Exploring the Potential of Aspergillus oryzae for Sustainable Mycoprotein Production Using Okara and Soy Whey as Cost-Effective Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Reagents
2.2. Microorganism Maintenance and Media Preparation
2.3. Enzymatic Activity of Aspergillus oryzae
2.4. Mycelial Biomass Production in Media Containing Soy By-Product
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results
3.1. Enzymatic Activity of A. oryzae
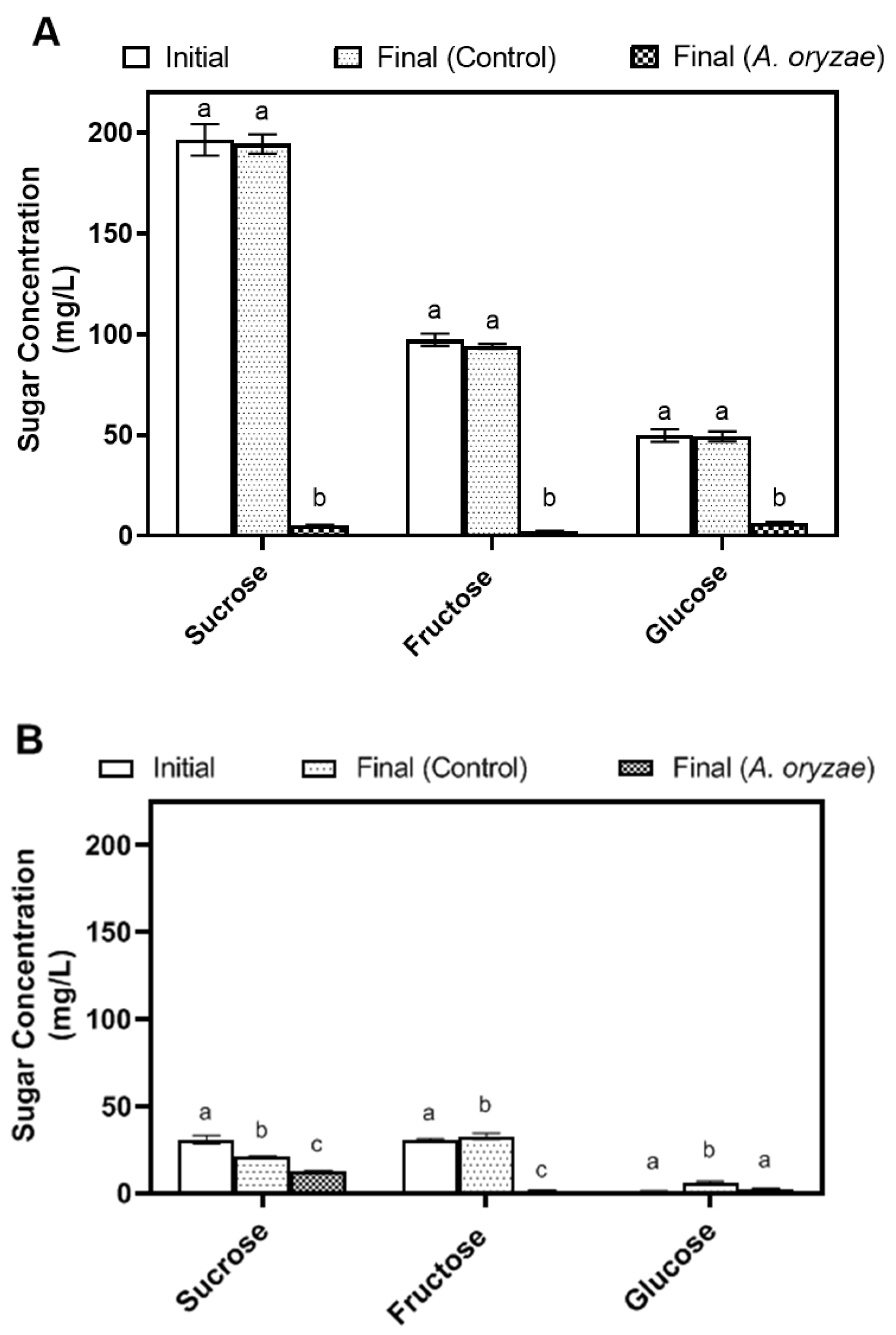
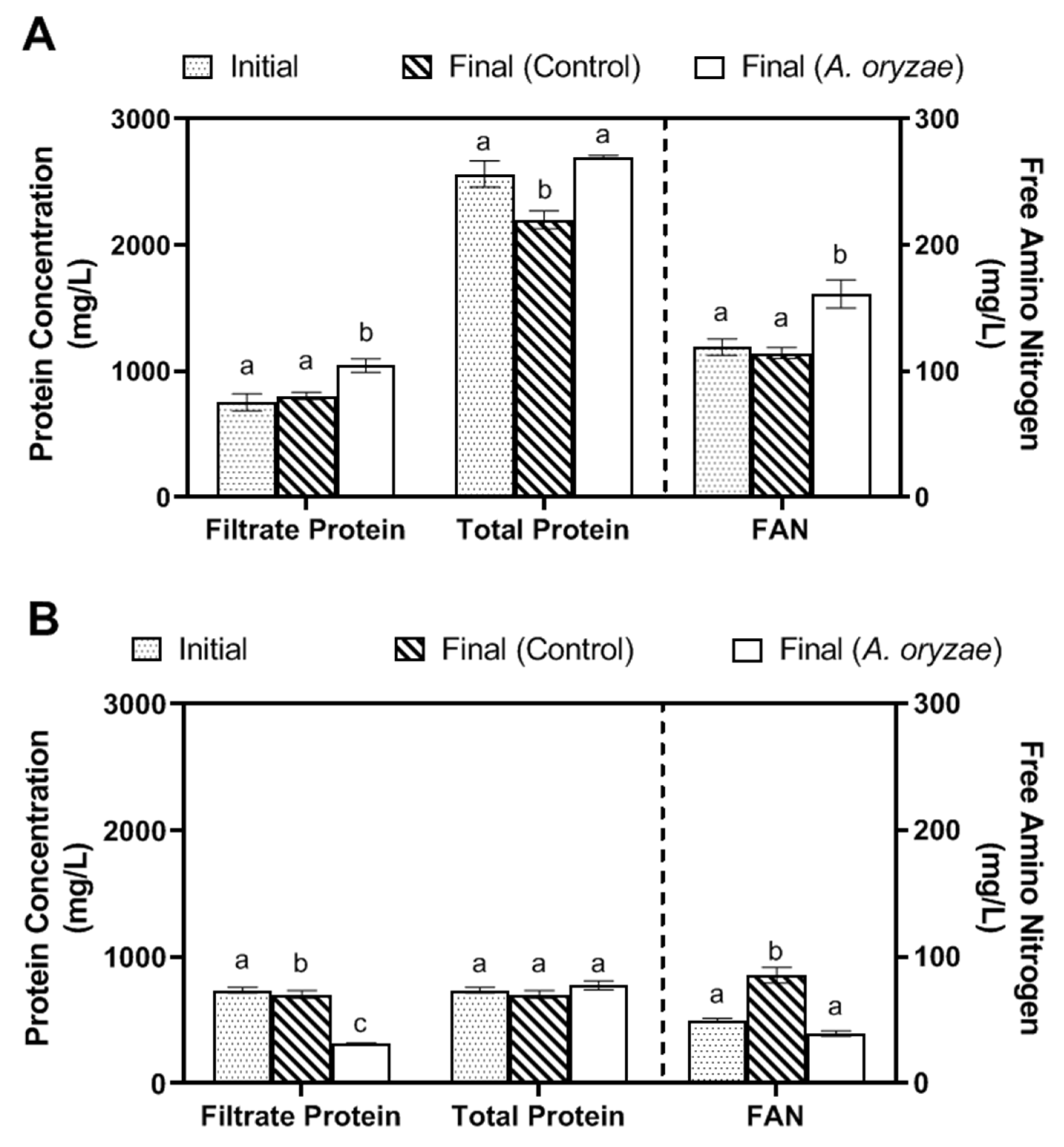
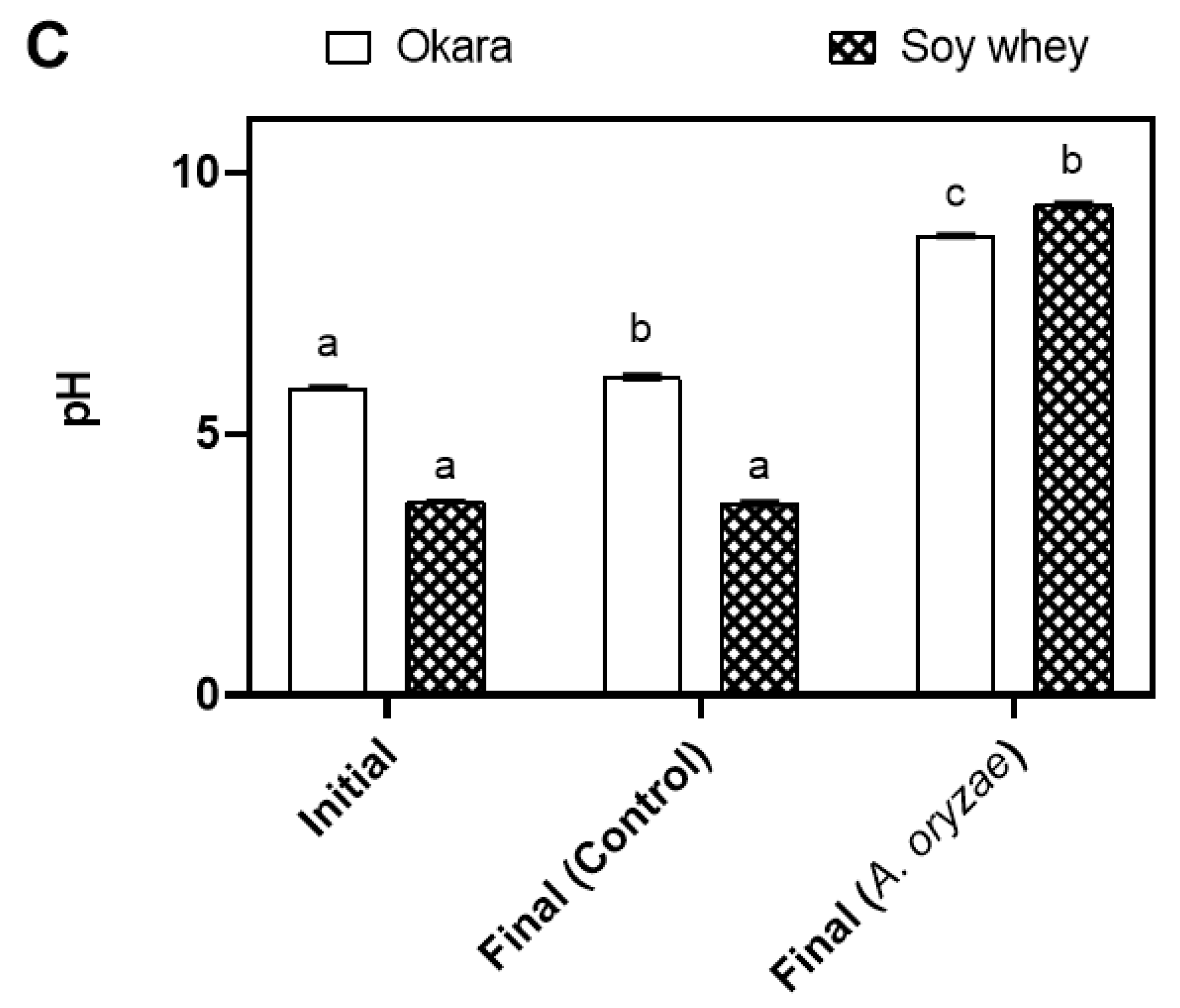
3.2. Metabolic Changes during A. oryzae Cultivation in Okara and Soy Whey Media under Agitated Conditions
3.3. Biomass Production in Okara and Soy Whey under Agitated Conditions
3.4. Static Conditions Promote the Production of Biomass in Okara but Reduce It in Soy Whey
3.5. Soy Whey Resulted in a Higher Biomass and Protein Yield per Substrate in Comparison to Okara
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appleton, K. Barriers to and Facilitators of the Consumption of Animal-Based Protein-Rich Foods in Older Adults. Nutrients 2016, 8, 187. [Google Scholar] [CrossRef]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and Supply of High-quality Food Protein for Human Consumption: Sustainability, Challenges, and Innovations. Ann. N. Y. Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef]
- Wu, G. Dietary Protein Intake and Human Health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef]
- Millward, D.J. Nutrition, Infection and Stunting: The Roles of Deficiencies of Individual Nutrients and Foods, and of Inflammation, as Determinants of Reduced Linear Growth of Children. Nutr. Res. Rev. 2017, 30, 50–72. [Google Scholar] [CrossRef]
- Castro-Nunez, A.; Buriticá, A.; Gonzalez, C.; Villarino, E.; Holmann, F.; Perez, L.; Del Río, M.; Sandoval, D.; Eufemia, L.; Löhr, K.; et al. The Risk of Unintended Deforestation from Scaling Sustainable Livestock Production Systems. Conserv. Sci. Pract. 2021, 3, e495. [Google Scholar] [CrossRef]
- Kraham, S.J. Environmental Impacts of Industrial Livestock Production. In International Farm Animal, Wildlife and Food Safety Law; Steier, G., Patel, K.K., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–40. ISBN 978-3-319-18001-4. [Google Scholar]
- Sakadevan, K.; Nguyen, M.-L. Livestock Production and Its Impact on Nutrient Pollution and Greenhouse Gas Emissions. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2017; Volume 141, pp. 147–184. ISBN 978-0-12-812423-9. [Google Scholar]
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Smetana, S.; Ristic, D.; Pleissner, D.; Tuomisto, H.L.; Parniakov, O.; Heinz, V. Meat Substitutes: Resource Demands and Environmental Footprints. Resour. Conserv. Recycl. 2023, 190, 106831. [Google Scholar] [CrossRef]
- Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Dugat-Bony, E.; Bonnarme, P.; Minervini, F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods 2022, 11, 2065. [Google Scholar] [CrossRef]
- Van Vliet, S.; Kronberg, S.L.; Provenza, F.D. Plant-Based Meats, Human Health, and Climate Change. Front. Sustain. Food Syst. 2020, 4, 128. [Google Scholar] [CrossRef]
- Vicente, F.A.; Ventura, S.P.M.; Passos, H.; Dias, A.C.R.V.; Torres-Acosta, M.A.; Novak, U.; Likozar, B. Crustacean Waste Biorefinery as a Sustainable Cost-Effective Business Model. Chem. Eng. J. 2022, 442, 135937. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Barco-Mendoza, G.A.; Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ochoa, M.A.V.; González-Aguilar, G.A. Single-Cell Protein Production as a Strategy to Reincorporate Food Waste and Agro By-Products Back into the Processing Chain. Bioengineering 2022, 9, 623. [Google Scholar] [CrossRef]
- Bajić, B.; Vučurović, D.; Vasić, Đ.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods 2022, 12, 107. [Google Scholar] [CrossRef]
- Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae Based Production of Single-Cell Protein. Curr. Opin. Biotechnol. 2022, 75, 102705. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The Potentials and Challenges of Using Microalgae as an Ingredient to Produce Meat Analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Meng, D.; Zhou, Z.; Zhang, Y.; Yang, R. Yeast Proteins: The Novel and Sustainable Alternative Protein in Food Applications. Trends Food Sci. Technol. 2023, 135, 190–201. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Farooq, S.; Alhamoud, Y.; Li, C.; Zhang, H. A Review on Mycoprotein: History, Nutritional Composition, Production Methods, and Health Benefits. Trends Food Sci. Technol. 2022, 121, 14–29. [Google Scholar] [CrossRef]
- Derbyshire, E.; Ayoob, K.-T. Mycoprotein: Nutritional and Health Properties. Nutr. Today 2019, 54, 7–15. [Google Scholar] [CrossRef]
- Finnigan, T.J.; Wall, B.T.; Wilde, P.J.; Stephens, F.B.; Taylor, S.L.; Freedman, M.R. Mycoprotein: The Future of Nutritious Nonmeat Protein, a Symposium Review. Curr. Dev. Nutr. 2019, 3, nzz021. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; Delange, J. Fungal Protein—What Is It and What Is the Health Evidence? A Systematic Review Focusing on Mycoprotein. Front. Sustain. Food Syst. 2021, 5, 581682. [Google Scholar] [CrossRef]
- Wiebe, M. Myco-Protein from Fusarium Venenatum: A Well-Established Product for Human Consumption. Appl. Microbiol. Biotechnol. 2002, 58, 421–427. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Khosravi-Darani, K. Response Surface Methodology for Mycoprotein Production by Fusarium Venenatum ATCC 20334. J. Bioprocess. Biotech. 2011, 1, 1. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Hosseini, S.M.; Assarehzadegan, M.; Khosravi-Darani, K.; Hosseini, H. Safety Assays and Nutritional Values of Mycoprotein Produced by Fusarium venenatum IR372C from Date Waste as Substrate. J. Sci. Food Agric. 2020, 100, 4433–4441. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Nair, R.B.; Andersson, D.; Lennartsson, P.R.; Taherzadeh, M.J. Vegan-Mycoprotein Concentrate from Pea-Processing Industry Byproduct Using Edible Filamentous Fungi. Fungal Biol. Biotechnol. 2018, 5, 5. [Google Scholar] [CrossRef]
- Karimi, S.; Mahboobi Soofiani, N.; Lundh, T.; Mahboubi, A.; Kiessling, A.; Taherzadeh, M.J. Evaluation of Filamentous Fungal Biomass Cultivated on Vinasse as an Alternative Nutrient Source of Fish Feed: Protein, Lipid, and Mineral Composition. Fermentation 2019, 5, 99. [Google Scholar] [CrossRef]
- Gmoser, R.; Fristedt, R.; Larsson, K.; Undeland, I.; Taherzadeh, M.J.; Lennartsson, P.R. From Stale Bread and Brewers Spent Grain to a New Food Source Using Edible Filamentous Fungi. Bioengineered 2020, 11, 582–598. [Google Scholar] [CrossRef]
- Borujeni, N.E.; Karimi, K.; Denayer, J.F.M.; Kumar, R. Apple Pomace Biorefinery for Ethanol, Mycoprotein, and Value-Added Biochemicals Production by Mucor Indicus. Energy 2022, 240, 122469. [Google Scholar] [CrossRef]
- Gamarra-Castillo, O.; Echeverry-Montaña, N.; Marbello-Santrich, A.; Hernández-Carrión, M.; Restrepo, S. Meat Substitute Development from Fungal Protein (Aspergillus oryzae). Foods 2022, 11, 2940. [Google Scholar] [CrossRef]
- Rousta, N.; Hellwig, C.; Wainaina, S.; Lukitawesa, L.; Agnihotri, S.; Rousta, K.; Taherzadeh, M.J. Filamentous Fungus Aspergillus Oryzae for Food: From Submerged Cultivation to Fungal Burgers and Their Sensory Evaluation—A Pilot Study. Foods 2021, 10, 2774. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the Microbial Community of Japanese Koji and Miso: A Review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lu, X.; Xing, L.; Ho, S.W.A.; Kwan, H.S. Genomic and Transcriptomic Comparison of Aspergillus oryzae Strains: A Case Study in Soy Sauce Koji Fermentation. J. Ind. Microbiol. Biotechnol. 2018, 45, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Daba, G.M.; Mostafa, F.A.; Elkhateeb, W.A. The Ancient Koji Mold (Aspergillus oryzae) as a Modern Biotechnological Tool. Bioresour. Bioprocess. 2021, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of Okara (Soybean Residue) for Food and Nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Privatti, R.T.; Rodrigues, C.E.D.C. An Overview of the Composition, Applications, and Recovery Techniques of the Components of Okara Aimed at the Biovalorization of This Soybean Processing Residue. Food Rev. Int. 2023, 39, 726–749. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mat, K.; Ishigaki, G.; Akashi, R. A Review of Okara (Soybean Curd Residue) Utilization as Animal Feed: Nutritive Value and Animal Performance Aspects. Anim. Sci. J. 2021, 92, e13594. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.-Y.; Liu, S.-Q. Soy Whey: More than Just Wastewater from Tofu and Soy Protein Isolate Industry. Trends Food Sci. Technol. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Cappuccino, J.G.; Welsh, C.T. Microbiology Books a La Carte Edition; Benjamin-Cummings Pub Co.: Menlo Park, CA, USA, 2016; ISBN 978-0-13-429867-2. [Google Scholar]
- Yassein, A.S.; Hassan, M.M.; Elamary, R.B. Prevalence of Lipase Producer Aspergillus Niger in Nuts and Anti-Biofilm Efficacy of Its Crude Lipase against Some Human Pathogenic Bacteria. Sci. Rep. 2021, 11, 7981. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, H.K.; Pandya, N.D. Screening, Identification of Alkaline Proteases Producing Fungi from Soil of Different Habitats of Amalner Tahsil [Maharashtra] and Their Applications. Int. J. Appl. Sci. Biotechnol. 2017, 5, 397–402. [Google Scholar] [CrossRef]
- Sukmawati, D.; Arman, Z.; Sondana, G.A.; Fikriyah, N.N.; Hasanah, R.; Afifah, Z.N.; Balqis, M.; Enshasy, H.E.; Husna, S.N.A.; Rahayu, S.; et al. Potential Amylase-Producing Yeast Isolated from Indigenous Fermented Beverages Originating from Bali, Indonesia. J. Phys. Conf. Ser. 2019, 1402, 055021. [Google Scholar] [CrossRef]
- Serna Saldívar, S.R.O. Cereal Grains: Laboratory Reference and Procedures Manual; Food Preservation Technology Series; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-5565-2. [Google Scholar]
- Wikandari, R.; Tanugraha, D.R.; Yastanto, A.J.; Manikharda; Gmoser, R.; Teixeira, J.A. Development of Meat Substitutes from Filamentous Fungi Cultivated on Residual Water of Tempeh Factories. Molecules 2023, 28, 997. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Zamani, A.; Taherzadeh, M.J. Edible Protein Production by Filamentous Fungi Using Starch Plant Wastewater. Waste Biomass Valorization 2019, 10, 2487–2496. [Google Scholar] [CrossRef]
- El-Enshasy, H.; Kleine, J.; Rinas, U. Agitation Effects on Morphology and Protein Productive Fractions of Filamentous and Pelleted Growth Forms of Recombinant Aspergillus Niger. Process Biochem. 2006, 41, 2103–2112. [Google Scholar] [CrossRef]
- Veiter, L.; Rajamanickam, V.; Herwig, C. The Filamentous Fungal Pellet—Relationship between Morphology and Productivity. Appl. Microbiol. Biotechnol. 2018, 102, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Jankovic, V.S.; Vucelic-Radovic, B.V. Bioactive Proteins and Energy Value of Okara as a Byproduct in Hydrothermal Processing of Soy Milk. J. Agric. Food Chem. 2013, 61, 9210–9219. [Google Scholar] [CrossRef]
- Asghar, A.; Afzaal, M.; Saeed, F.; Ahmed, A.; Ateeq, H.; Shah, Y.A.; Islam, F.; Hussain, M.; Akram, N.; Shah, M.A. Valorization and Food Applications of Okara (Soybean Residue): A Concurrent Review. Food Sci. Nutr. 2023, 11, 3631–3640. [Google Scholar] [CrossRef]
- Rukmi, I.; Pujiyanto, S.; Mulyani, N.S.; Faidah, N.; Ayu, L. The Effect of Coconut Water and Tofu Wastewater as Nitrogen Source on the Production of Alkali Protease from Aspergillus Flavus DUCC K225. J. Phys. Conf. Ser. 2019, 1217, 012127. [Google Scholar] [CrossRef]
- Widyarani; Butar Butar, E.S.; Dara, F.; Hamidah, U.; Sriwuryandari, L.; Hariyadi, H.R.; Sintawardani, N. Distribution of Protein Fractions in Tofu Whey Wastewater and Its Potential Influence on Anaerobic Digestion. IOP Conf. Ser. Earth Environ. Sci. 2019, 277, 012012. [Google Scholar] [CrossRef]
- Song, X.; Qi, X.; Hao, B.; Qu, Y. Studies of Substrate Specificities of Lipases from Different Sources. Eur. J. Lipid Sci. Technol. 2008, 110, 1095–1101. [Google Scholar] [CrossRef]
- Griebeler, N.; Polloni, A.E.; Remonatto, D.; Arbter, F.; Vardanega, R.; Cechet, J.L.; Di Luccio, M.; De Oliveira, D.; Treichel, H.; Cansian, R.L.; et al. Isolation and Screening of Lipase-Producing Fungi with Hydrolytic Activity. Food Bioprocess Technol. 2011, 4, 578–586. [Google Scholar] [CrossRef]
- Shreya; Bhati, N.; Sharma, A.K. Isolation, Screening, and Optimization of Culture Parameters for Enhanced Lipase Production from Aspergillus Eucalypticola Strain CBS 122,712 and Its Application. Vegetos 2022, 36, 70–78. [Google Scholar] [CrossRef]
- Geoffry, K.; Achur, R.N. Screening and Production of Lipase from Fungal Organisms. Biocatal. Agric. Biotechnol. 2018, 14, 241–253. [Google Scholar] [CrossRef]
- El-Nahrawy, S. Culture Conditions for Production of Cellulase by Aspergillus Tubingensis KY615746 Using Rice Straw Waste. Environ. Biodivers. Soil Secur. 2017. [Google Scholar] [CrossRef]
- Dutt, D.; Kumar, A. Optimization of Cellulase Production under Solid-State Fermentation by Aspergillus Flavus (AT-2) and Aspergillus Niger (AT-3) and Its Impact on Stickies and Ink Particle Size of Sorted Office Paper. Cellul. Chem. Technol. 2014, 48, 285–298. [Google Scholar]
- Makhuvele, R.; Ncube, I.; Jansen Van Rensburg, E.L.; La Grange, D.C. Isolation of Fungi from Dung of Wild Herbivores for Application in Bioethanol Production. Braz. J. Microbiol. 2017, 48, 648–655. [Google Scholar] [CrossRef]
- Svensson, S.E.; Bucuricova, L.; Ferreira, J.A.; Souza Filho, P.F.; Taherzadeh, M.J.; Zamani, A. Valorization of Bread Waste to a Fiber- and Protein-Rich Fungal Biomass. Fermentation 2021, 7, 91. [Google Scholar] [CrossRef]
- Beauvais, A.; Schmidt, C.; Guadagnini, S.; Roux, P.; Perret, E.; Henry, C.; Paris, S.; Mallet, A.; Prévost, M.-C.; Latgé, J.P. An Extracellular Matrix Glues Together the Aerial-Grown Hyphae of Aspergillus Fumigatus. Cell. Microbiol. 2007, 9, 1588–1600. [Google Scholar] [CrossRef]
- Ibrahim, D. Effect of Agitation Speed on the Morphology of Aspergillus niger HFD5A-1 Hyphae and Its Pectinase Production in Submerged Fermentation. World J. Biol. Chem. 2015, 6, 265. [Google Scholar] [CrossRef]
- Darah, I.; Sumathi, G.; Jain, K.; Lim, S.H. Influence of Agitation Speed on Tannase Production and Morphology of Aspergillus niger FETL FT3 in Submerged Fermentation. Appl. Biochem. Biotechnol. 2011, 165, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M. Fungal Morphology and Metabolite Production in Submerged Mycelial Processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef]
- Sar, T.; Larsson, K.; Fristedt, R.; Undeland, I.; Taherzadeh, M.J. Demo-Scale Production of Protein-Rich Fungal Biomass from Potato Protein Liquor for Use as Innovative Food and Feed Products. Food Biosci. 2022, 47, 101637. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Ghasemian, A.; Saraeian, A.; Resalati, H.; Lennartsson, P.R.; Taherzadeh, M.J. Using Spent Sulfite Liquor for Valuable Fungal Biomass Production by Aspergilus Oryzae. Nord. Pulp Pap. Res. J. 2017, 32, 630–638. [Google Scholar] [CrossRef]
- Karimi, S.; Mahboobi Soofiani, N.; Mahboubi, A.; Ferreira, J.A.; Lundh, T.; Kiessling, A.; Taherzadeh, M.J. Evaluation of Nutritional Composition of Pure Filamentous Fungal Biomass as a Novel Ingredient for Fish Feed. Fermentation 2021, 7, 152. [Google Scholar] [CrossRef]
- Pleissner, D.; Kwan, T.H.; Lin, C.S.K. Fungal Hydrolysis in Submerged Fermentation for Food Waste Treatment and Fermentation Feedstock Preparation. Bioresour. Technol. 2014, 158, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, C.; Xu, X.; Kang, F.; Chen, Y.; Zhou, M. Components Analysis and Flour Preparation of Tofu Whey. Adv. J. Food Sci. Technol. 2016, 12, 574–578. [Google Scholar] [CrossRef]
- Fung, W.-Y.; Woo, Y.-P.; Liong, M.-T. Optimization of Growth of Lactobacillus Acidophilus FTCC 0291 and Evaluation of Growth Characteristics in Soy Whey Medium: A Response Surface Methodology Approach. J. Agric. Food Chem. 2008, 56, 7910–7918. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.B.; Taherzadeh, M.J. Valorization of Sugar-to-Ethanol Process Waste Vinasse: A Novel Biorefinery Approach Using Edible Ascomycetes Filamentous Fungi. Bioresour. Technol. 2016, 221, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Sar, T.; Ozturk, M.; Taherzadeh, M.J.; Ferreira, J.A. New Insights on Protein Recovery from Olive Oil Mill Wastewater through Bioconversion with Edible Filamentous Fungi. Processes 2020, 8, 1210. [Google Scholar] [CrossRef]
- Uwineza, C.; Mahboubi, A.; Atmowidjojo, A.; Ramadhani, A.; Wainaina, S.; Millati, R.; Wikandari, R.; Niklasson, C.; Taherzadeh, M.J. Cultivation of Edible Filamentous Fungus Aspergillus Oryzae on Volatile Fatty Acids Derived from Anaerobic Digestion of Food Waste and Cow Manure. Bioresour. Technol. 2021, 337, 125410. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Gani, A.; Mulana, F.; Daimon, H. Treatment and Utilization of Industrial Tofu Waste in Indonesia. Asian J. Chem. 2016, 28, 501–507. [Google Scholar] [CrossRef]
- Xu, D.; Li, C.; Zhao, M.; Fengc, Y.; Sun, L.; Wang, Y. Assessment on the Improvement of Soy Sauce Fermentation by Aspergillus Oryzae HG76. Biocatal. Agric. Biotechnol. 2013, 2, 344–351. [Google Scholar] [CrossRef]
- Lee, S.-M.; Chen, Z.-Y.; Sheu, S.-C.; Chen, C.-W. Optimizing Brewing Beer Production Using Aspergillus oryzae Solid-State Fermentation of Sorghum Koji as an Adjunct. Int. J. Food Prop. 2023, 26, 3065–3081. [Google Scholar] [CrossRef]
- Yuan, H.-W.; Zhang, C.; Chen, S.-Y.; Zhao, Y.; Tie, Y.; Yin, L.-G.; Chen, J.; Wu, Q.-D.; Wang, Y.-T.; Xu, Z.; et al. Effect of Different Moulds on Oenological Properties and Flavor Characteristics in Rice Wine. LWT 2023, 173, 114201. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, J.; Wang, L.; Dong, M.; Xia, X. Biotransformation of Soy Whey into a Novel Functional Beverage by Cordyceps Militaris SN-18. Food Prod. Process. Nutr. 2021, 3, 13. [Google Scholar] [CrossRef]
- Liang, J.; Xu, N.; Nedele, A.-K.; Rigling, M.; Zhu, L.; Zhang, Y.; Stöppelmann, F.; Hannemann, L.; Heimbach, J.; Kohlus, R.; et al. Upcycling of Soy Whey with Ischnoderma benzoinum toward Production of Bioflavors and Mycoprotein. J. Agric. Food Chem. 2023, 71, 9070–9079. [Google Scholar] [CrossRef] [PubMed]
- Arini, A.M.S.; Afifah, D.N.; Dieny, F.F. The Effect of Tempeh Gembus Substitution on Protein Content, Calcium, Protein Digestibility and Organoleptic Quality of Meatballs. Curr. Res. Nutr. Food Sci. J. 2019, 7, 828–841. [Google Scholar] [CrossRef]
- Pilafidis, S.; Tsouko, E.; Sougleri, G.; Diamantopoulou, P.; Gkatzionis, K.; Ioannou, Z.; Sarris, D. Submerged Cultivation of Selected Macro-Fungi to Produce Mycelia Rich in β-Glucans and Other Bioactive Compounds, Valorizing Side Streams of the Food Industry. Carbon Resour. Convers. 2023, 7, 100198. [Google Scholar] [CrossRef]
- Ahlborn, J.; Stephan, A.; Meckel, T.; Maheshwari, G.; Rühl, M.; Zorn, H. Upcycling of Food Industry Side Streams by Basidiomycetes for Production of a Vegan Protein Source. Int. J. Recycl. Org. Waste Agric. 2019, 8, 447–455. [Google Scholar] [CrossRef]
| Microorganism | Zone of Activity (mm) | Activity (U/mL) | ||
|---|---|---|---|---|
| Amylase | Protease | Lipase | Cellulase | |
| Aspergillus oryzae | 9 ± 1.45 | 1.3 ± 0.47 | 0 | 0 |
| Pseudomonas aeruginosa | 2 ± 0 | 6.3 ± 1.25 | 3 ± 0 | n.a. |
| Trichoderma sp. | n.a. | n.a. | n.a. | 3.64 ± 0.12 |
| Retentate Concentration (g DW/L) | Retentate Protein Content | |||
|---|---|---|---|---|
| Media | Fungal Biomass | Residual Soy Pulp | Total Retentate | (mg/mg DW) |
| Okara (control) | n.d. | 12.86 ± 0.21 | 12.86 ± 0.21 a | 0.109 ± 0.01 a |
| Okara (A. oryzae) | n.d. | n.d. | 7.77 ± 0.4 b | 0.213 ± 0.04 b |
| Soy Whey (control) | n.a. | n.a. | n.a. | n.a. |
| Soy Whey (A. oryzae) | 0.867 ± 0.09 | n.a. | 0.867 ± 0.09 c | 0.531 ± 0.04 c |
| Parameters | Agitated | Static |
|---|---|---|
| Biomass concentration (g DW/L) | n.d. | 2.02 ± 0.06 |
| Residual soy pulp concentration (g DW/L) | n.d. | 9.16 ± 0.04 |
| Total retentate concentration (g DW/L) | 7.77 ± 0.4 a | 11.18 ± 0.08 b |
| Biomass protein (mg/mg DW) | n.d. | 0.271 ± 0.008 |
| Soy pulp protein (mg/mg DW) | n.d. | 0.106 ± 0.01 |
| Total retentate protein (mg/L) | 1653 ± 32.6 a | 1518 ± 16.5 b |
| Parameters | Initial | Final Agitated | Final Static |
|---|---|---|---|
| pH | 5.88 ± 0.03 a | 8.79 ± 0.02 b | 8.78 ± 0.04 b |
| Glucose (mg/L) | 49.89 ± 2.58 a | 6.43 ± 0.5 b | 2.08 ± 0.11 b |
| Fructose (mg/L) | 97.24 ± 2.5 a | 2.48 ± 0.15 b | 3.4 ± 0.33 b |
| Sucrose (mg/L) | 196.41 ± 6.35 a | 5.17 ± 0.33 b | 4.13 ± 0.29 b |
| Filtrate Protein (mg/L) | 754 ± 54.3 a | 1044 ± 43.7 b | 1555 ± 107 c |
| Total Protein Content (mg/L) * | 2529 ± 82.6 a | 2696 ± 11 a | 3071 ± 61.4 b |
| Parameters | Initial | Final Agitated | Final Static |
|---|---|---|---|
| pH | 3.71 ± 0.01 a | 9.38 ± 0.03 b | 8.4 ± 0.17 c |
| Glucose (mg/L) | 1.35 ± 0.11 a | 2.63 ± 0.4 b | 1.89 ± 0.42 ab |
| Fructose (mg/L) | 27.82 ± 1.95 a | 1.67 ± 0.16 b | 1.18 ± 0.03 b |
| Sucrose (mg/L) | 31.29 ± 2.6 a | 13.42 ± 0.12 b | 8.94 ± 0.66 b |
| Filtrate Protein (mg/L) | 787 ± 16.9 a | 317 ± 4.25 b | 222 ± 3.29 c |
| Total Protein Content (mg/L) * | 787 ± 16.9 a | 777 ± 29 b | 388 ± 0.64 b |
| Parameters | Agitated | Static |
|---|---|---|
| Biomass concentration (g DW/L) | 0.867 ± 0.09 a | 0.783 ± 0.02 a |
| Biomass protein (mg/mg DW) | 0.531 ± 0.004 a | 0.213 ± 0.005 b |
| Biomass protein (mg/L) | 459.8 ± 33 a | 166.9 ± 3.6 b |
| Parameters | Okara | Soy Whey | ||
|---|---|---|---|---|
| Agitated | Static | Agitated | Static | |
| Biomass yield/substrate (mg DW/g DW) | n.d. | 114.7 ± 3.68 a | 408.8 ± 47.1 b | 369.2 ± 9.9 b |
| Biomass protein yield/substrate (mg DW/g DW) | n.d. | 31.07 ± 0.88 a | 216.9 ± 15.7 b | 78.73 ± 1.68 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devanthi, P.V.P.; Pratama, F.; Pramanda, I.T.; Bani, M.D.; Kadar, A.D.; Kho, K. Exploring the Potential of Aspergillus oryzae for Sustainable Mycoprotein Production Using Okara and Soy Whey as Cost-Effective Substrates. J. Fungi 2024, 10, 555. https://doi.org/10.3390/jof10080555
Devanthi PVP, Pratama F, Pramanda IT, Bani MD, Kadar AD, Kho K. Exploring the Potential of Aspergillus oryzae for Sustainable Mycoprotein Production Using Okara and Soy Whey as Cost-Effective Substrates. Journal of Fungi. 2024; 10(8):555. https://doi.org/10.3390/jof10080555
Chicago/Turabian StyleDevanthi, Putu Virgina Partha, Ferren Pratama, Ihsan Tria Pramanda, Mario Donald Bani, Adinda Darwati Kadar, and Katherine Kho. 2024. "Exploring the Potential of Aspergillus oryzae for Sustainable Mycoprotein Production Using Okara and Soy Whey as Cost-Effective Substrates" Journal of Fungi 10, no. 8: 555. https://doi.org/10.3390/jof10080555
APA StyleDevanthi, P. V. P., Pratama, F., Pramanda, I. T., Bani, M. D., Kadar, A. D., & Kho, K. (2024). Exploring the Potential of Aspergillus oryzae for Sustainable Mycoprotein Production Using Okara and Soy Whey as Cost-Effective Substrates. Journal of Fungi, 10(8), 555. https://doi.org/10.3390/jof10080555






