Serine Rejuvenated Degenerated Volvariella volvacea by Enhancing ROS Scavenging Ability and Mitochondrial Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
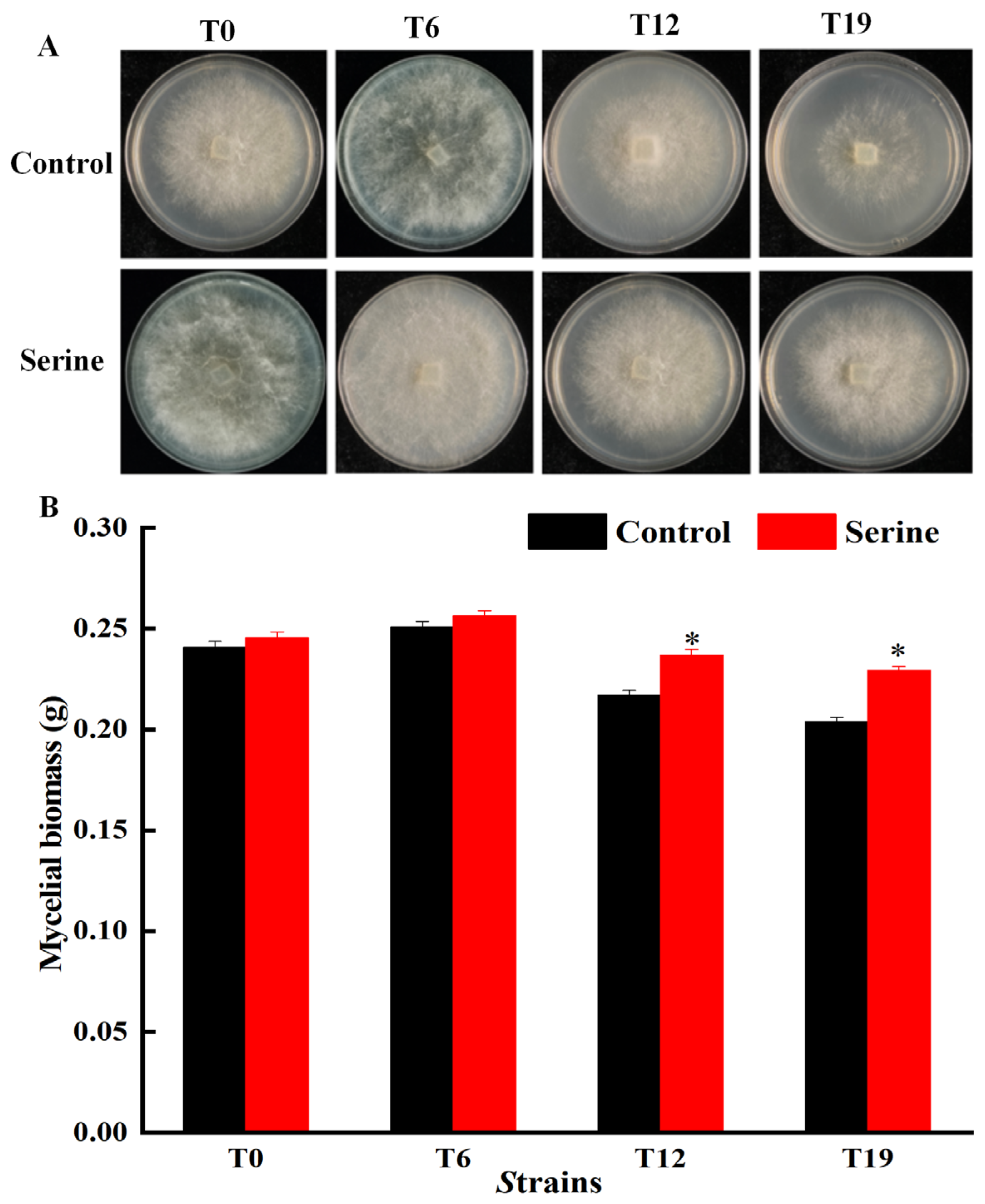
2.2. Observation of Colonies Morphology
2.3. Determination of Mycelial Biomass
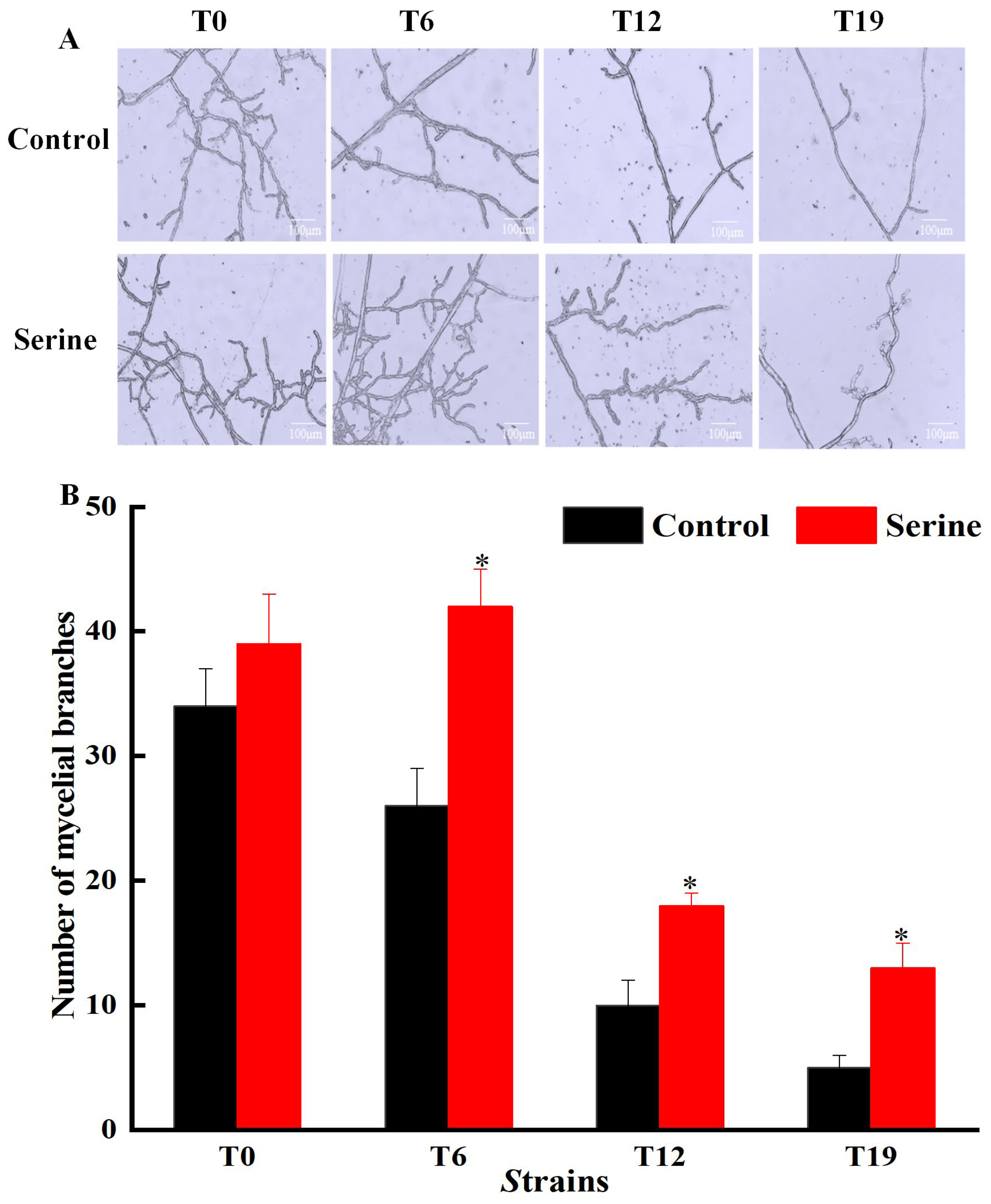
2.4. Determination of Mycelial Branching
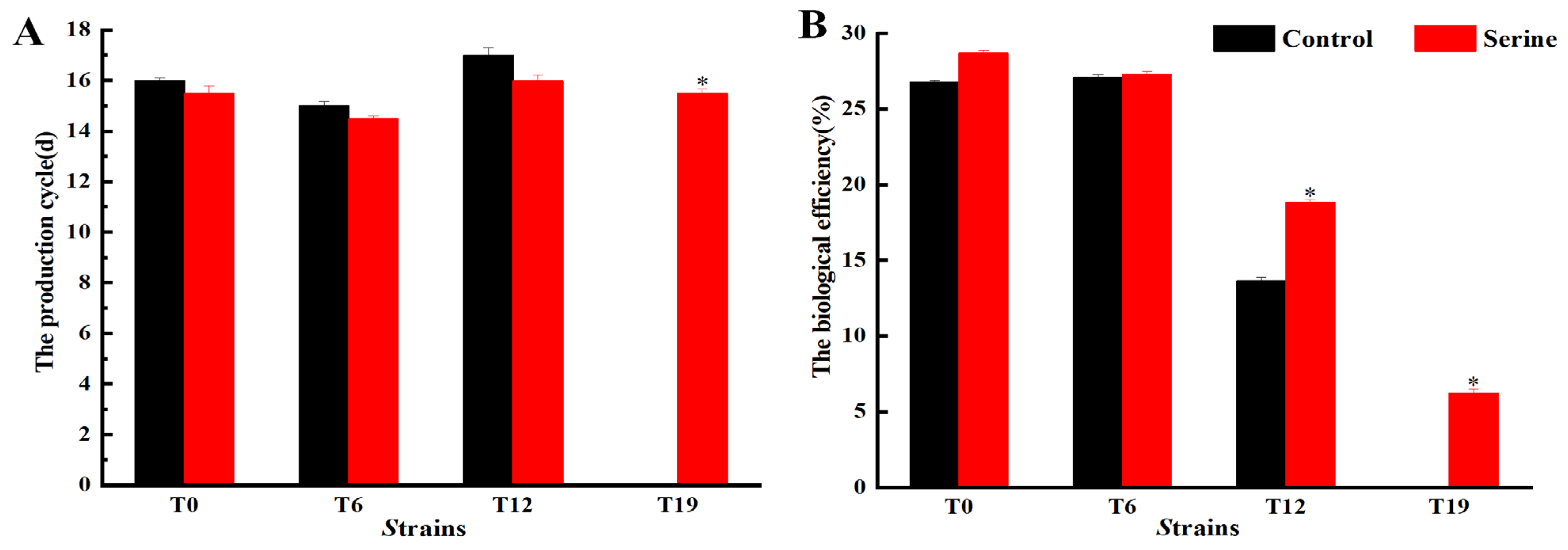
2.5. Cultivation Test
2.6. Estimation of Antioxidant Enzyme Gene Expressions by Real-Time Quantitative PCR (RT-qPCR)
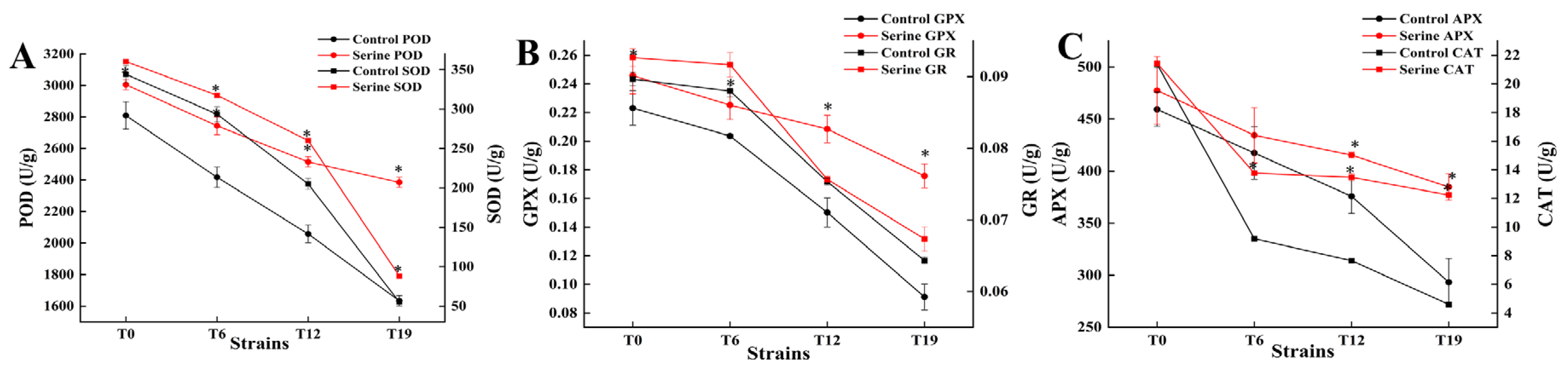
2.7. Determination of Antioxidant Enzyme Activity and ROS Content
2.8. Nitrouble Tetrazolium Chloride (NBT) Staining
2.9. Mitochondrial Staining
2.10. Measurement of Mitochondrial Membrane Potential (MMP)
2.11. Determination of Strain Energy
2.12. Determination of Malondialdehyde Content (MDA)
2.13. Determination of Matrix-Degrading Enzyme Activity
2.14. Data Processing
3. Results
3.1. Effect of Serine on Mycelial Characteristics of V. volvacea
3.2. Effect of Serine on Fruiting Body Characteristics of V. volvacea
3.3. Effect of Serine on the Relative Expression of Antioxidant Enzyme Genes in V. volvacea
3.4. Effect of Serine on the Antioxidase Activity of V. volvacea
3.5. Effect of Serine on ROS Content in V. volvacea
3.6. Effect of Serine on Mitochondria of V. volvacea
3.7. Effect of Serine on Mycelial Energy of V. volvacea
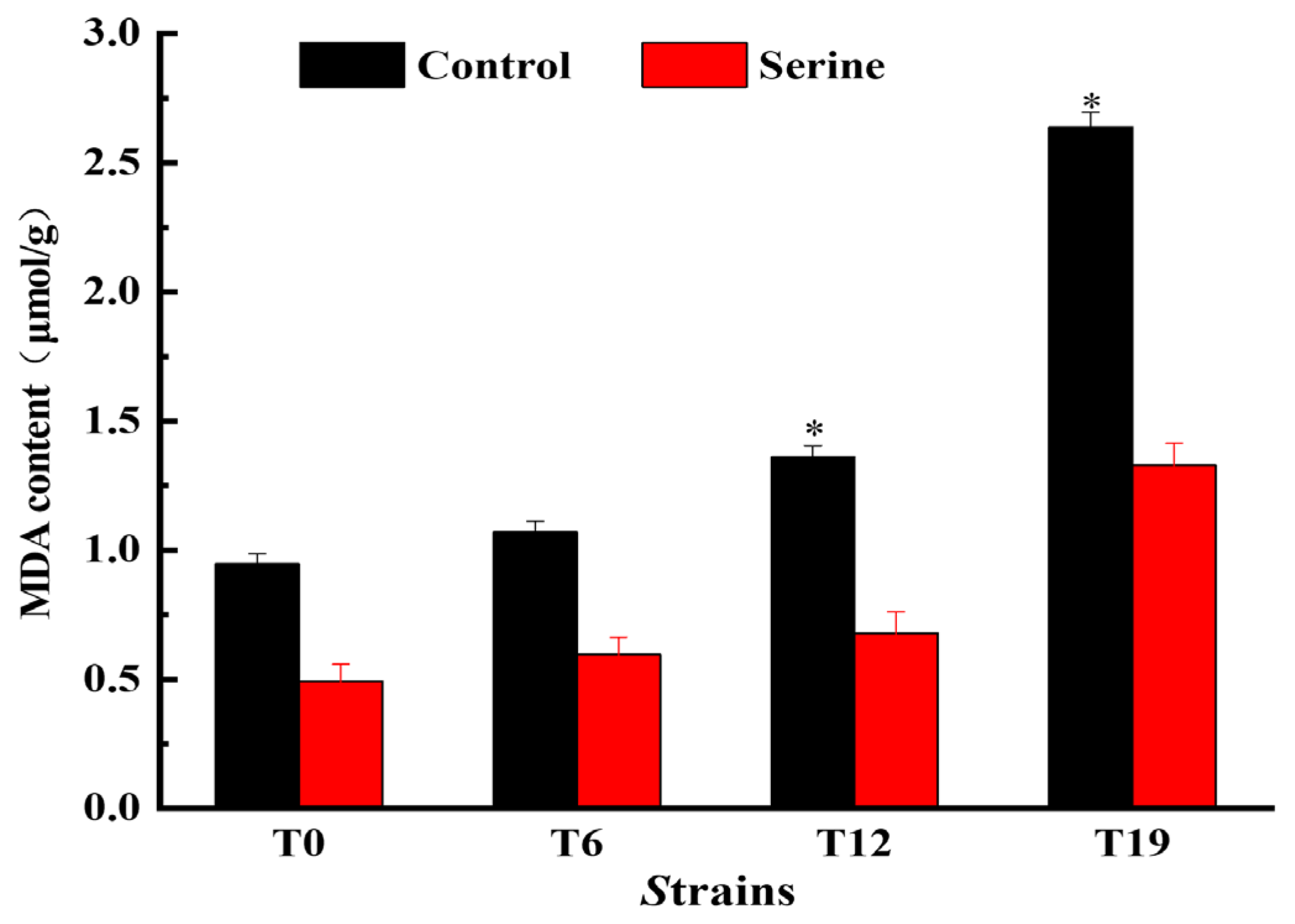
3.8. Effect of Serine on MDA Content of V. volvacea
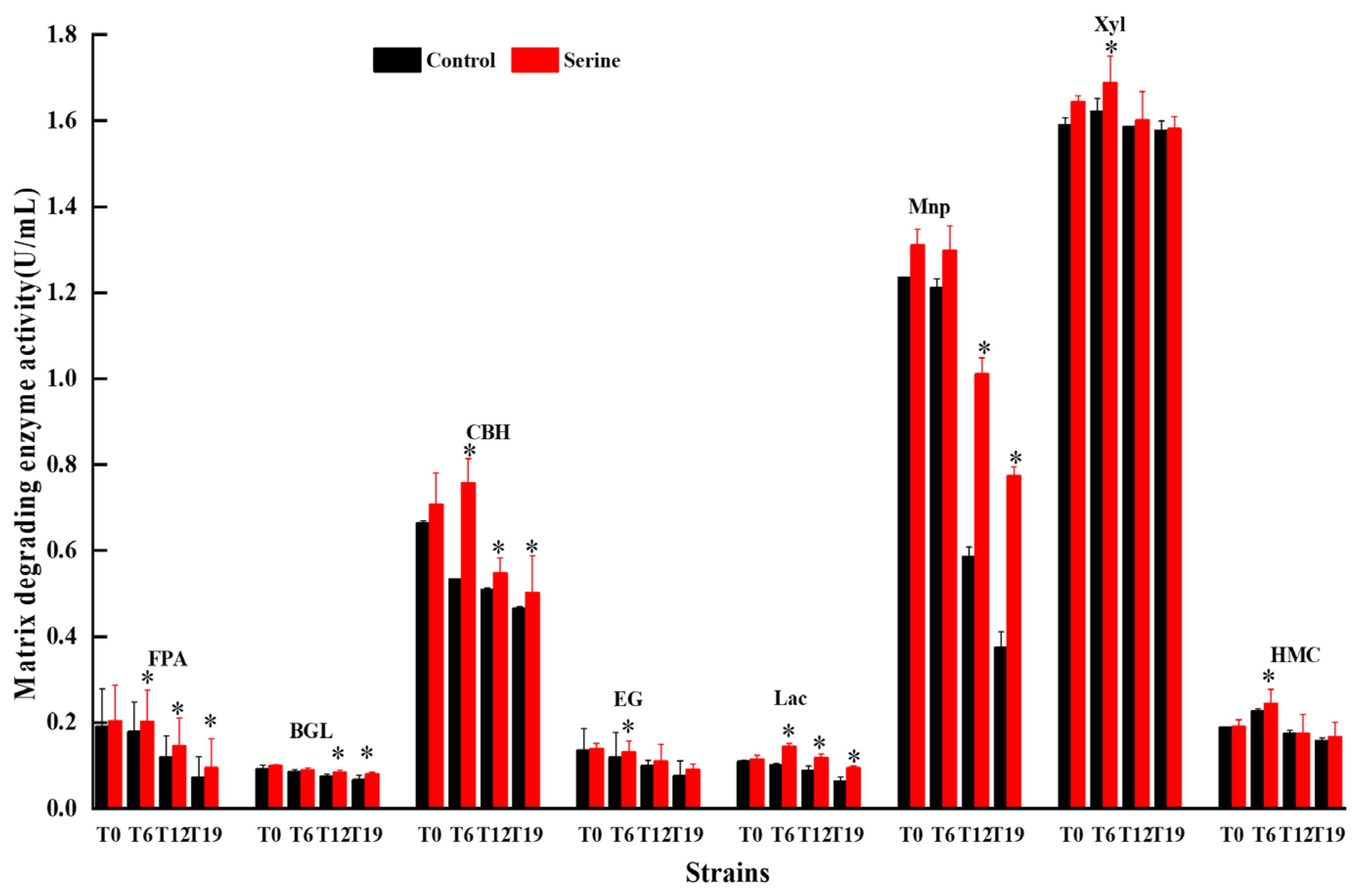
3.9. Effect of Serine on the Matrix-Degrading Enzymes of V. volvacea
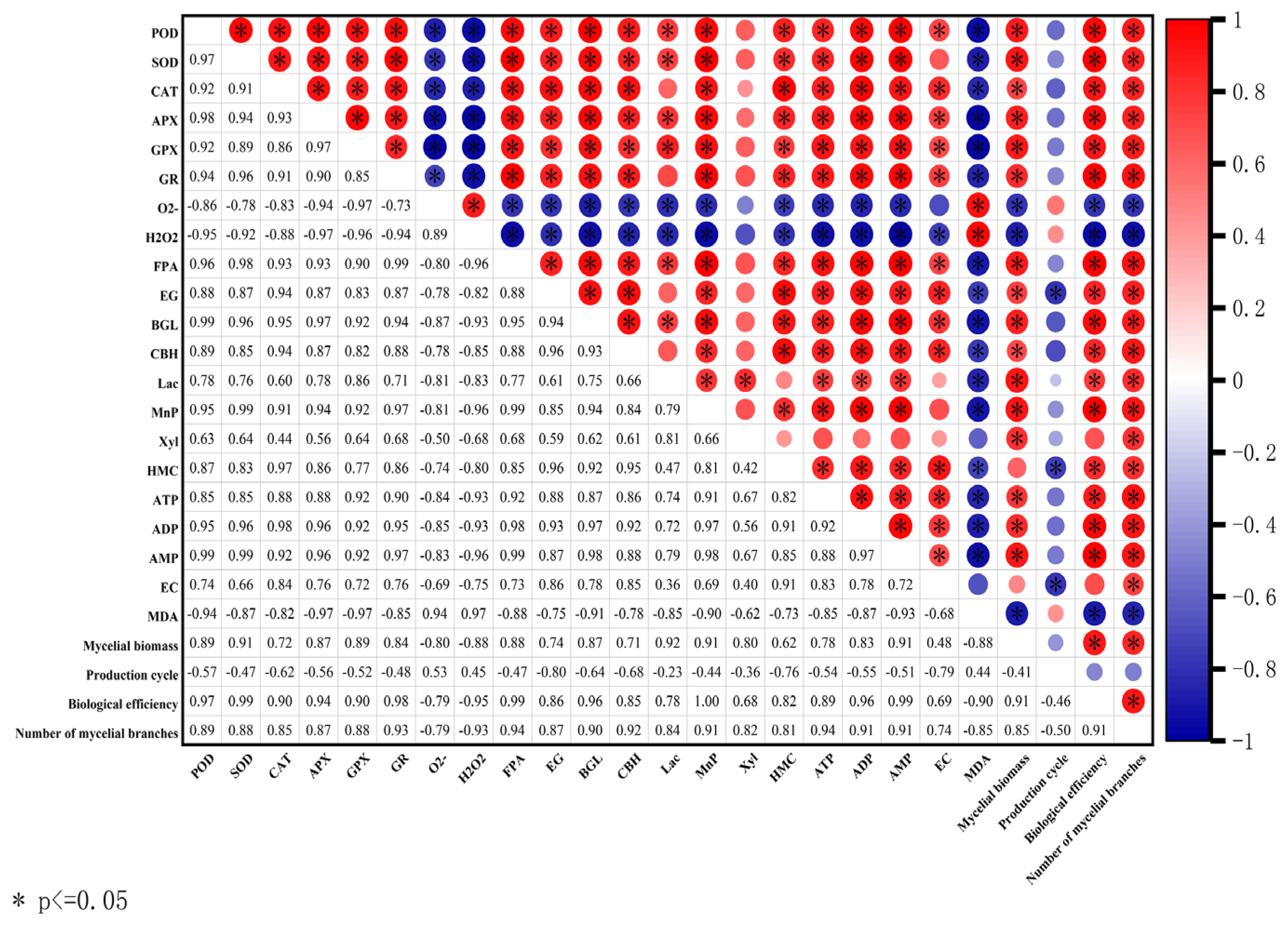
3.10. Correlation between Main Characteristics of V. volvacea
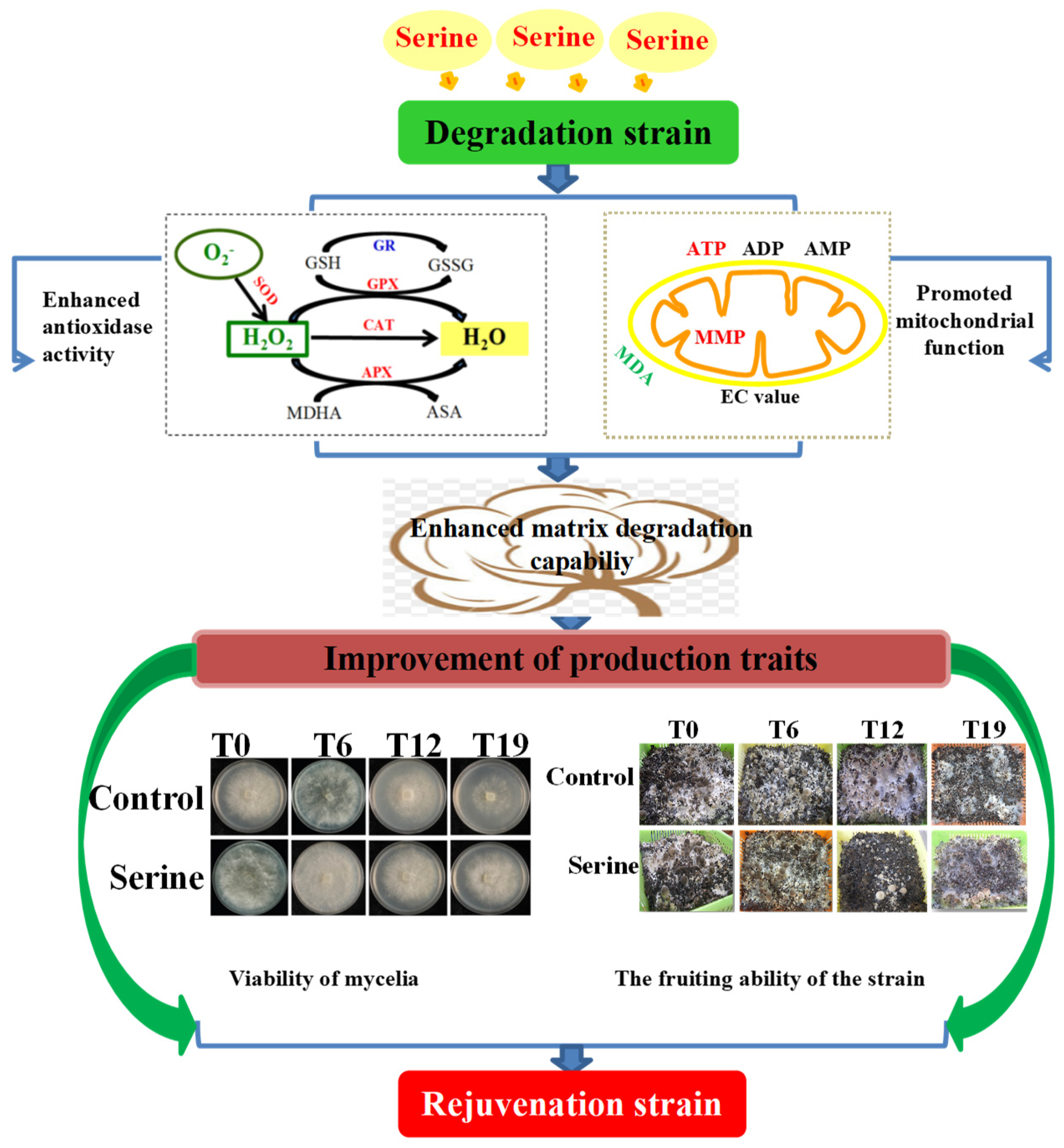
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mingyi, Y.; Belwal, T.; Devkota, H.P.; Li, L.; Luo, Z. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 94–110. [Google Scholar] [CrossRef]
- Xu, X.; Xu, R.; Jia, Q.; Feng, T.; Huang, Q.; Ho, C.T.; Song, S. Identification of dihydro-β-ionone as a key aroma compound in addition to C8 ketones and alcohols in Volvariella volvacea mushroom. Food Chem. 2019, 293, 333–339. [Google Scholar] [CrossRef]
- Liu, Y.C.; Liang, X.W.; Mo, W.P.; Zhuo, L.J.; Hu, H.P.; Huang, H.P.; Xie, Y.Z. Research Advances on Preservation of Edible Mushroom. Edible Fungi China 2018, 37, 1–6. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, C.; Zhao, Y.; Liu, S.; Wang, H.; Wang, C.; Guo, L.; Chen, M. Changes in Mannitol Content, Regulation of Genes Involved in Mannitol Metabolism, and the Protective Effect of Mannitol on Volvariella volvacea at Low Temperature. BioMed Res. Int. 2019, 2019, 1493721. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wang, Q.; Cheng, Z.; Yun, J.; Zhang, W.; Zhao, F. Effect of exogenous amino acids on the rejuvenation of degraded strains of Volvariella volvacea. Food Ferment. Ind. 2021, 47, 30–36. [Google Scholar]
- Parker, S.J.; Metallo, C.M. Chasing One-Carbon Units to Understand the Role of Serine in Epigenetics. Mol. Cell 2016, 61, 185–186. [Google Scholar] [CrossRef]
- Esch, B.M.; Limar, S.; Bogdanowski, A.; Gournas, C.; More, T.; Sundag, C.; Walter, S.; Heinisch, J.J.; Ejsing, C.S.; André, B.; et al. Uptake of exogenous serine is important to maintain sphingolipid homeostasis in Saccharomyces cerevisiae. PLoS Genet. 2020, 16, e1008745. [Google Scholar] [CrossRef]
- Garcia, A.L.; Madrid, R.; Gimeno, V. The effects of amino acids fertilization incorporated to the nutrient solution on mineral composition and growth in tomato seedlings. Span. J. Agric. Res. 2011, 9, 852–861. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, F.; Li, H.; Li, X. Influence of earthworm mucus and amino acids on tomato seedling growth and cadmium accumulation. Environ. Pollut. 2009, 157, 2737–2742. [Google Scholar] [CrossRef]
- Ko, Y.H.; Chun, J.; Yang, H.E.; Kim, D.H. Hypoviral-regulated HSP90 co-chaperone p23 (CpCop23) determines the colony morphology, virulence, and viral response of chestnut blight fungus Cryphonectria parasitica. Mol. Plant Pathol. 2023, 24, 413–424. [Google Scholar] [CrossRef]
- Scheid, S.S.; Faria, M.G.I.; Velasquez, L.G.; do Valle, J.S.; Gonçalves, A.C., Jr.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron biofortification and availability in the mycelial biomass of edible and medicinal basidiomycetes cultivated in sugarcane molasses. Sci. Rep. 2020, 10, 12875. [Google Scholar] [CrossRef]
- Guo, F.; Shan, Z.; Yu, J.; Xu, G.; Zhang, Z. The Cysteine-Rich Repeat Protein TaCRR1 Participates in Defense against Both Rhizoctonia cerealis and Bipolaris sorokiniana in Wheat. Int. J. Mol. Sci. 2020, 21, 5698. [Google Scholar] [CrossRef]
- Li, H.; He, Z.; Jiang, Y.; Kan, J.; Peng, T.; Zhong, M.; Hu, Z. Bioconversion of bamboo shoot shells through the cultivation of the edible mushrooms Volvariella volvacea. Ecotoxicology 2021, 30, 1476–1486. [Google Scholar] [CrossRef]
- Lei, M.; Guo, L.; Zhang, Y.; Yan, X.; Jiang, F.; Sun, B. Effectiveness of anaerobic treatment combined with microperforated film packaging in reducing Agaricus bisporus postharvest browning. Postharvest Biol. Technol. 2024, 211, 112833. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Guo, J.; Deng, D.; He, G.; Ji, C.; Wang, R.; Long, H.; Wang, J.; et al. Effect of shiitake mushrooms polysaccharide and chitosan coating on softening and browning of shiitake mushrooms (Lentinus edodes) during postharvest storage. Int. J. Biol. Macromol. 2022, 218, 816–827. [Google Scholar] [CrossRef]
- Parkhey, S.; Naithani, S.C.; Keshavkant, S. ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol. Biochem. 2012, 57, 261–267. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Ma, L.; Guo, Y.; Dou, X.; Yan, S.; Zhang, B.; Roman, A. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate intestinal epithelial barrier dysfunction caused by oxidative stress via Nrf2 signaling-mediated mitochondrial pathway. Int. J. Nanomed. 2019, 14, 4491–4502. [Google Scholar] [CrossRef]
- Moghadasi Boroujeni, S.; Koontz, A.; Tseropoulos, G.; Kerosuo, L.; Mehrotra, P.; Bajpai, V.K.; Selvam, S.R.; Lei, P.; Bronner, M.E.; Andreadis, S.T. Neural crest stem cells from human epidermis of aged donors maintain their multipotency in vitro and in vivo. Sci. Rep. 2019, 9, 9750. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yang, S.; Cheng, Y.; Chen, F.; Pan, S.; Fan, G. Antifungal activity and action mode of pinocembrin from propolis against Penicillium italicum. Food Sci. Biotechnol. 2012, 21, 1533–1539. [Google Scholar] [CrossRef]
- Pan, Q.; Li, Y.; Zhang, J.; Hu, T.; Hou, Y.; Tang, S. Mechanisms of oxidative response during biodegradation of malathion by S. oneidensis MR-1. Environ. Sci. Pollut. Res. Int. 2024, 31, 16832–16845. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Su, X.; Luo, H.; Ma, R.; Yao, B.; Ma, F. Deciphering lignocellulose deconstruction by the white rot fungus Irpex lacteus based on genomic and transcriptomic analyses. Biotechnol. Biofuels 2018, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, Y.; Shao, Y.; Huang, B. A Novel Technique for Rejuvenation of Degenerated Caterpillar Medicinal Mushroom, Cordyceps militaris (Ascomycetes), a Valued Traditional Chinese Medicine. Int. J. Med. Mushrooms 2017, 19, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Giménez, A. Supplementation in mushroom crops and its impact on yield and quality. AMB Express 2018, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, Y.; Chen, M.; Li, Z. Improved fruiting of the straw mushroom (Volvariella volvacea) on cotton waste supplemented with sodium acetate. Appl. Microbiol. Biotechnol. 2017, 101, 8533–8541. [Google Scholar] [CrossRef] [PubMed]
- Sardar, H.; Anjum, M.A.; Hussain, S.; Ali, S.; Shaheen, M.R.; Ahsan, M.; Ejaz, S.; Ahmad, K.S.; Naz, S.; Shafique, M. Deciphering the role of moringa leaf powder as a supplement in the cotton waste substrate for the growth and nutrition of king oyster mushroom. Sci. Hortic. 2022, 293, 110694. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Sun, P.P.; Li, H.R.; Zhu, Z.Y. Effects of Na2SeO3 on growth, metabolism, antioxidase and enzymes involved in polysaccharide synthesis of Cordyceps militaris. Process Biochem. 2020, 97, 64–71. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, H.; Zhou, C.; Gong, M.; Li, Y.; Shao, Y.; Wu, Y.; Bao, D. Exogenous γ-Aminobutyric Acid (GABA) Enhanced Response to Abiotic Stress in Hypsizygus marmoreus by Improving Mycelial Growth and Antioxidant Capacity. Metabolites 2024, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. PCA 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Shinogi, T.; Suzuki, T.; Kurihara, T.; Narusaka, Y.; Park, P. Microscopic detection of reactive oxygen species generation in the compatible and incompatible interactions of Alternaria alternata Japanese pear pathotype and host plants. J. Gen. Plant Pathol. 2003, 69, 7–16. [Google Scholar] [CrossRef]
- Javvaji, P.K.; Dhali, A.; Francis, J.R.; Kolte, A.P.; Mech, A.; Roy, S.C.; Mishra, A.; Bhatta, R. An Efficient Nitroblue Tetrazolium Staining and Bright-Field Microscopy Based Method for Detecting and Quantifying Intracellular Reactive Oxygen Species in Oocytes, Cumulus Cells and Embryos. Front. Cell Dev. Biol. 2020, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.W.; Wang, R.; Gao, Q.; Liu, M.X.; Bao, D.P.; Wang, Y. Studies on the addition of exogenous amino acids to restore or prevent the degradation of filamentous fungi. J. Biol. 2017, 34, 108–111. [Google Scholar]
- Wang, C.; Butt, T.M.; Leger, R.J.S. Colony sectorization of Metarhizium anisopliae is a sign of ageing. Microbiology 2005, 151, 3223–3236. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pischetsrieder, M.; St. Leger, R.J.; Wang, C. Associated links among mtDNA glycation, oxidative stress and colony sectorization in Metarhizium anisopliae. Fungal Genet. Biol. 2008, 45, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. Serine and Functional Metabolites in Cancer. Trends Cell Biol. 2017, 27, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, L.; Zuo, S.; Zhang, Y.; Wan, D.; Long, C.; Huang, P.; Wu, X.; Wu, C.; Liu, G.; et al. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lee, K.; Reid, M.A.; Sanderson, S.M.; Qiu, C.; Li, S.; Liu, J.; Locasale, J.W. Serine Availability Influences Mitochondrial Dynamics and Function through Lipid Metabolism. Cell Rep. 2018, 22, 3507–3520. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Xia, Y.; Zheng, P.; Wang, C. Increasing oxidative stress tolerance and subculturing stability of Cordyceps militaris by overexpression of a glutathione peroxidase gene. Appl. Microbiol. Biotechnol. 2013, 97, 2009–2015. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 Enhances Tolerance of Wheat to Salt Stress through Improvement of Antioxidative Defense System and Gene Expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Jazwinski, S.M. Yeast longevity and aging—The mitochondrial connection. Mech. Ageing Dev. 2005, 126, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Berry, B.J.; Vodičková, A.; Müller-Eigner, A.; Meng, C.; Ludwig, C.; Kaeberlein, M.; Peleg, S.; Wojtovich, A.P. Optogenetic rejuvenation of mitochondrial membrane potential extends C. elegans lifespan. Nat. Aging 2023, 3, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Bratic, I.; Trifunovic, A. Mitochondrial energy metabolism and ageing. Biochim. Biophys. Acta 2010, 1797, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar] [CrossRef]
- Yale, W.; Yingming, X.; Xuefeng, L.; Liping, L.; Qingqing, H. Soil addition of MnSO4 reduces wheat Cd accumulation by simultaneously increasing labile Mn and decreasing labile Cd concentrations in calcareous soil: A two-year pot study. Chemosphere 2023, 317, 137900. [Google Scholar]
- Mostofa, M.G.; Hossain, M.A.; Fujita, M. Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: Oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 2015, 252, 461–475. [Google Scholar] [CrossRef]
- Park, Y.J.; Baek, J.H.; Lee, S.; Kim, C.; Rhee, H.; Kim, H.; Seo, J.S.; Park, H.R.; Yoon, D.E.; Nam, J.Y.; et al. Whole genome and global gene expression analyses of the model mushroom Flammulina velutipes reveal a high capacity for lignocellulose degradation. PLoS ONE 2014, 9, e93560. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.T.; Hu, S.; Yu, X.Y.; Jin, L.; Zhu, Y.J.; Jin, F.J. Studies of Cellulose and Starch Utilization and the Regulatory Mechanisms of Related Enzymes in Fungi. Polymers 2020, 12, 530. [Google Scholar] [CrossRef]
- Mamimin, C.; Chanthong, S.; Leamdum, C.; O-Thong, S.; Prasertsan, P. Improvement of empty palm fruit bunches biodegradability and biogas production by integrating the straw mushroom cultivation as a pretreatment in the solid-state anaerobic digestion. Bioresour. Technol. 2021, 319, 124227. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Fernández, F.J.; Montiel-González, A.M.; Sánchez, C.; Díaz-Godínez, G. Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Appl. Microbiol. Biotechnol. 2008, 81, 675–679. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, H.; Wu, X.; Wang, Q.; Chen, M.; Feng, Z.; Chen, H. The functions of glutathione peroxidase in ROS homeostasis and fruiting body development in Hypsizygus marmoreus. Appl. Microbiol. Biotechnol. 2020, 104, 10555–10570. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhu, J.; Wang, Y.; Yun, J.; Zhang, Y.; Zhao, F. Serine Rejuvenated Degenerated Volvariella volvacea by Enhancing ROS Scavenging Ability and Mitochondrial Function. J. Fungi 2024, 10, 540. https://doi.org/10.3390/jof10080540
Wang Q, Zhu J, Wang Y, Yun J, Zhang Y, Zhao F. Serine Rejuvenated Degenerated Volvariella volvacea by Enhancing ROS Scavenging Ability and Mitochondrial Function. Journal of Fungi. 2024; 10(8):540. https://doi.org/10.3390/jof10080540
Chicago/Turabian StyleWang, Qiaoli, Jianing Zhu, Yonghui Wang, Jianmin Yun, Yubin Zhang, and Fengyun Zhao. 2024. "Serine Rejuvenated Degenerated Volvariella volvacea by Enhancing ROS Scavenging Ability and Mitochondrial Function" Journal of Fungi 10, no. 8: 540. https://doi.org/10.3390/jof10080540
APA StyleWang, Q., Zhu, J., Wang, Y., Yun, J., Zhang, Y., & Zhao, F. (2024). Serine Rejuvenated Degenerated Volvariella volvacea by Enhancing ROS Scavenging Ability and Mitochondrial Function. Journal of Fungi, 10(8), 540. https://doi.org/10.3390/jof10080540






