.; Melloul, E.; Gustin, M.-P.; Hénaff, L.; Guillot, C.; Dupont, D.; Wallon, M.; Cassier, P.; Dananché, C.; Bénet, T.; et al. Investigation of the relationships between clinical and environmental isolates of Aspergillus fumigatus by multiple-locus variable number tandem repeat analysis during major demolition work in a french hospital. Clin. Infect. Dis. 2019, 68, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Diba, K.; Jangi, F.; Makhdoomi, K.; Moshiri, N.; Mansouri, F. Aspergillus diversity in the environments of nosocomial infection cases at a university hospital. J. Med. Life 2019, 12, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Herrera, E.O.; Frias-De-Leon, M.G.; Duarte-Escalante, E.; Calderon-Ezquerro, M.d.C.; Jimenez-Martinez, M.d.C.; Acosta-Altamirano, G.; Rivera-Becerril, F.; Toriello, C.; Reyes-Montes, M.d.R. Fungal diversity and Aspergillus in hospital environments. Ann. Agric. Environ. Med. 2016, 23, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, M.; Zhu, J.; Gerrits van den Ende, B.; Chen, A.J.; Al-Hatmi, A.M.S.; Li, L.; Zhang, Q.; Xu, J.; Liao, W.; et al. Aspergillus species in lower respiratory tract of hospitalized patients from Shanghai, China: Species Diversity and Emerging Azole Resistance. Infect. Drug Resist. 2020, 13, 4663–4672. [Google Scholar] [CrossRef] [PubMed]
- Géry, A.; Rioult, J.-P.; Heutte, N.; Séguin, V.; Bonhomme, J.; Garon, D. First characterization and description of Aspergillus series Versicolores in French Bioaerosols. J. Fungi 2021, 7, 676. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.; Abdolrasouli, A.; Dunne, K.; Sewell, T.R.; Zhang, Y.; Ballard, E.; Brackin, A.P.; van Rhijn, N.; Chown, H.; Tsitsopoulou, A.; et al. Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat. Microbiol. 2022, 7, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Stemler, J.; Többen, C.; Lass-Flörl, C.; Steinmann, J.; Ackermann, K.; Rath, P.-M.; Simon, M.; Cornely, O.A.; Koehler, P. Diagnosis and Treatment of invasive aspergillosis caused by non-fumigatus Aspergillus spp. J. Fungi 2023, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.K.; Tiwari, S.; Shankar, J. Resistance Mechanism and Proteins in Aspergillus species against antifungal agents. Mycology 2019, 10, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Wirmann, L.; Ross, B.; Reimann, O.; Steinmann, J.; Rath, P.-M. Airborne Aspergillus fumigatus spore concentration during demolition of a building on a hospital site, and patient risk determination for invasive aspergillosis including azole resistance. J. Hosp. Infec. 2018, 100, e91–e97. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Myong, J.-P.; Kim, W.-B.; Park, C.; Lee, S.J.; Lee, S.H.; Lee, D.-G. Profiles of environmental mold: Indoor and outdoor air sampling in a hematology hospital in Seoul, south Korea. Int. J. Environ. Res. Public Health 2018, 15, 2560. [Google Scholar] [CrossRef] [PubMed]
- Andrade Júnior, F.P.D.; Barbosa, V.; Medeiros, C.; Cruz, J.; Filho, A. Presença de Aspergillus em hospitais brasileiros: Uma revisão integrativa. J. Med. Health Promot. 2019, 4, 1242–1253. [Google Scholar]
- Loeffert, S.T.; Melloul, E.; Dananché, C.; Hénaff, L.; Bénet, T.; Cassier, P.; Dupont, D.; Guillot, J.; Botterel, F.; Wallon, M.; et al. Monitoring of Clinical strains and environmental fungal aerocontamination to prevent invasive aspergillosis infections in hospital during large deconstruction work: A protocol study. BMJ Open 2017, 7, e018109. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Hayden, R.T.; Larone, D.H. Larone’s Medically Important Fungi: A Guide to Identification; Wiley: Hoboken, NY, USA, 2018. [Google Scholar]
- Ferrer, C.; Colom, F.; Frasés, S.; Mulet, E.; Abad, J.L.; Alió, J.L. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S Ribosomal DNA Typing in Ocular Infections. J. Clin. Microbiol. 2001, 39, 2873–2879. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, V.E.; Márquez, R.; Turissini, D.A.; Goldman, W.E.; Matute, D.R. Genome sequences reveal cryptic speciation in the human pathogen Histoplasma capsulatum. mBio 2017, 8, e01339-17. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Nirenberg, H.I.; Aoki, T.; Cigelnik, E. A Multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Božić, J.; Ilić, P.; Ilić, S. Indoor Air Quality in the Hospital: The influence of heating, ventilating and conditioning systems. Braz. Arch. Biol. Technol. 2019, 62, e19180295. [Google Scholar] [CrossRef]
- Brazil. Ministério da Saúde. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2003/rdc0009_16_01_2003.html (accessed on 12 November 2023).
- Pantoja, L.D.M.; Couto, M.S.; Leitão Junior, N.P.; Sousa, B.L.; Mourão, C.I.; Paixão, G.V. Fungal biodiversity of air in hospitals in the city of Fortaleza, Ceará, Brazil. Rev. Bras. Promoç. Saúde 2012, 25, 192–196. [Google Scholar] [CrossRef]
- Gheith, S.; Ranque, S.; Bannour, W.; Ben Youssef, Y.; Khelif, A.; Ben Said, M.; Njah, M.; Saghrouni, F. Hospital environment fungal contamination and aspergillosis risk in acute leukaemia patients in Sousse (Tunisia). Mycoses 2015, 58, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Belizario, J.A.; Lopes, L.G.; Pires, R.H. Fungi in the indoor air of critical hospital areas: A review. Aerobiologia 2021, 37, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.K.P.; Nascimento, J.P.M.; Araújo, M.A.D.S.; Pedrosa, K.P.D.S.; Tenorio, B.M.; Pires, L.L.S.; Lima, G.B.C.; Barboza, R.I.D.S.; Silva Filho, E.A. Airborne Fungi in neonatal intensive care unit of a public hospital in Brazil. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1210–1219. [Google Scholar] [CrossRef]
- Hassan, A.; Zeeshan, M. Microbiological indoor air quality of hospital buildings with different ventilation systems, cleaning frequencies and occupancy levels. Atmos. Pol. Res. 2022, 13, 101382. [Google Scholar] [CrossRef]
- Sivagnanasundaram, P.; Amarasekara, R.W.K.; Madegedara, R.M.D.; Ekanayake, A.; Magana-Arachchi, D.N. Assessment of airborne bacterial and fungal communities in selected areas of teaching hospital, Kandy, Sri Lanka. BioMed. Res. Int. 2019, 2019, e7393926. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Samaei, M.R. The effect of temperature on airborne filamentous fungi in the indoor and outdoor space of a hospital. Environ. Sci. Pollut. Int. Res. 2019, 26, 16868–16876. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Kano, R.; Baddley, J.W.; Moser, S.A.; Marr, K.A.; Alexander, B.D.; Andes, D.; Kontoyiannis, D.P.; Perrone, G.; Peterson, S.; et al. Molecular identification of Aspergillus species collected for the transplant-associated infection surveillance network. J. Clin. Microbiol. 2009, 47, 3138–3141. [Google Scholar] [CrossRef] [PubMed]
- Kluge, S.; Strauß, R.; Kochanek, M.; Weigand, M.A.; Rohde, H.; Lahmer, T. Aspergillosis: Emerging risk groups in critically ill patients. Med. Mycol. 2022, 60, myab064. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ryu, S.H.; Lee, J.Y.; Kim, H.J.; Kwak, S.H.; Jung, J.; Lee, J.; Sung, H.; Kim, S.-H. Airborne fungal spores and invasive aspergillosis in hematologic units in a tertiary hospital during construction: A prospective cohort study. Antimicrob. Resist. Infect. Control. 2019, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Reboux, G.; Rocchi, S.; Laboissière, A.; Ammari, H.; Bochaton, M.; Gardin, G.; Rame, J.-M.; Millon, L. Survey of 1012 Moldy dwellings by culture fungal analysis: Threshold proposal for asthmatic patient management. Indoor Air 2019, 29, 5–16. [Google Scholar] [CrossRef]
- Mobin, M.; do Amparo, M. Fungus microbiota in air conditioners in intensive care units in Teresina, Piauí. Rev. Soc. Bras. Med. Trop. 2006, 39, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Calumby, R.J.N.; Silva, J.A.; da Silva, D.P.; de Farias Moreira, R.T.; dos Santos Araujo, M.A.; de Almeida, L.M.; Grillo, L.A.M.; Alvino, V. Isolamento e identificação da microbiota fúngica anemófila em unidade de terapia intensiva/isolation and identification of anemophilic fungal microbiota in an intensive care unit. Braz. J. Dev. 2019, 5, 19708–19722. [Google Scholar] [CrossRef]
- De Oliveira, M.T.; Batista, N.K.R.; Gil, E.d.S.; Silva, M.R.R.; Costa, C.R.; Bara, M.T.F.; Torres, I.M.S. Risks associated with pathogenic fungi isolated from surgical centers, intensive care units, and materials sterilization centers in hospitals. Risks associated with pathogenic fungi isolated from critical hospital areas. Med. Mycol. 2020, 58, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Barreiros, G.; Akiti, T.; Magalhães, A.C.G.; Nouér, S.A.; Nucci, M. Effect of the implosion and demolition of a hospital building on the concentration of fungi in the air. Mycoses 2015, 58, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.L.; Mota, F.V.; Ferreira, G.F.; Mendes, J.F.; Pereira, E.C.; Freitas, C.H.; Vieira, J.N.; Villarreal, J.P.; Nascente, P.S. Airborne fungi in an intensive care unit. Braz. J. Biol. 2017, 78, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Gits-Muselli, M.; Hamane, S.; Verillaud, B.; Cherpin, E.; Denis, B.; Bondeelle, L.; Touratier, S.; Alanio, A.; Garcia-Hermoso, D.; Bretagne, S. Different repartition of the cryptic species of black Aspergilli According to the anatomical sites in human infections, in a French university hospital. Med. Mycol. 2021, 59, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Demuyser, T.; De Cock, E.; Sermijn, E. Airborne Aspergillus fumigatus contamination in an intensive care unit: Detection, management and control. J. Infect. Public Health 2019, 12, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kikuchi, T.; Kato, J.; Koda, Y.; Sakurai, M.; Kikumi, O.; Inose, R.; Murata, M.; Hasegawa, N.; Nakayama, H.; et al. Seasonal Changes in indoor airborne fungal concentration in a hematology ward. J. Infec. Chemother. 2020, 26, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Shelton, B.G.; Kirkland, K.H.; Flanders, W.D.; Morris, G.K. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 2002, 68, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Panackal, A.A.; Li, H.; Kontoyiannis, D.P.; Mori, M.; Perego, C.A.; Boeckh, M.; Marr, K.A. Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2010, 50, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Korfanty, G.; Heifetz, E.; Xu, J. Assessing thermal adaptation of a global sample of Aspergillus fumigatus: Implications for climate change effects. Front. Public Health. 2023, 11, 1059238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Gorman, C.M.; Fuller, H.T.; Dyer, P.S. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 2009, 457, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.B.A.; Silva, M.; Lyra, L.; Luz, E.A.; Uno, J.; Takada, H.; Miyaji, M.; Nishimura, K.; Schreiber, A.Z. Antifungal susceptibility and pathogenic potential of environmental isolated filamentous fungi compared with colonizing agents in immunocompromised patients. Mycopathologia 2005, 160, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wickes, B.L.; Wiederhold, N.P. Molecular diagnostics in medical mycology. Nat. Commun. 2018, 9, 5135. [Google Scholar] [CrossRef] [PubMed]
- Géry, A.; Séguin, V.; Eldin de Pécoulas, P.; Bonhomme, J.; Garon, D. Aspergilli series Versicolores: Importance of species identification in the clinical setting. Crit. Rev. Microbiol. 2022, 49, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Negri, C.E.; Gonçalves, S.S.; Xafranski, H.; Bergamasco, M.D.; Aquino, V.R.; Castro, P.T.O.; Colombo, A.L. Cryptic and rare Aspergillus species in Brazil: Prevalence in clinical samples and in vitro susceptibility to triazoles. J. Clin. Microbiol. 2014, 52, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Y.; Li, Y.; Osherov, N.; Goldman, G.H.; Verweij, P.E.; Zheng, B.; Li, R.; Chen, W.; Liang, T.; et al. Triazole-Resistant Aspergillus Luchuensis, an Industrially Important Black Aspergillus spp. Used in fermentation in East Asia, isolated from the patient with invasive pulmonary aspergillosis in China. Emerg. Microbes Infect. 2022, 11, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, N.; Kocsubé, S.; Szekeres, A.; Raghavan, A.; Narendran, V.; Vágvölgyi, C.; Panneer Selvam, K.; Babu Singh, Y.R.; Kredics, L.; Varga, J.; et al. Keratitis caused by Aspergillus pseudotamarii. Med. Mycol. Case Rep. 2013, 2, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B.; Kumar, P. Common environmental allergens causing respiratory allergy in India. Indian J. Pediatr. 2002, 69, 245–250. [Google Scholar] [CrossRef]
- Kariyawasam, R.M.; Dingle, T.C.; Kula, B.E.; Vandermeer, B.; Sligl, W.I.; Schwartz, I.S. Defining COVID-19–associated pulmonary aspergillosis: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Katsurayama, A.M.; Martins, L.M.; Iamanaka, B.T.; Fungaro, M.H.P.; Silva, J.J.; Frisvad, J.C.; Pitt, J.I.; Taniwaki, M.H. Occurrence of Aspergillus section Flavi and aflatoxins in brazilian rice: From field to market. Int. J. Food Microbiol. 2018, 266, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence (5′-3′) | Reference |
|---|---|---|
| β-tubulin 2a | GGTAACCAAATCGGTGCTGCTTTC | Glass and Donaldson, 1995 [19] |
| β-tubulin 2b | ACCCTCAGTGTAGTGACCCTTGGC | Glass and Donaldson, 1995 [19] |
| Calmodulin 1 | GARTWCAAGGAGGCCTTCTC | O’Donnell et al., 2000 [20] |
| Calmodulin 2a | TTTTTGCATCATGAGTTGGAC | O’Donnell et al., 2000 [20] |
| Calmodulin 11 | ACCATGATGGCGCGCAAG | O’Donnell et al., 2000 [20] |
| Calmodulin 22 | TCCTTCATCTTGCGCGCC | O’Donnell et al., 2000 [20] |
| CFU/m3 of Filamentous Fungi/100 L/min | |||
|---|---|---|---|
| Date | N (%) | Mean per Plate (SD) | p Value * |
| 12 January | 253 (6.04) | 9.3 (6.6) | |
| 26 January | 448 (10.46) | 16.7 (9.2) | |
| 9 February | 202 (4.98) | 7.4 (5.0) | |
| 23 February | 325 (7.55) | 12.5 (9.2) | |
| 9 March | 431 (10.33) | 15.9 (7.8) | <0.001 |
| 6 August | 442 (10.80) | 17.0 (6.7) | |
| 20 August | 498 (12.22) | 19.1 (8.9) | |
| 7 December | 593 (11.86) | 18.8 (7.6) | |
| 14 December | 1103 (25.73) | 42.4 (12.5) | |
| Date | Flavi N (%) | Nidulantes N (%) | Nigri N (%) | Terrei N (%) | Fumigati N (%) | Indeterminate N (%) | Total of Aspergillus N (%) | p Value |
|---|---|---|---|---|---|---|---|---|
| 12 January | 0 (0.0) | 0 (0.0) | 5 (55.5) | 0 (0.0) | 4 (44.4) | 0 (0.0) | 9 (2.8) | <0.001 |
| 26 January | 8 (17.8) | 5 (11.1) | 26 (57.8) | 4 (8.9) | 2 (4.4) | 0 (0.0) | 45 (13.9) | |
| 9 February | 0 (0.0) | 26 (86.7) | 4 (13.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 30 (9.3) | |
| 23 February | 1 (2.6) | 18 (47.4) | 18 (47.4) | 1 (2.6) | 0 (0.0) | 0 (0.0) | 38 (11.7) | |
| 9 March | 11 (33.3) | 0 (0.0) | 18 (54.5) | 2 (6.1) | 0 (0.0) | 2 (6.1) | 33 (10.2) | |
| 6 August | 1 (1.5) | 13 (27.6) | 4 (6.0) | 1 (1.5) | 48 (71.6) | 0 (0.0) | 67 (20.7) | |
| 20 August | 9 (26.4) | 3 (8.8) | 5 (14.7) | 1 (2.9) | 16 (47.1) | 0 (0.0) | 34 (10.5) | |
| 7 December | 5 (11.9) | 13 (30.9) | 5 (11.9) | 1 (2.4) | 18 (42.9) | 0 (0.0) | 42 (13.0) | |
| 14 December | 3 (11.5) | 12 (46.2) | 4 (15.4) | 0 (0.0) | 7 (26.9) | 0 (0.0) | 26 (8.0) | |
| Total | 38 (11.7) | 90 (27.8) | 89 (27.4) | 10 (3.1) | 95 (29.3) | 2 (0.6) | 324 (100) |
| Aspergillus spp. (Section) | WINTER N (%) | SPRING N (%) | SUMMER N (%) | Total of Aspergillus N (%) | p Value |
|---|---|---|---|---|---|
| Flavi | 10 (26.3) | 8 (21.0) | 20 (52.6) | 38 (11.7) | <0.001 |
| Nidulantes | 16 (17.8) | 25 (27.8) | 49 (54.4) | 90 (27.8) | |
| Nigri | 9 (10.1) | 9 (10.1) | 71 (79.8) | 89 (27.4) | |
| Terrei | 2 (20.0) | 1 (10.0) | 7 (70.0) | 10 (3.1) | |
| Fumigati | 64 (67.4) | 25 (26.3) | 6 (6.3) | 95 (29.3) | |
| Indeterminate | 0 (0.0) | 0 (0.0) | 2 (100.0) | 2 (0.6) | |
| Total | 101 (31.2) | 68 (21.0) | 155 (47.8) | 324 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemos, M.S.C.d.; Higa Junior, M.G.; Paniago, A.M.M.; Melhem, M.d.S.C.; Takahashi, J.P.F.; Fava, W.S.; Venancio, F.A.; Martins, N.M.; Chang, M.R. Aspergillus in the Indoor Air of Critical Areas of a Tertiary Hospital in Brazil. J. Fungi 2024, 10, 538. https://doi.org/10.3390/jof10080538
Lemos MSCd, Higa Junior MG, Paniago AMM, Melhem MdSC, Takahashi JPF, Fava WS, Venancio FA, Martins NM, Chang MR. Aspergillus in the Indoor Air of Critical Areas of a Tertiary Hospital in Brazil. Journal of Fungi. 2024; 10(8):538. https://doi.org/10.3390/jof10080538
Chicago/Turabian StyleLemos, Michele Scardine Corrêa de, Minoru German Higa Junior, Anamaria Mello Miranda Paniago, Marcia de Souza Carvalho Melhem, Juliana Possato Fernandes Takahashi, Wellington Santos Fava, Fabio Antonio Venancio, Nayara Moreno Martins, and Marilene Rodrigues Chang. 2024. "Aspergillus in the Indoor Air of Critical Areas of a Tertiary Hospital in Brazil" Journal of Fungi 10, no. 8: 538. https://doi.org/10.3390/jof10080538







