Characterization, Molecular Mechanism of Prochloraz-Resistance in Fusarium fujikuroi and Development of Loop-Mediated Isothermal Amplification Rapid Detection Technique Based on the S312T Genotype of Resistances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Resistance Monitoring and Sensitivity Determination of Fusarium fujikuroi to Prochloraz
2.2. Characterization of Prochloraz-Resistant Isolates and Prochloraz-Sensitive Isolates
2.2.1. Mycelial Growth Rate
2.2.2. In Vitro Conidial Production and Germination
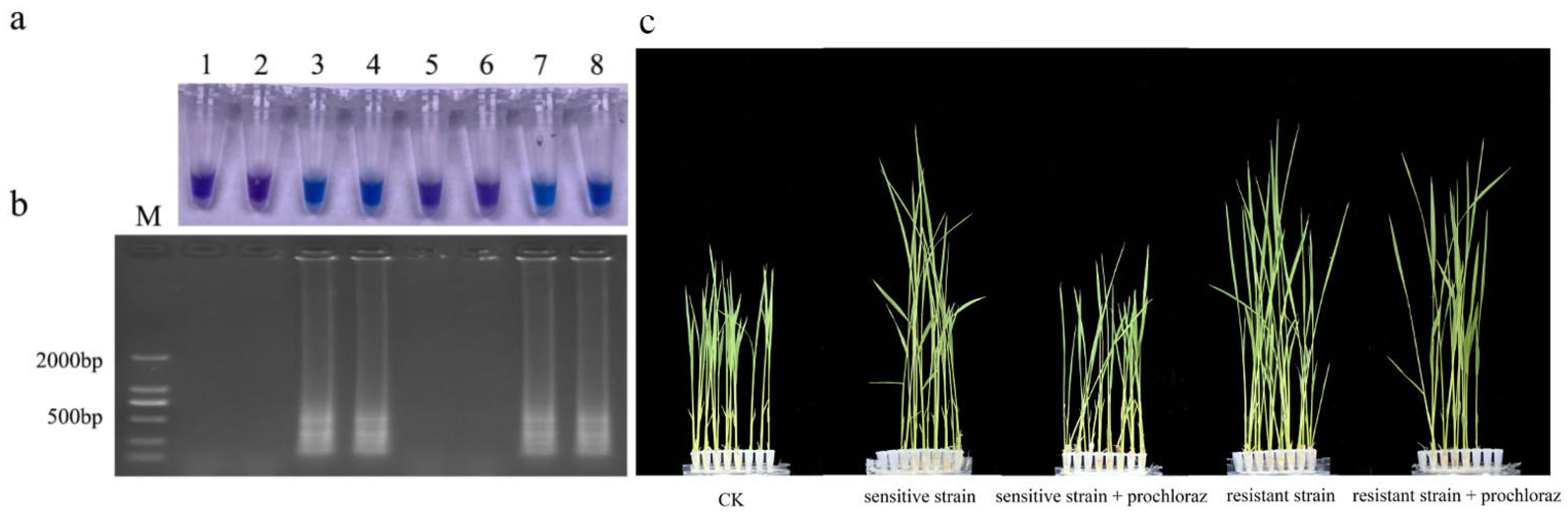
2.2.3. Pathogenicity on Rice Seedlings
2.3. Cloning and Sequencing of Ffcyp51b in Resistant and Sensitive Isolates
2.4. Primer Design
2.5. PCR Amplification and Sequencing
2.6. Detection of LAMP Specificity
2.7. Detection of LAMP Sensitivity
2.8. Detection Limit of Resistance Frequency in Different Sensitive: Resistant Isolate Ratios
2.9. Detection of Resistant Isolates from Rice Seeds and Seedlings
3. Results
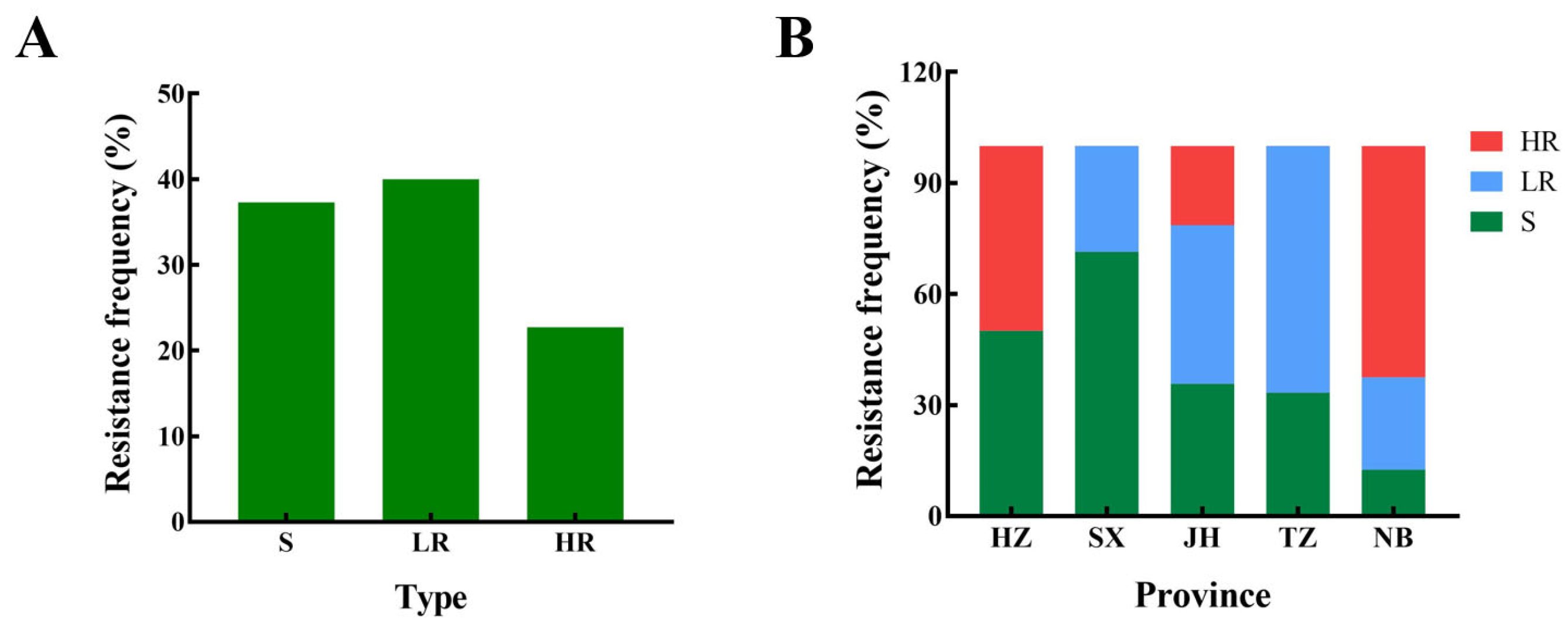
3.1. Resistance of F. fujikuroi to Prochloraz
3.2. Characterization of Prochloraz-Resistant Isolates
3.3. Sequence Analysis of Ffcyp51 in F. fujikuroi
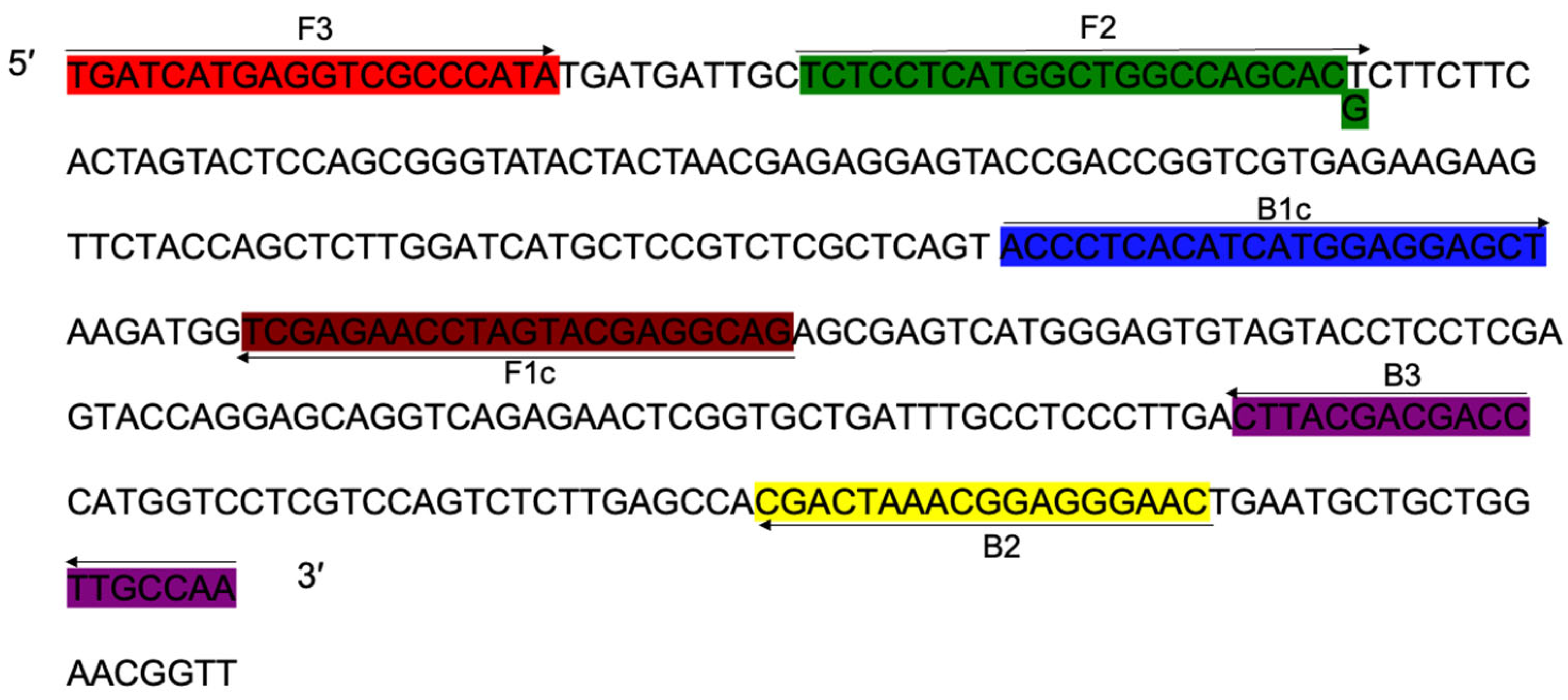
3.4. Primer Design
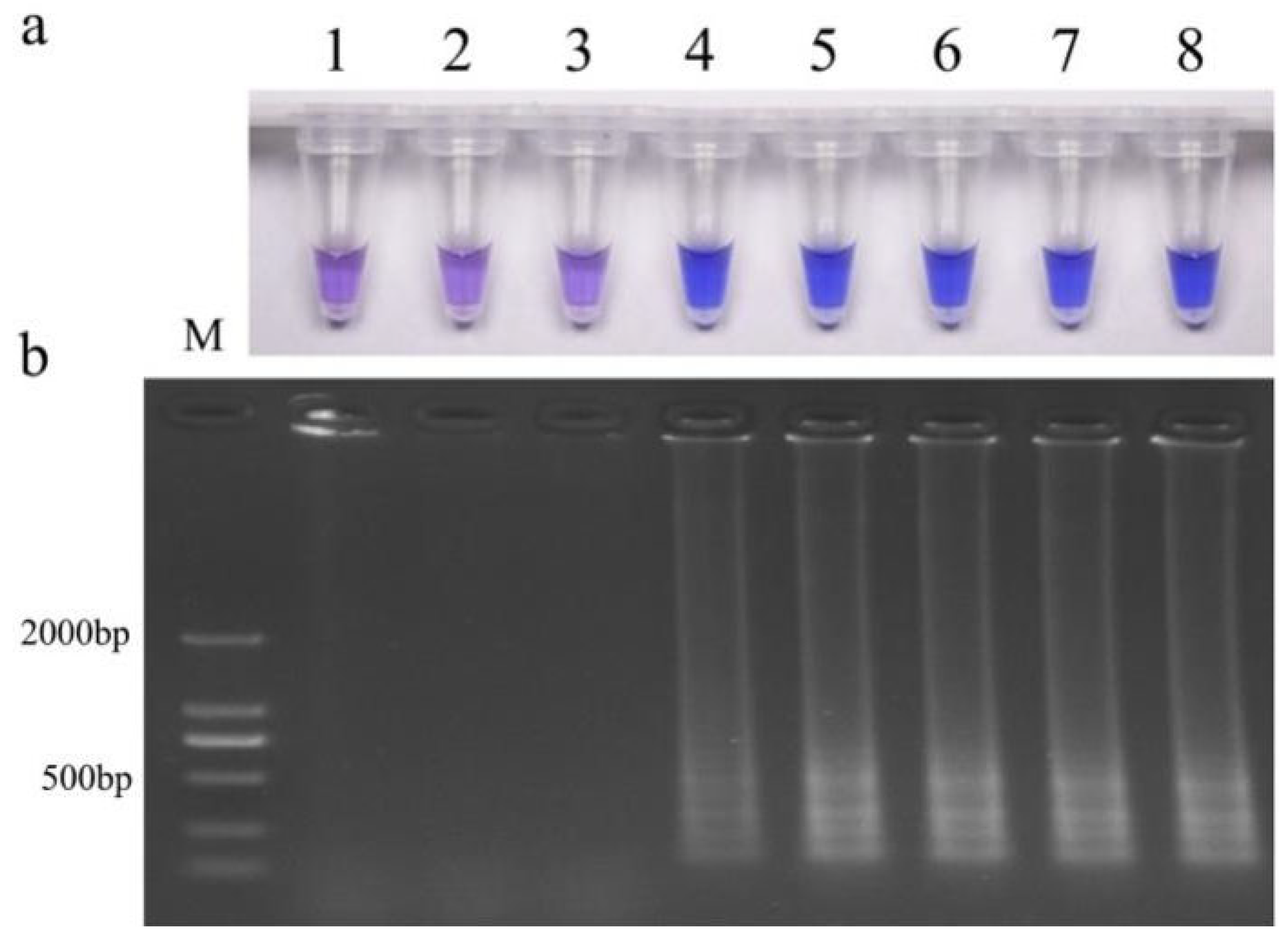
3.5. LAMP Specificity
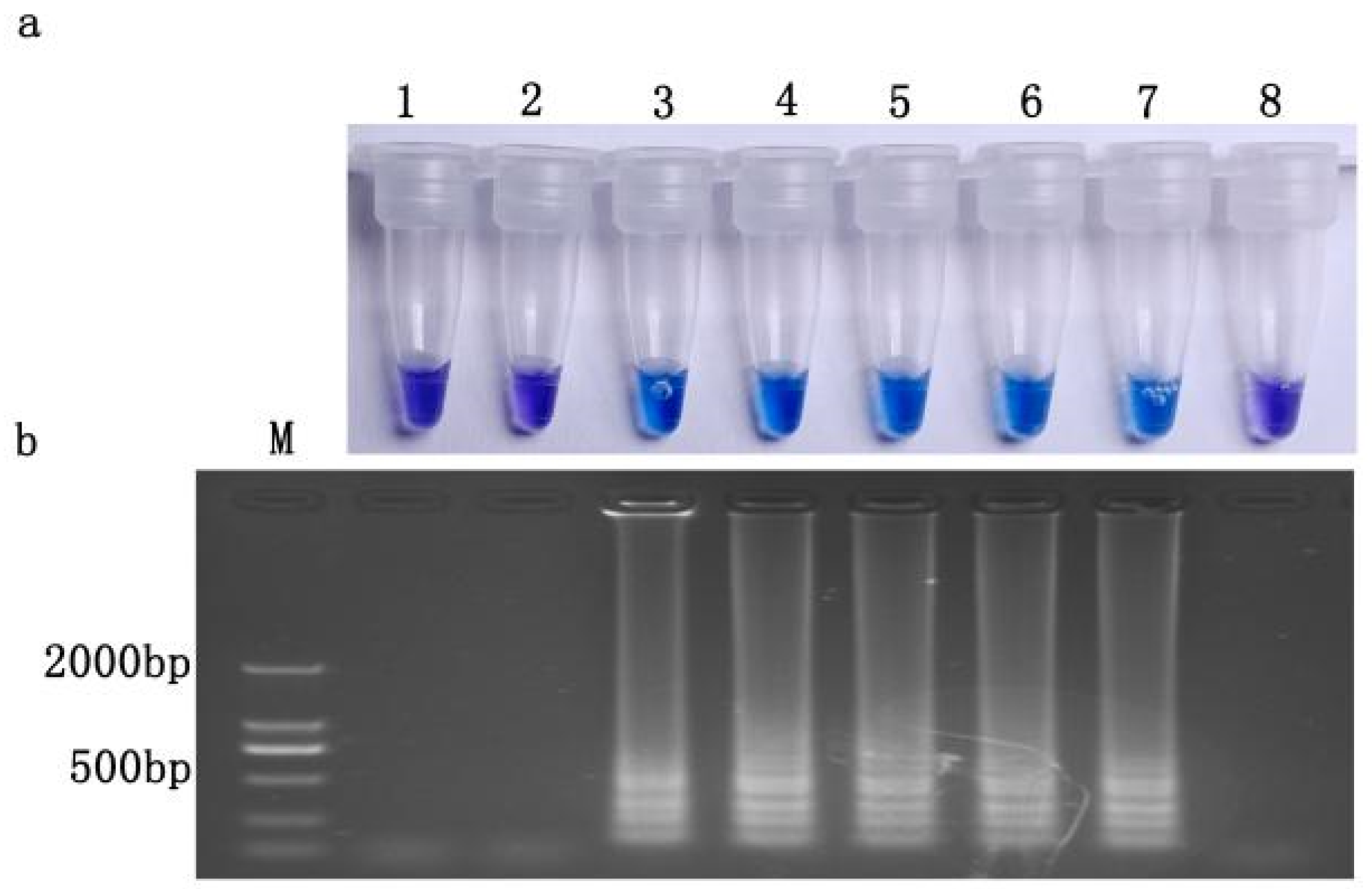
3.6. LAMP Sensitivity
3.7. Detection Limit of Resistance Frequency
3.8. Detection of Prochloraz-Resistant Isolates from Seeds and Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amatulli, M.T.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Molecular identification of Fusarium spp. associated with bakanae disease of rice in Italy and assessment of their pathogenicity. Plant Pathol. 2010, 59, 839–844. [Google Scholar] [CrossRef]
- Sultana, S.; Kitajima, M.; Kobayashi, H.; Nakagawa, H.; Shimizu, M.; Kageyama, K.; Suga, H. A natural variation of fumonisin gene cluster associated with fumonisin production difference in Fusarium fujikuroi. Toxins 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Kitajima, M.; Nagumo, R.; Tsukiboshi, T.; Uegaki, R.; Nakajima, T.; Kushiro, M.; Nakagawa, H.; Shimizu, M.; Kageyama, K.; et al. A single nucleotide polymorphism in the translation elongation factor 1α gene correlates with the ability to produce fumonisin in Japanese Fusarium fujikuroi. Fungal Biol. 2014, 118, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Pedrozo, M.R.; Fenoglio, M.J.; Little, C. First report of seedborne Fusarium fujikuroi and its potential to cause pre- and post-emergent damping-off on soybean (Glycine max) in the United States. Plant Dis. 2015, 22, 2–4. [Google Scholar] [CrossRef]
- Shen, Y.; Xiao, D.; Hu, X.; Wen, R. First report of leaf spot on Lasia spinosa caused by Fusarium fujikuroi in China. Plant Dis. 2020, 104, 2525. [Google Scholar] [CrossRef]
- Nur, I.M.Z.; Razak, A.; Salleh, B. Bakanae disease of rice in Malaysia and Indonesia: Etiology of the causal agent based on morphological, physiological and pathogenicity characteristics. J. Plant Prot. Res. 2008, 48, 475–485. [Google Scholar]
- Nicolli, C.P.; Haidukowski, M.; Susca, A.; Gomes, L.B.; Logrieco, A.; Stea, G.; Del Ponte, E.M.; Moretti, A.; Pfenning, L.H. Fusarium fujikuroi species complex in Brazilian rice: Unveiling increased phylogenetic diversity and toxigenic potential. Int. J. Food Microbiol. 2020, 330, 108667. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.L.; Huang, K.J.; Chen, S.Y.; Kuo, Y.F. Detecting bakanae disease in rice seedlings by machine vision. Comput. Electron. Agric. 2016, 121, 404–411. [Google Scholar] [CrossRef]
- Piombo, E.; Bosio, P.; Acquadro, A.; Abbruscato, P.; Spadaro, D. Different phenotypes, similar genomes: Three newly sequenced Fusarium fujikuroi strains induce different symptoms in rice depending on temperature. Phytopathology 2020, 110, 656–665. [Google Scholar] [CrossRef]
- Matic, S.; Gullino, M.L.; Spadaro, D. The puzzle of bakanae disease through interactions between Fusarium fujikuroi and rice. Front. Biosci. 2017, 9, 333–344. [Google Scholar]
- Saito, H.; Sasaki, M.; Nonaka, Y.; Tanaka, J.; Tokunaga, T.; Kato, A.; Thuy, T.T.T.; Vang, L.V.; Tuong, L.M.; Kanematsu, S.; et al. Spray application of nonpathogenic Fusaria onto rice flowers controls bakanae disease (caused by Fusarium fujikuroi) in the next plant generation. Appl. Environ. Microb. 2021, 87, e01959–20. [Google Scholar] [CrossRef] [PubMed]
- Vinggaard, A.M.; Hass, U.; Dalgaard, M.; Andersen, H.R.; Bonefeld-Jørgensen, E.; Christiansen, S.; Laier, P.; Poulsen, M.E. Prochloraz: An imidazole fungicide with multiple mechanisms of action. Int. J. Androl. 2006, 9, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.P.; Li, J.S.; Wang, J.X.; Wu, L.Y.; Wang, Y.F.; Chen, C.J.; Zhou, M.G.; Hou, Y.P. Effects of the dinitroaniline fungicide fluazinam on Fusarium fujikuroi and rice. Pestic. Biochem. Physiol. 2018, 152, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.A.; McDonald, B.A.; Brunner, P.C. Mutations in the CYP51 gene reduce DMI sensitivity in Parastagonospora nodorum populations in Europe and China. Pest Manag. Sci. 2017, 73, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, Y.H.; Hong, S.K.; Lee, Y.K.; Nam, Y.J.; Lee, J.G.; Han, S. Monitoring for the resistance to prochloraz of Fusarium species causing bakanae disease in Korea. Korean J. Mycol. 2015, 43, 112–117. [Google Scholar] [CrossRef]
- Liu, Y.F.; Chen, Z.Y.; Zhou, B.H.; Liu, R.; Lu, J. Detection of resistance of Fusarium moniliforme to rice seed soaking agents in some rice areas of Jiangsu Province. Jiangsu J. Agri. 2002, 3, 190–192. [Google Scholar]
- Fan, J.; Urban, M.; Parker, J.E.; Brewer, H.C.; Kelly, S.L.; Hammond-Kosack, K.E.; Fraaije, B.A.; Liu, X.; Cools, H.J. Characterization of the sterol 14α-demethylases of Fusarium graminearum identifies a novel genus-specific CYP51 function. New Phytol. 2013, 198, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, J.; Yun, Y.; Yin, Y.; Ma, Z. A sterol C-14 reductase encoded by FgERG24B is responsible for the intrinsic resistance of Fusarium graminearum to amine fungicides. Microbiology 2011, 157, 1665–1675. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Loza-Reyes, E.; Atkins, S.L.; Fraaije, B.A. The CYP51C gene, a reliable marker to resolve interspecific phylogenetic relationships within the Fusarium species complex and a novel target for species-specific PCR. Int. J. Food Microbiol. 2010, 144, 301–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, C.X.; Zhai, X.Y.; Jamieson, P.A.; Zhang, C.Q. Mutation in cyp51b and overexpression of cyp51a and cyp51b confer multiple resistant to DMIs fungicide prochloraz in Fusarium fujikuroi. Pest Manag. Sci. 2021, 77, 824–833. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Q.; Yuan, K.; Li, Y.; Shi, M.; Miao, J.; Liu, X. Monitoring and characterization of prochloraz resistance in Fusarium fujikuroi in China. Pestic. Biochem. Physiol. 2022, 187, 105189. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ebihara, A.; Sakahara, Y.; Matsumoto, S.; Ueno, R.; Bao, W.; Kimura, M.; Fuji, S.I.; Shimizu, M.; Kageyama, K.; et al. Synergistic effect of amino acid substitutions in CYP51B for prochloraz resistance in Fusarium fujikuroi. Pestic. Biochem. Physiol. 2023, 189, 105291. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, H.; Mao, C.; Wu, J.; Zhang, C. Activity of a SDHI fungicide penflufen and the characterization of natural-resistance in Fusarium fujikuroi. Pestic. Biochem. Physiol. 2021, 179, 104960. [Google Scholar] [CrossRef] [PubMed]
- Buddhachat, K.; Sripairoj, N.; Ritbamrung, O.; Inthima, P.; Ratanasut, K.; Boonsrangsom, T.; Rungrat, T.; Pongcharoen, P.; Sujipuli, K. RPA-assisted Cas12a system for detecting pathogenic Xanthomonas oryzae, a causative agent for bacterial leaf blight disease in rice. Rice Sci. 2022, 29, 340–352. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- Wu, J.Y.; Hu, X.R.; Zhang, C.Q. Molecular detection of QoI resistance in Colletotrichum gloeosporioides causing strawberry anthracnose based on Loop-mediated isothermal amplification assay. Plant Dis. 2019, 103, 1319–1325. [Google Scholar] [CrossRef]
- Hou, Y.P.; Mao, X.W.; Lin, S.P.; Song, X.S.; Duan, Y.B.; Wang, J.X.; Zhou, M.G. Activity of a novel succinate dehydrogenase inhibitor fungicide pyraziflumid against Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2018, 145, 22–28. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, Y.; Li, T.; Zhao, D.; Cao, J.; Shi, Y.; Wang, J.; Zhou, M. Development of a rapid and high-throughput molecular method for detecting the F200Y mutant genotype in benzimidazole-resistant isolates of Fusarium asiaticum. Pest Manag. Sci. 2016, 72, 2128–2135. [Google Scholar] [CrossRef]
- Chen, S.; Schnabel, G.; Yuan, H.; Luo, C. LAMP detection of the genetic element ‘Mona’ associated with DMI resistance in Monilinia fructicola. Pest Manag. Sci. 2019, 75, 779–786. [Google Scholar] [CrossRef]
- Forcelini, B.B.; Lee, S.; Oliveira, M.S.; Peres, N.A. Development of high-throughput snp genotyping assays for rapid detection of strawberry Colletotrichum species and the G143A mutation. Phytopathology 2018, 108, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yan, L.; Liang, W.; Yang, Q. Paralogous Cyp51s mediate the differential sensitivity of Fusarium oxysporum to sterol demethylation inhibitors. Pest Manag. Sci. 2019, 75, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.; Van Diepeningen, A.D.; Curfs-Breuker, I.; De Hoog, G.S.; Meis, J.F. Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J. Antimicrob. Chemother. 2015, 70, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Denschlag, C.; Vogel, R.F.; Niessen, L. Hyd5 gene-based detection of the major gushing-inducing Fusarium spp. in a loop-mediated isothermal amplification (LAMP) assay. Int. J. Food Microbiol. 2012, 156, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Duan, X.; Peng, Y.; Rui, Y. Rapid detection of a novel B1-β-lactamase gene, blaAFM-1 using a loop-mediated isothermal amplification (LAMP) assay. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.Y.; Zhang, C.Q. Establishment of a rapid detection method for rice blast fungus based on one-step loop-mediated isothermal amplification (LAMP). Plant Dis. 2019, 103, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.F.; Tomlinson, J.; Hodgetts, J.; Spadaro, D.; Gullino, M.L.; Boonham, N. Development of loop-mediated isothermal amplification assays for the detection of seedborne fungal pathogens Fusarium fujikuroi and Magnaporthe oryzae in Rice Seed. Plant Dis. 2018, 102, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Waqas Younas, M.; Yang, J.; Zhu, H.; Miao, J.; Gu, B.; Liu, X. Characterization of prochloraz resistance in Fusarium fujikuroi from Heilongjiang province in China. Plant Dis. 2022, 106, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Myeong, U.K.; Hyo, S.; Kang, J.; Lee, Y.H.; Kim, H.T. Detection for the resistance of Fusarium spp. isolated from rice seeds to prochloraz and cross-resistance to other fungicides inhibiting sterol biosynthesis. Korean J. Pestic. Sci. 2008, 12, 276–281. [Google Scholar]
- Forcelini, B.B.; Rebello, C.S.; Wang, N.Y.; Peres, N.A. Fitness, competitive ability, and mutation stability of isolates of Colletotrichum acutatum from strawberry resistant to QoI fungicides. Phytopathology 2018, 108, 462–468. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Fraaije, B.A. Fitness penalties in the evolution of fungicide resistance. Phytopathology 2018, 56, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Wanjala, B.W.; Ateka, E.M.; Miano, D.W.; Fuentes, S.; Perez, A.; Low, J.W.; Kreuze, J.F. Loop-mediated isothermal amplification assays for on-site detection of the main sweet potato infecting viruses. J. Virol. Methods 2021, 298, 114301. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Yaqinuddin, A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med. Hypotheses 2020, 141, 109786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Dai, D.J.; Wang, H.D.; Zhang, C.Q. One-step loop-mediated isothermal amplification (LAMP) for the rapid and sensitive detection of Fusarium fujikuroi in bakanae disease through NRPS31, an important gene in the gibberellic acid bio-synthesis. Sci. Rep. 2019, 9, 3726. [Google Scholar] [CrossRef] [PubMed]
- Amira, M.B.; Faize, M.; Karlsson, M.; Dubey, M.; Frąc, M.; Panek, J.; Fumanal, B.; Gousset-Dupont, A.; Julien, J.L.; Chaar, H.; et al. Fungal x-intrinsic protein aquaporin from Trichoderma atroviride: Structural and functional considerations. Biomolecules 2021, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Shen, X.; Li, Z.; Wang, J.; Li, X.; Xu, Z.; Shen, Y.; Lei, Y.; Huang, X.; Wang, X.; et al. Antibody generation and rapid immunochromatography using time-resolved fluorescence microspheres for propiconazole: Fungicide abused as growth regulator in vegetable. Foods 2022, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Masiello, M.; Somma, S.; Ghionna, V.; Logrieco, A.F.; Moretti, A. In vitro and in field response of different fungicides against aspergillus flavus and Fusarium species causing ear rot disease of maize. Toxins 2019, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Hu, S.; Wu, Y.; Wu, C.; Yang, Y.; Gu, B.; Du, H. A sers platform for rapid detection of drug resistance of non-Candida albicans using Fe3O4@PEI and triangular silver nanoplates. Int. J. Nanomed. 2022, 17, 3531–3541. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, Z.; Gao, J.; Tian, E.; Yu, W.; Li, H.; Zhang, J.; Xie, R.; Zhao, X.; Chen, A. Rapid and visual identification of Chlorophyllum molybdites with loop-mediated isothermal amplification method. Front. Microbiol. 2021, 12, 638315. [Google Scholar] [CrossRef]


| Component (Concentration) | Final Concentration | Volume (μL) per 25 μL Reaction |
|---|---|---|
| Bst DNA (8 U·μL−1) | 0.16 U·μL−1 | 0.5 |
| 10× Thermo Pol | 2.5 | |
| Mg2+ (25 mM) | 4 mM | 4 |
| dNTP (10 mM) | 1 mM | 2.5 |
| F3/B3 (10 μM) | 0.3 μM | 0.75 |
| F1P/B1P (10 μM) | 0.8 μM | 2 |
| Betine (5 M) | 0.6 M | 3 |
| HNB (3.75 mM) | 150 μM | 1 |
| Sample DNA | 100 ng·μL−1 | 1 |
| ddH2O | 5 |
| Year | Fungus | Isolates | EC50 (μg/mL) | CODON | Resistance | |
|---|---|---|---|---|---|---|
| 312 | 511 | |||||
| 2018 | Fusarium fujikuroi | JS16 [20] | 0.033 | TCT (Ser) | S | |
| JH18 | 0.080 | TCT (Ser) | S | |||
| JH1 [20] | 2.258 | ACT (Thr) | R | |||
| JH8 | 2.390 | ACT (Thr) | R | |||
| SX23 [20] | 4.050 | ACT (Thr) | R | |||
| SX24 [20] | 1.530 | ACT (Thr) | R | |||
| SX30 [20] | 2.781 | ACT (Thr) | R | |||
| SX30JS16Cyp51b [20] | 0.098 | TCT (Ser) | S | |||
| JS16JH1Cyp51b [20] | 2.109 | ACT (Thr) | R | |||
| Ustilaginoidea virens | ACCC2711 | |||||
| 2022 | F. fujikuroi | F1 | 0.242 | ACT (Thr) | TCC(S) | R |
| F4 | 1.162 | ACT (Thr) | TCC(S) | R | ||
| F54 | 0.852 | ACT (Thr) | TCC(S) | R | ||
| F68 | 0.105 | ACT (Thr) | TCC(S) | R | ||
| F72 | 0.232 | ACT (Thr) | TCC(S) | R | ||
| F5 | 0.028 | TCT (Ser) | TTC(F) | S | ||
| F8 | 0.037 | TCT (Ser) | TTC(F) | S | ||
| F9 | 0.045 | TCT (Ser) | TTC(F) | S | ||
| F10 | 0.016 | TCT (Ser) | TTC(F) | S | ||
| F25 | 0.023 | TCT (Ser) | TTC(F) | S | ||
| Isolates | Mycelial Growth Rate (mm/d) * | Conidium Production (×106/mL) | Germination Rate (%) | ||
|---|---|---|---|---|---|
| 4 h | 8 h | 12 h | |||
| F9(S) ** | (10.19 ± 0.49) a | (8.23 ± 0.56) a | (9.78 ± 1.35) a | (44.50 ± 0.94) a | (90.89 ± 0.86) a |
| F10(S) | |||||
| F25(S) | |||||
| F59(S) | |||||
| F1(R) | (8.27 ± 0.073) b | (6.74 ± 0.60) b | (5.22 ± 2.38) b | (30.50 ± 2.08) b | (80.75 ± 1.46) b |
| F4(R) | |||||
| F68(R) | |||||
| F72(R) | |||||
| Isolates | Average Height (cm) * | Excessive Growth Rate (%) |
|---|---|---|
| CK | (15.30 ± 0.28) c | |
| F9(S) ** | (19.09 ± 0.40) b | (24.74 ± 0.11) b |
| F10(S) | ||
| F25(S) | ||
| F59(S) | ||
| F1(R) | (21.16 ± 0.38) a | (38.23 ± 0.29) a |
| F4(R) | ||
| F68(R) | ||
| F72(R) |
| Primer Name | Type | Sequence (5′-3′) |
|---|---|---|
| F3 | Forward outer | TGATCATGAGGTCGCCCATA |
| B3 | Backward outer | TTGGCAAGGTCGTCGTAAG |
| FIP (F1c-F2) | Forward inner primer | GACGGAGCATGATCCAAGAGCTTCATGGCTGGCCAGCACG |
| BIP (B1c-B2) | Backward inner primer | ACCCTCACATCATGGAGGAGCTTCAAGGGAGGCAAATCAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, C.; Dong, D.; Mao, C.; Zhang, Q.; Zhang, C. Characterization, Molecular Mechanism of Prochloraz-Resistance in Fusarium fujikuroi and Development of Loop-Mediated Isothermal Amplification Rapid Detection Technique Based on the S312T Genotype of Resistances. J. Fungi 2024, 10, 560. https://doi.org/10.3390/jof10080560
Ge C, Dong D, Mao C, Zhang Q, Zhang C. Characterization, Molecular Mechanism of Prochloraz-Resistance in Fusarium fujikuroi and Development of Loop-Mediated Isothermal Amplification Rapid Detection Technique Based on the S312T Genotype of Resistances. Journal of Fungi. 2024; 10(8):560. https://doi.org/10.3390/jof10080560
Chicago/Turabian StyleGe, Chenyang, Daixing Dong, Chengxing Mao, Qianqian Zhang, and Chuanqing Zhang. 2024. "Characterization, Molecular Mechanism of Prochloraz-Resistance in Fusarium fujikuroi and Development of Loop-Mediated Isothermal Amplification Rapid Detection Technique Based on the S312T Genotype of Resistances" Journal of Fungi 10, no. 8: 560. https://doi.org/10.3390/jof10080560






