Phenotypic and Genotypic Characterization of Resistance and Virulence Markers in Candida spp. Isolated from Community-Acquired Infections in Bucharest, and the Impact of AgNPs on the Highly Resistant Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains Identification and Analysis
2.2. Antibiotic Susceptibility Testing
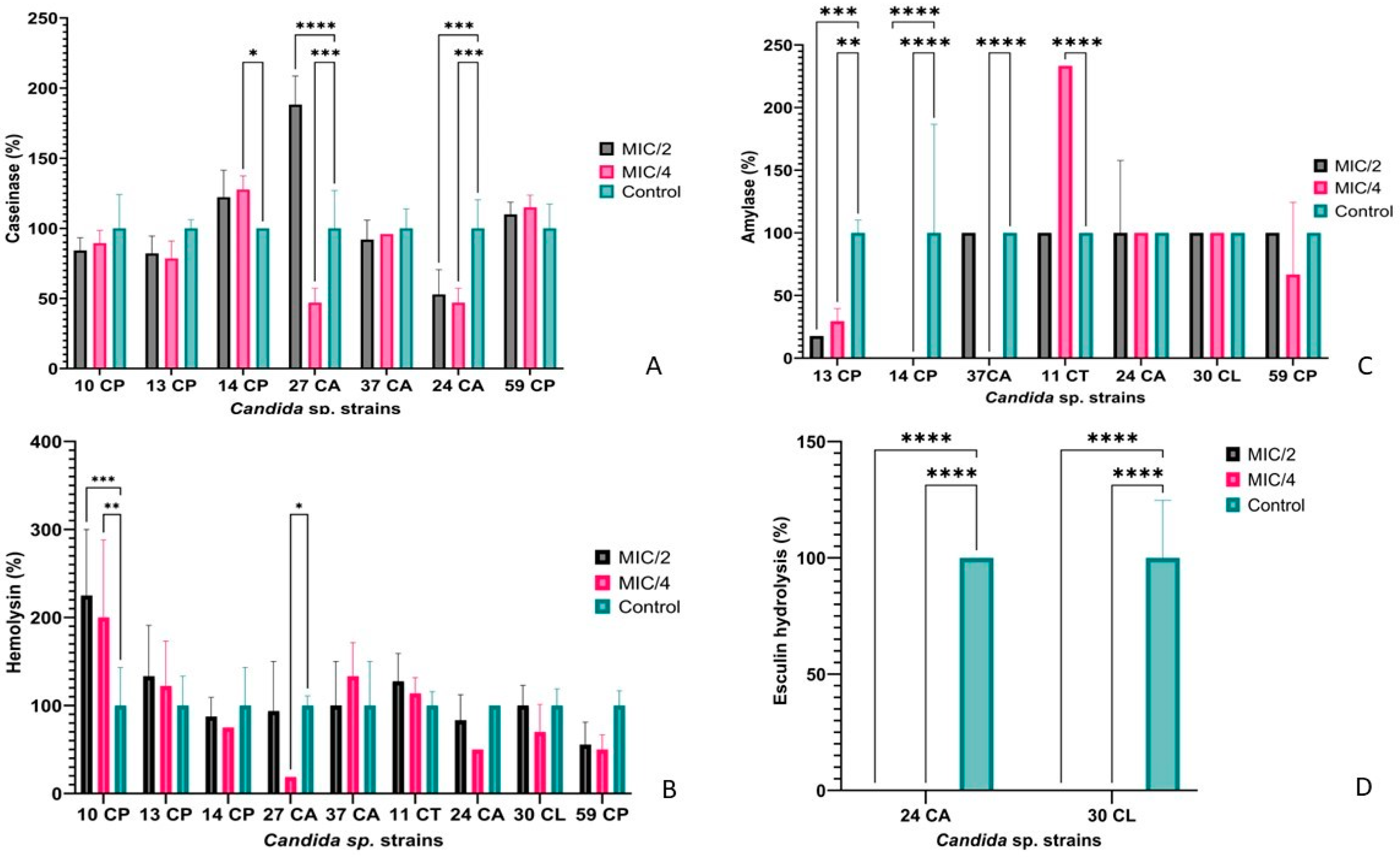
2.3. Soluble Virulence Factors: Enzyme and Organic Acid Production
2.4. Biofilm Formation Capacity
2.5. Investigation of Adherence Capacity on HeLa Cells
2.6. Genotypic Investigation of Virulence Markers Involved in Adherence, Lipolytic Activity, or Genes Encoding β-1,3-glucan Synthase
2.7. Evaluation of Antimicrobial and Anti-Biofilm Activity, and the Influence of AgNP Solutions (AgNPs) on Metabolic Activity
2.7.1. Synthesis and Characterization of AgNPs
2.7.2. Qualitative Antimicrobial Activity
2.7.3. Quantitative Antimicrobial Activity
2.7.4. The Influence of AgNPs on Adherence Capacity
2.7.5. The Influence of AgNPs on the Viability of Microorganisms in Biofilms
2.8. Extracellular Nitric Oxide Quantification
2.9. Statistical Analysis
3. Results
3.1. Phenotypic and Genotypic Features of Resistance and Virulence Markers in Candida spp. Isolates
3.2. Antimicrobial and Anti-Biofilm Activity, and the Impact of AgNP Solutions on the Adherence Capacity and Metabolic Activity
3.2.1. Qualitative and Quantitative Antimicrobial Activity of AgNPs against Candida spp. Strains
3.2.2. Anti-Biofilm Activity of AgNPs
3.2.3. The Impact of AgNPs on Metabolic Activity in Selected Candida spp. Isolates
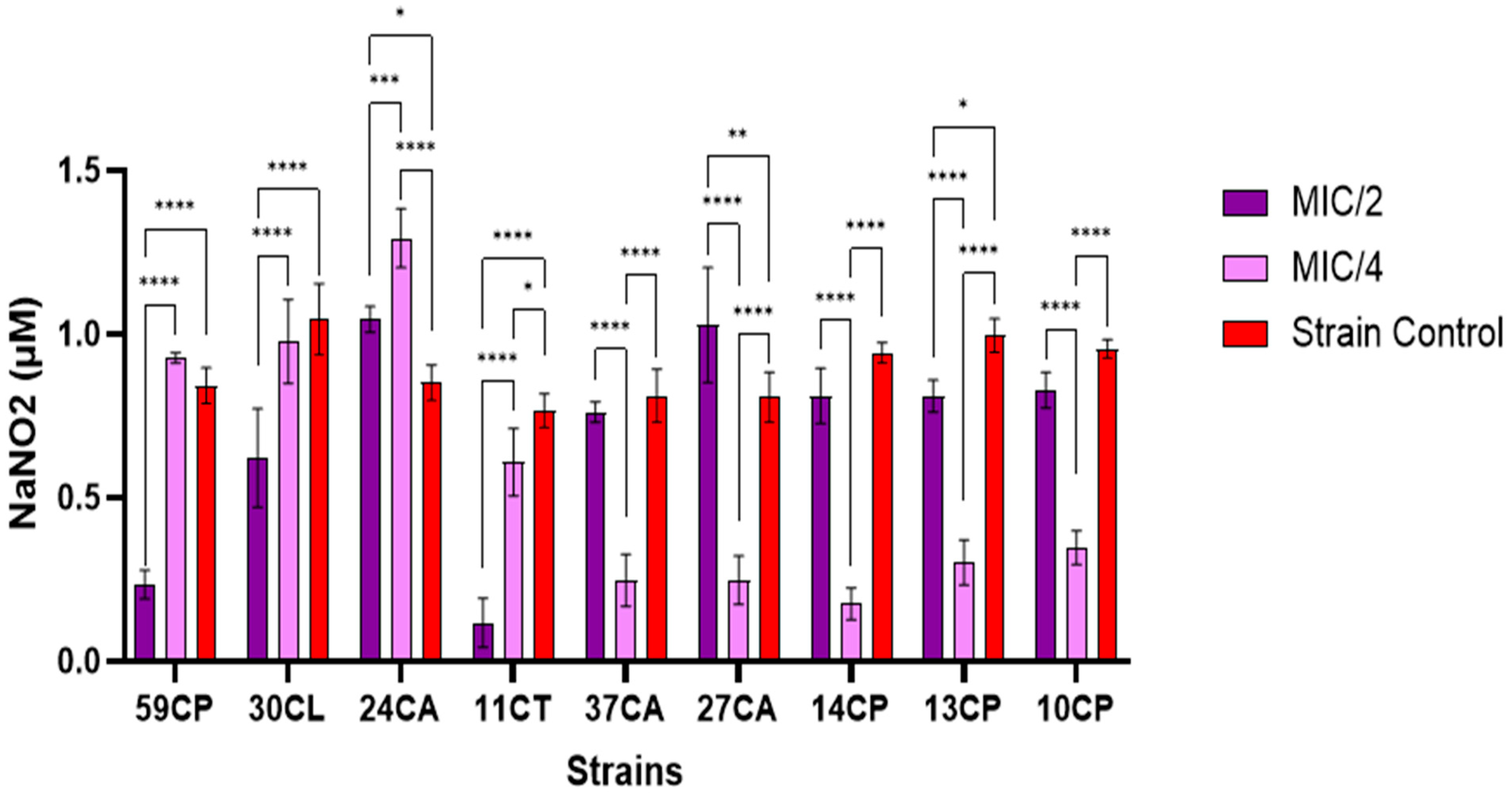
3.2.4. Extracellular Nitric Oxide Content
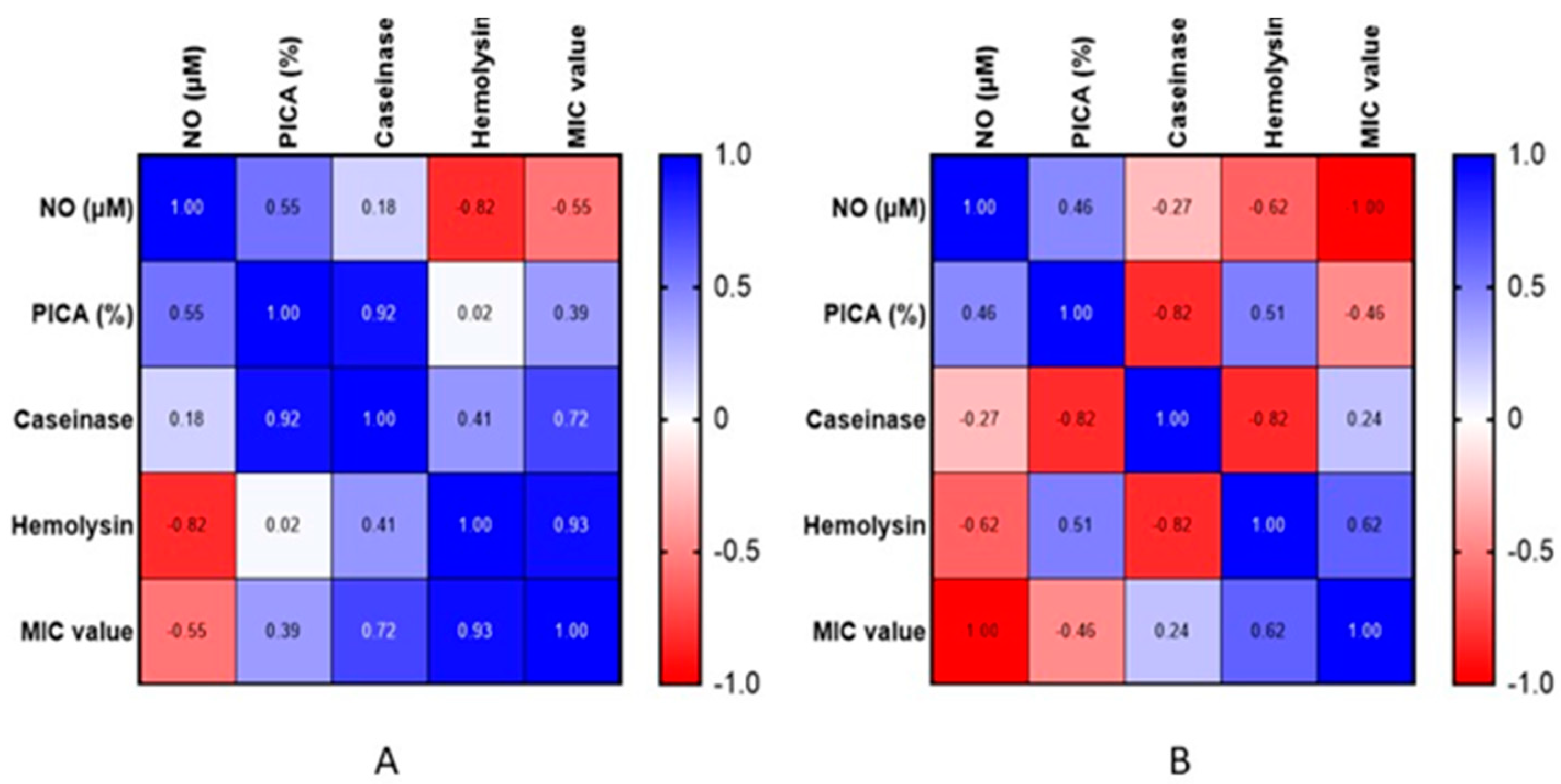
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef]
- Freitas, C.G.; Felipe, M.S. Candida albicans and Antifungal Peptides. Infect. Dis. Ther. 2023, 12, 2631–2648. [Google Scholar] [CrossRef]
- Ferngren, G.; Yu, D.; Unalan-Altintop, T.; Dinnétz, P.; Özenci, V. Epidemiological patterns of candidaemia: A comprehensive analysis over a decade. Mycoses 2024, 64, e13729. [Google Scholar] [CrossRef]
- Richardson, J.P.; Moyes, D.L. Adaptive immune responses to Candida albicans infection. Virulence 2015, 6, 327–337. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Esaiassen, E.; Fjalstad, J.W.; Juvet, L.K.; van den Anker, J.N.; Klingenberg, C. Antibiotic exposure in neonates and early adverse outcomes: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 1858–1870. [Google Scholar] [CrossRef]
- Czechowicz, P.; Nowicka, J.; Gościniak, G. Virulence Factors of Candida spp. and Host Immune Response Important in the Pathogenesis of Vulvovaginal Candidiasis. Int. J. Mol. Sci. 2022, 23, 5895. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Q.; Zhu, F.; An, Y. Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: A retrospective study from 2011 to 2017 in a teaching hospital in China. Antimicrob. Resist. Infect. Control 2019, 8, 89. [Google Scholar] [CrossRef]
- Saiprom, N.; Wongsuk, T.; Oonanant, W.; Sukphopetch, P.; Chantratita, N.; Boonsilp, S. Characterization of Virulence Factors in Candida Species Causing Candidemia in a Tertiary Care Hospital in Bangkok, Thailand. J. Fungi 2023, 9, 353. [Google Scholar] [CrossRef]
- Yang, Y.L. Virulence factors of Candida species. J. Microbiol. Immunol. Infect. 2003, 36, 223–228. [Google Scholar]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011, 19, 241–247. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 2000, 13, 122–143. [Google Scholar] [CrossRef]
- Tournu, H.; Van Dijck, P. Candida biofilms and the host: Models and new concepts for eradication. Int. J. Microbiol. 2012, 2012, 845352. [Google Scholar] [CrossRef]
- Maubon, D.; Garnaud, C.; Calandra, T.; Sanglard, D.; Corne, M. Resistance of Candida spp. to antifungal drugs in the ICU: Where are we now? Intensive Care Med. 2014, 40, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Mechanisms of echinocandin antifungal drug resistance. Ann. New York Acad. Sci. 2015, 1354, 1–11. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-resistant Candida: Epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 2017, 216 (Suppl. S3), S445–S451. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilm Development. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Nada, H.G.; El-Behery, R.R.; Gobara, M.; El-Sayyad, G.S. Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC Advances 2020, 10, 9274–9289. [Google Scholar] [CrossRef]
- Ford, C.B.; Funt, J.M.; Abbey, D.; Issi, L.; Guiducci, C.; Martinez, D.A.; Regev, A. The evolution of drug resistance in clinical isolates of Candida albicans. eLife 2015, 4, e00662. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline—Second Edition; CLSI document M44-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Wanjare, S.; Suryawanshi, R.; Bhadade, A.; Mehta, P. Utility of Chromagar Medium for Antifungal Susceptibility Testing of Candida Species Against Fluconazole and Voriconazole in Resource Constrained Settings. Bombay Hosp. J. 2015, 57, 147–152. [Google Scholar]
- Siopi, M.; Pachoulis, I.; Leventaki, S.; Spruijtenburg, B.; Meis, J.F.; Pournaras, S.; Vrioni, G.; Tsakris, A.; Meletiadis, J. Evaluation of the Vitek 2 system for antifungal susceptibility testing of Candida auris using a representative international panel of clinical isolates: Overestimation of amphotericin B resistance and underestimation of fluconazole resistance. J. Clin. Microbiol. 2024, 62, e01528-23. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A. Comparison of Etest with the CLSI broth microdilution method for antifungal susceptibility testing of common and emerging Candida spp. J. Clin. Microbiol. 2007, 45, 2197–2203. [Google Scholar]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanglard, D.; Odds, F.C. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2002, 2, 73–85. [Google Scholar] [CrossRef]
- Marichal, P.; Koymans, L.; Willemsens, S.; Bellens, D.; Verhasselt, P.; Luyten, W.; Borgers, M.; Ramaekers, F.C.; Odds, F.C.; Vanden Bossche, H. Contribution of mutations in the cytochrome P450 14α-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 1997, 143, 1291–1298. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Garcia-Effron, G.; Lass-Flörl, C.; Lopez, A.G.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M.; Perlin, D.S. Echinocandin susceptibility testing of Candida species: Comparison of results from disk diffusion and Etest with CLSI MIC data. J. Clin. Microbiol. 2010, 48, 1592–1599. [Google Scholar]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Calderone, R.A.; Fonzi, W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef]
- Buchan, B.W.; Ledeboer, N.A. Advances in identification of clinical yeast isolates by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, A.; Davidson, A.D.; Johnson, E.M.; Maiden, M.C.; Shaw, D.J.; Gow, N.A.; Odds, F.C. Multilocus sequence typing for differentiation of strains of Candida tropicalis. J. Clin. Microbiol. 2004, 42, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.; Sivalingam, V.; Tang, H.M.; Montgomery, J.M.; Chen, S.C.-A.; Halliday, C.L. Molecular Diagnostics for Invasive Fungal Diseases: Current and Future Approaches. J. Fungi 2024, 10, 447. [Google Scholar] [CrossRef]
- Tosiano, M.A.; Lanni, F.; Mitchell, A.P.; McManus, C.J. Roles of P-body factors in Candida albicans filamentation and stress response. Cold Spring Harb. Lab. 2024. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef]
- Czajka, K.M.; Venkataraman, K.; Brabant-Kirwan, D.; Santi, S.A.; Verschoor, C.; Appanna, V.D.; Singh, R.; Saunders, D.P.; Tharmalingam, S. Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species. Cells 2023, 12, 2655. [Google Scholar] [CrossRef]
- Czernel, G.; Bloch, D.; Matwijczuk, A.; Cieśla, J.; Kędzierska-Matysek, M.; Florek, M.; Gagoś, M. Biodirected Synthesis of Silver Nanoparticles Using Aqueous Honey Solutions and Evaluation of Their Antifungal Activity against Pathogenic Candida spp. Int. J. Mol. Sci. 2021, 22, 7715. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotech. 2015, 13, 91. [Google Scholar] [CrossRef]
- AlJindan, R.; AlEraky, D.M. Silver Nanoparticles: A Promising Antifungal Agent against the Growth and Biofilm Formation of the Emergent Candida auris. J. Fungi 2022, 8, 744. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alghofaily, M.; Alfraih, J.; Alsaud, A.; Almazrua, N.; Sumague, T.S.; Auda, S.H.; Alsalleeh, F. The Effectiveness of Silver Nanoparticles Mixed with Calcium Hydroxide against Candida albicans: An Ex Vivo Analysis. Microorganisms 2024, 12, 289. [Google Scholar] [CrossRef]
- Kim, K.J.; Sung, W.S.; Suh, B.K.; Moon, S.K.; Choi, J.S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Núñez, N.V.; Ixtepan-Turrent, L.; Rodríguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotech. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.R.; Silva, S.; Negri, M.; Gorup, L.F.; de Camargo, E.R.; Oliveira, R.; Henriques, M. Silver nanoparticles: Influence of stabilizing agent and diameter on antifungal activity against Candida albicans and Candida glabrata biofilms. Lett. Appl. Microbiol. 2013, 58, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Zbořil, R. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Corbu, V.M.; Dumbravă, A.Ş.; Marinescu, L.; Motelica, L.; Chircov, C.; Surdu, A.V.; Gheorghe-Barbu, I.; Pecete, I.; Balotescu, I.; Popa, M.; et al. Alternative mitigating solutions based on inorganic nanoparticles for the preservation of cultural heritage. Front. Mater. 2023, 10, 1272869. [Google Scholar] [CrossRef]
- Truşcă, B.S.; Gheorghe-Barbu, I.; Manea, M.; Ianculescu, E.; Barbu, I.C.; Măruțescu, L.G.; Dițu, L.-M.; Chifiriuc, M.-C.; Lazăr, V. Snapshot of Phenotypic and Molecular Virulence and Resistance Profiles in Multidrug-Resistant Strains Isolated in a Tertiary Hospital in Romania. Pathogens 2023, 12, 609. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Herlea, V.; Cernat, R.; Bulai, D.; Balotescu, M.; Moraru, M. Microbiologie Generala Manual de Lucrari Practice; Editura Universitatii Bucuresti: Bucharest, Romania, 2004. [Google Scholar]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. J. Pathol. Microbiol. Immunol. 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Sadik, O.; Ditu, L.M.; Gheorghe, I.; Holban, A.M.; Curutiu, C.; Gradisteanu Parcalabioru, G.; Avram, I.; Banu, O.; Chifiriuc, M.C. Phenotypic and genotypic evaluation of adherence and biofilm development in Candida albicans respiratory tract isolates from hospitalized patients. Rev. Română Med. Lab. 2019, 27, 73–84. [Google Scholar] [CrossRef]
- Csutak, O.; Stoica, I.; Vassu, T. Molecular identification and antimicrobial activity of two new Kluyveromyces lodderae and Saccharomyces cerevisiae strains. Biointerface Res. Appl. Chem. 2014, 4, 873–878. [Google Scholar]
- Corbu, V.M.; Gheorghe, I.; Marinaș, I.C.; Geană, E.I.; Moza, M.I.; Csutak, O.; Chifiriuc, M.C. Demonstration of Allium sativum Inhibitory Effect on Biodeteriogenic Microbial Strain Growth, Biofilm Development, and Enzymatic and Organic Acid Production. Molecules 2021, 26, 7195. [Google Scholar] [CrossRef]
- Muthamil, S.; Devi, V.A.; Balasubramaniam, B.; Balamurugan, K.; Pandian, S.K. Green synthesized silver nanoparticles demonstrating enhanced In Vitro and In Vivo antibiofilm activity against Candida spp. J. Basic Microbiol. 2018, 58, 343–357. [Google Scholar] [CrossRef]
- Dhabalia, D.; Ukkund, S.J.; Syed, U.T.; Uddin, W.; Kabir, M.A. Antifungal activity of biosynthesized silver nanoparticles from Candida albicans on the strain lacking the CNP41 gene. Mater. Res. Express 2020, 7, 125401. [Google Scholar] [CrossRef]
- Gheorghe-Barbu, I.; Corbu, V.M.; Vrancianu, C.O.; Marinas, I.C.; Popa, M.; Dumbravă, A.Ș.; Niță-Lazăr, M.; Pecete, I.; Muntean, A.A.; Popa, M.I.; et al. Phenotypic and Genotypic Characterization of Recently Isolated Multidrug-Resistant Acinetobacter baumannii Clinical and Aquatic Strains and Demonstration of Silver Nanoparticle Potency. Microorganisms 2023, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Trafny, E.A.; Lewandowski, R.; Zawistowska-Marciniak, I.; Stępińska, M. Use of MTT assay for determination of the biofilm formation capacity of microorganisms in metalworking fluids. World J. Microbiol. Biotechnol. 2013, 29, 1635–1643. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Cano Aristizábal, V.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Shahina, Z.; Ndlovu, E.; Persaud, O.; Sultana, T.; Dahms, T.E.S. Candida albicans Reactive Oxygen Species (ROS)-Dependent Lethality and ROS-Independent Hyphal and Biofilm Inhibition by Eugenol and Citral. Microbiol. Spectr. 2022, 10, e0318322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brocca, S.; Grandori, R.; Longhi, S.; Uversky, V. Liquid–Liquid Phase Separation by Intrinsically Disordered Protein Regions of Viruses: Roles in Viral Life Cycle and Control of Virus–Host Interactions. Int. J. Mol. Sci. 2020, 21, 9045. [Google Scholar] [CrossRef] [PubMed]
- Barraud, N.; Hassett, D.J.; Hwang, S.; Rice, S.A.; Kjelleberg, S.; Webb, J.S. Involvement of Nitric Oxide in Biofilm Dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef]
- Hu, Z.; Wessels, H.J.C.T.; van Alen, T.; Jetten, M.S.; Kartal, B. Nitric oxide-dependent anaerobic ammonium oxidation. Nat. Commun. 2019, 10, 1244. [Google Scholar] [CrossRef]
- Christofidou, M.; Spiliopoulou, A.; Vamvacopoulou, S.; Dimitracopoulos, G.; Anastassiou, E.; Junie, L.M.; Costache, C.; Colosi, I.; Ciobanca, P.T. Species distribution and antifungal susceptibility of Candida isolates collected from hospitalized patients in Romania and Greece. Sci. Parasitol. 2008, 1, 61–67. [Google Scholar]
- Taei, M.; Chadeganipour, M.; Mohammadi, R. An alarming rise of non-albicans Candida species and uncommon yeasts in the clinical samples; a combination of various molecular techniques for identification of etiologic agents. BMC Res. Notes 2019, 12, 779. [Google Scholar] [CrossRef]
- Das, K.H.; Mangayarkarasi, V.; Sen, M. Antifungal resistant in non-albicans Candida species are emerging as a threat to antenatal women with vulvovaginal candidiasis. Biomed. Pharmacol. J. 2019, 12, 1369–1378. [Google Scholar] [CrossRef]
- Liu, F.; Zhong, L.; Zhou, F.; Zheng, C.; Zhang, K.; Cai, J.; Zhou, H.; Tang, K.; Dong, Z.; Cui, W.; et al. Clinical features, strain distribution, antifungal resistance and prognosis of patients with non-albicans candidemia: A retrospective observational study. Infect. Drug Resist. 2021, 14, 3233–3246. [Google Scholar] [CrossRef]
- Aydın Kurç, M.; Kaya, A.D.; Erfan, G.; Albayrak, Ş. Distribution and antifungal susceptibility profiles of candida species isolated from dermatomycosis patients. J. Health Sci. Med. JHSM 2024, 7, 290–295. [Google Scholar] [CrossRef]
- Najee, H.; Gheorghe, I.; Pircalabioru, G.G.; Chifiriuc, M.C.; Lazar, V. Genotypic characterization of adhesion and biofilm development genes in Candida albicans strains isolated from different clinical specimens. Biointerface Res. Appl. Chem. 2018, 8, 3590–3593. [Google Scholar]
- Sadik, O.; Ditu, L.M.; Gheorghe, I.; Pircalabioru, G.G.; Bleotu, C.; Banu, O.; Merezeanu, N.; Lupu, A.C.; Lazar, V.; Chifiriuc, M.C. Adherence and Biofilm Formation in Candida albicans Strains Isolated from Different Infection Sites in Hospitalized Patients. Rev. Chim. 2017, 68, 2832–2835. [Google Scholar] [CrossRef]
- Singh, D.P.; Kumar Verma, R.; Sarswat, S.; Saraswat, S. Non-Candida albicans Candida species: Virulence factors and species identification in India. Curr. Med. Mycol. 2021, 7, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, G.; Ebrahimi-Rad, M.; Shams-Ghahfarokhi, M.; Jahanshiri, Z.; Ardakani, E.M.; Eslamifar, A.; Mousavi, S.F.; Razzaghi-Abyaneh, M. Cutaneous candidiasis in Tehran-Iran: From epidemiology to multilocus sequence types, virulence factors and antifungal susceptibility of etiologic Candida species. Iran J. Microbiol. 2019, 11, 267–279. [Google Scholar] [CrossRef]
- Mello, V.G.; Escudeiro, H.; Weckwerth, A.C.V.B.; Andrade, M.I.; Fusaro, A.E.; de Moraes, E.B.; Baptista, I.M.F.D. Virulence Factors and Antifungal Susceptibility in Candida Species Isolated from Dermatomycosis Patients. Mycopathologia 2020, 186, 71–80. [Google Scholar] [CrossRef]
- Nenoff, P.; Krüger, C.; Paasch, U.; Ginter-Hanselmayer, G. Mycology—An update Part 3: Dermatomycoses: Topical and systemic therapy. J. Dtsch. Dermatol. Ges. 2015, 13, 387–410. [Google Scholar] [CrossRef]
- Hassan, A.; Mansour, K.; Mahmoud, H. Biosynthesis of silver nanoparticles(Ag-Nps) (a model of metals) by Candida albicans and its antifungal activity on Some fungal pathogens (Trichophyton mentagrophytes and Candida albicans). N. Y. Sci. J. 2013, 6, 27–34. [Google Scholar]
- Choi, J.S.; Lee, J.W.; Shin, U.C.; Lee, M.W.; Kim, D.J.; Kim, S.W. Inhibitory activity of silver nanoparticles synthesized using Lycopersicon esculentum against biofilm formation in Candida species. Nanomaterials 2019, 9, 1512. [Google Scholar] [CrossRef]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Rohde, M.M.; Snyder, C.M.; Sloop, J.; Solst, S.R.; Donati, G.L.; Spitz, D.R.; Furdui, C.M.; Singh, R. The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Part. Fibre Toxicol. 2021, 18, 37. [Google Scholar] [CrossRef]
- Zielinska, E.; Tukaj, C.; Radomski, M.W.; Inkielewicz-Stepniak, I. Molecular Mechanism of Silver Nanoparticles-Induced Human Osteoblast Cell Death: Protective Effect of Inducible Nitric Oxide Synthase Inhibitor. PLoS ONE 2016, 11, e0164137. [Google Scholar] [CrossRef]
- Perween, N.; Khan, H.M.; Fatima, N. Silver nanoparticles: An upcoming therapeutic agent for the resistant Candida infections. J. Microbiol. Exp. 2019, 7, 49–54. [Google Scholar] [CrossRef]
- Ahamad, I.; Bano, F.; Anwer, R.; Srivastava, P.; Kumar, R.; Fatma, T. Antibiofilm Activities of Biogenic Silver Nanoparticles Against Candida albicans. Front. Microbiol. 2022, 12, 741493. [Google Scholar] [CrossRef] [PubMed]
- Masoumizadeh, M.; Madani, M.; Fatahian, S.; Shakib, P. Effect of Silver Nanoparticles (AgNPs) on Candida albicans, Candida dubliniensis and Candida guilliermondii. Curr. Drug Ther. 2022, 17, 50–55. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural Analysis of Candida albicans When Exposed to Silver Nanoparticles. PLoS ONE 2014, 9, e108876. [Google Scholar] [CrossRef]
- Górka, K.; Kubiński, K. Antifungal Activity against Human and Plant Mycopathogens, and Green Synthesis of Silver Nanoparticles Exhibiting Such Activity. Appl. Sci. 2024, 14, 115. [Google Scholar] [CrossRef]
- Elnagar Rasha, M.; Elshaer, M.; Abd El-Raouf, M.A. Silver Nanoparticles Display Inhibitory Effect against Drug-Resistant Pathogenic Candida Isolates from Different Clinical Specimens. Egypt. J. Bot. 2023, 63, 305–314. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, X.; Li, M.; Lu, Y.; Ai, C.; Jiang, C.; Liu, Y.; Pan, Z.; Shi, J. Antifungal activity of silver nanoparticles synthesized by iturin against Candida albicans In vitro and In Vivo. Appl. Microbiol. Biotechnol. 2021, 105, 3759–3770. [Google Scholar] [CrossRef]
- Rossoni, R.D.; Barbosa, J.O.; Vilela, S.F.; Jorge, A.O.; Junqueira, J.C. Comparison of the hemolytic activity between C. albicans and non-albicans Candida species. Braz. Oral Res. 2013, 27, 484–489. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Silva, S.; Negri, M.; Gorup, L.F.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver colloidal nanoparticles: Effect on matrix composition and structure of Candida albicans and Candida glabrata biofilms. J. Appl. Microbiol. 2013, 114, 1175–1183. [Google Scholar] [CrossRef]
- Jia, D.; Sun, W. Silver nanoparticles offer a synergistic effect with fluconazole against fluconazole-resistant Candida albicans by abrogating drug efflux pumps and increasing endogenous ROS. Infect. Genet. Evol. 2021, 93, 104937. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of Antifungal Properties of Metal Nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef]
- Falcão, C.M.C.; Andrade, A.; Holanda, V.N.; de Figueiredo, R.C.B.Q.; Ximenes, E.A.; Gomes, A.S.L. Activity of poly(methacrylic acid)-silver nanoparticles on fluconazole-resistant Candida albicans strains: Synergistic and cytotoxic effects. J. Appl. Microbiol. 2022, 132, 4300–4309. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Siddiqui, M.I. Anticandidal activity of biosynthesized silver nanoparticles: Effect on growth, cell morphology, and key virulence attributes of Candida species. Int. J. Nanomed. 2019, 14, 4667–4679. [Google Scholar] [CrossRef]
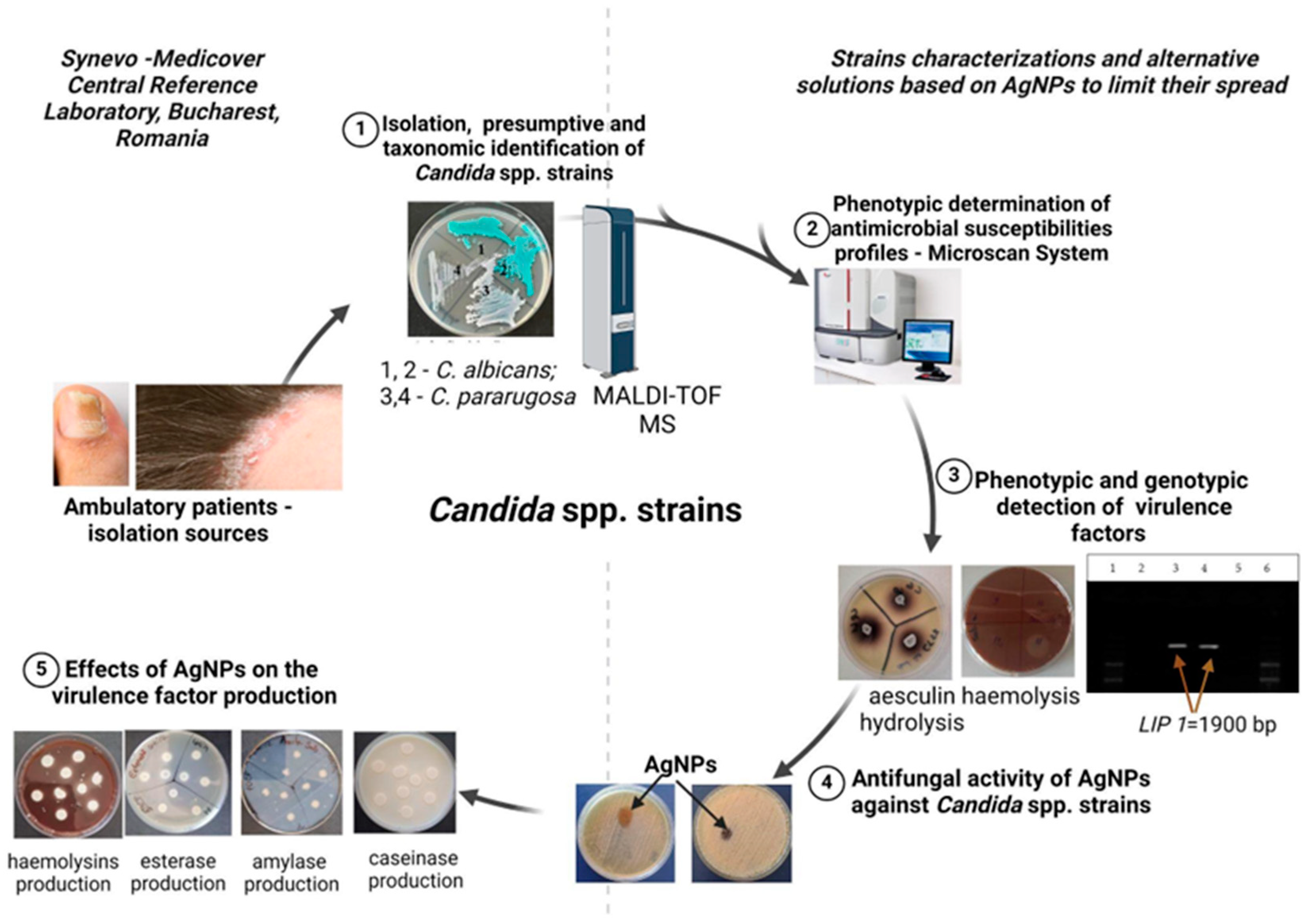

| Species/Strain Number | Isolation Sources | Patient Ages | Sex | Resistance Profiles |
|---|---|---|---|---|
| C. albicans (n = 18) | Left and right foot, right and left hand nail, face scales | 26–73 years | Male and female | Amphotericin B (CMI > 2 µg/mL) (22%), clotrimazole (CMI > 1 µg/mL) (27%), nystatin (CMI > 1.25 µg/mL) (17%), econazole (CMI > 2 µg/mL) (11%), miconazole (CMI > 2 µg/mL) (6%), |
| C. parapsilosis (n = 28) | Left and right foot, right and left hand nail, scalp | 25–77 years | Male and female | Amphotericin B (CMI > 2 µg/mL) (29%), clotrimazole (CMI > 1 µg/mL) (24%), nystatin (CMI > 1.25 µg/mL) (18%), econazole (CMI > 2 µg/mL) (11%), miconazole (CMI > 2 µg/mL) (7%), itraconazole (CMI > 1 µg/mL) (4%), |
| C. tropicalis (n = 2) | Left foot | 31–72 years | Male and female | Clotrimazole (CMI > 1 µg/mL), econazole (CMI > 2 µg/mL) and nystatin (CMI > 1.25 µg/mL) (50/50/%) |
| C. guilliermondii (n = 6) | Left foot, right and left hand nail, scalp | 42–75 years | Male and female | Nystatin (17%) (CMI > 1.25 µg/mL) |
| C. lusitaniae (n = 2) | Right hand nail | 42 to 54 years | Female | Nystatin (CMI > 1.25 µg/mL) and amphotericin B (CMI > 2 µg/mL) (100%) |
| C. krusei (n = 1) | Left hand nail | 55 years | Female | - |
| C. metapsilosis (n = 1) | Right hand nail | 31 years | Female | - |
| C. haemulonii (n = 1) | Left hand nail | 47 years | Female | Clotrimazole (CMI > 1 µg/mL) and econazole (CMI > 2 µg/mL) (100%) |
| C. famata (n = 1) | Left foot | 45 years | Female | - |
| C. pararugosa (n = 2) | Left and right hand nail | 15–28 years | Female | - |
| Species/Strain Number | Virulence Factors/Metabolic Enzymes | Genotypic Traits |
|---|---|---|
| VMs, Adherence, Lipolytic Activity | ||
| C. albicans (n = 18) | Aesculin hydrolysis, hemolysins, amylase, lipase | ALS1, ALS3, HWP1 |
| C. parapsilosis (n = 28) | Aesculin hydrolysis, caseinase hemolysins, amylase, lipase | N/A |
| C. tropicalis (n = 2) | Aesculin hydrolysis, caseinase hemolysins, amylase | LIP 1 |
| C. guilliermondii (n = 6) | Aesculin hydrolysis, caseinase hemolysins, amylase, lipase | N/A |
| C. lusitaniae (n = 2) | Aesculin hydrolysis, caseinase hemolysins, amylase, lipase | N/A |
| C. krusei (n = 1) | Aesculin hydrolysis, amylase | N/A |
| C. metapsilosis (n = 1) | Aesculin hydrolysis, caseinase | N/A |
| C. haemulonii (n = 1) | - | N/A |
| C. famata (n = 1) | Caseinase | N/A |
| C. pararugosa (n = 2) | Aesculin hydrolysis | N/A |
| Species/Strain Number | Biofilm Formation Capacity on Inert Substratum | Adherence Pattern to HeLa Cells | AIn |
|---|---|---|---|
| C. albicans (n = 18) | NP(4) | Diffuse adherence | 39.5% |
| W (6) | |||
| M (1) | |||
| S (7) | |||
| C. parapsilosis (n = 28) | NP (9) | Localized adherence | 50% |
| W (10) | |||
| S (9) | |||
| C. tropicalis (n = 2) | W(1) | Aggregative adherence | 35% |
| S (1) | |||
| C. guilliermondii (n = 6) | NP (3) | Localized adherence | 28.5% |
| W (3) | |||
| C. lusitaniae (n = 2) | S (2) | Not tested | Not tested |
| C. krusei (n = 1) | W (1) | Localized adherence | 26% |
| C. metapsilosis (n = 1) | NP (1) | Not tested | Not tested |
| C. haemulonii (n = 1) | NP (1) | Not tested | Not tested |
| C. famata (n = 1) | NP (1) | Not tested | Not tested |
| C. pararugosa (n = 2) | NP(1) | Not tested | Not tested |
| W(1) |
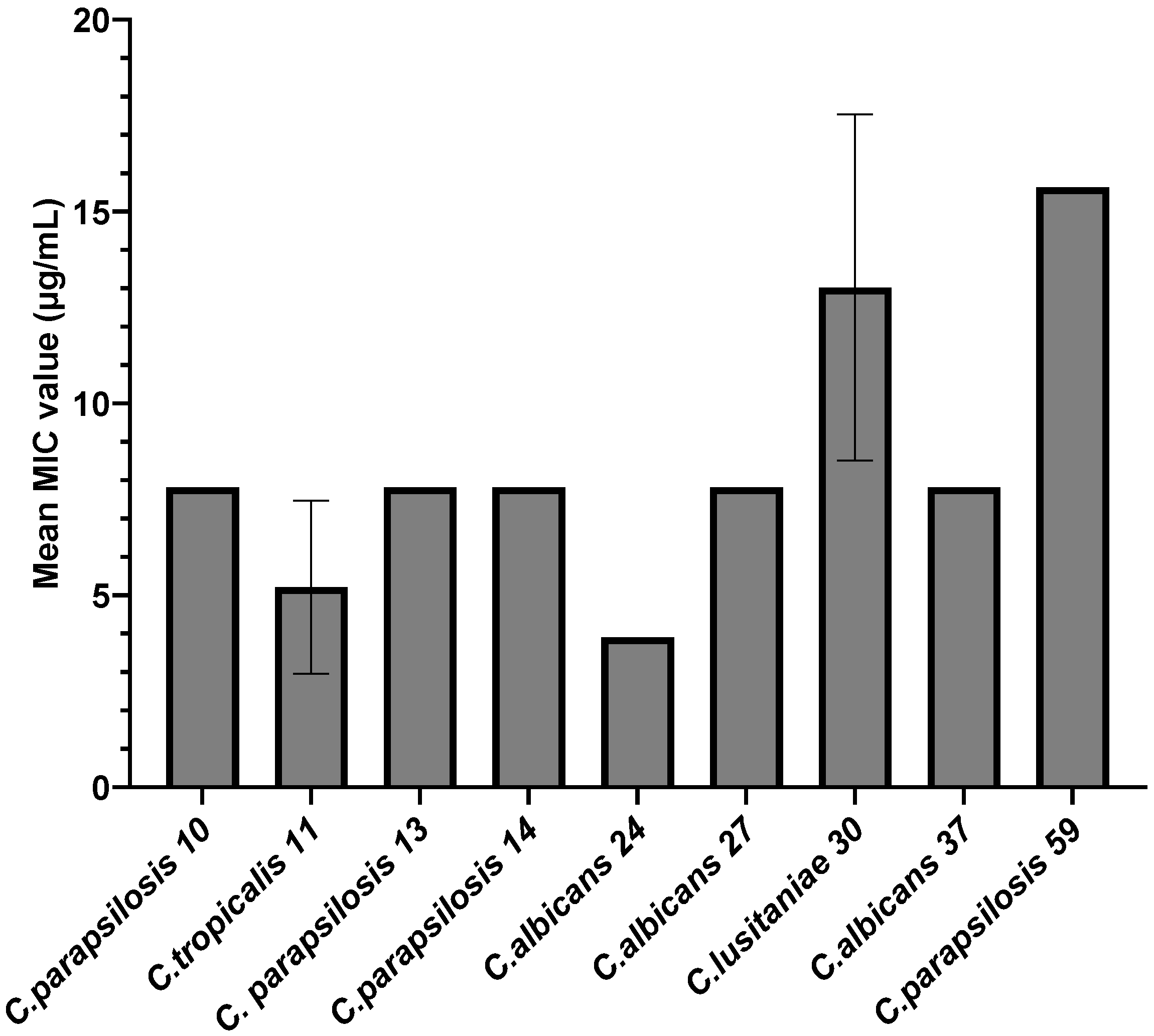
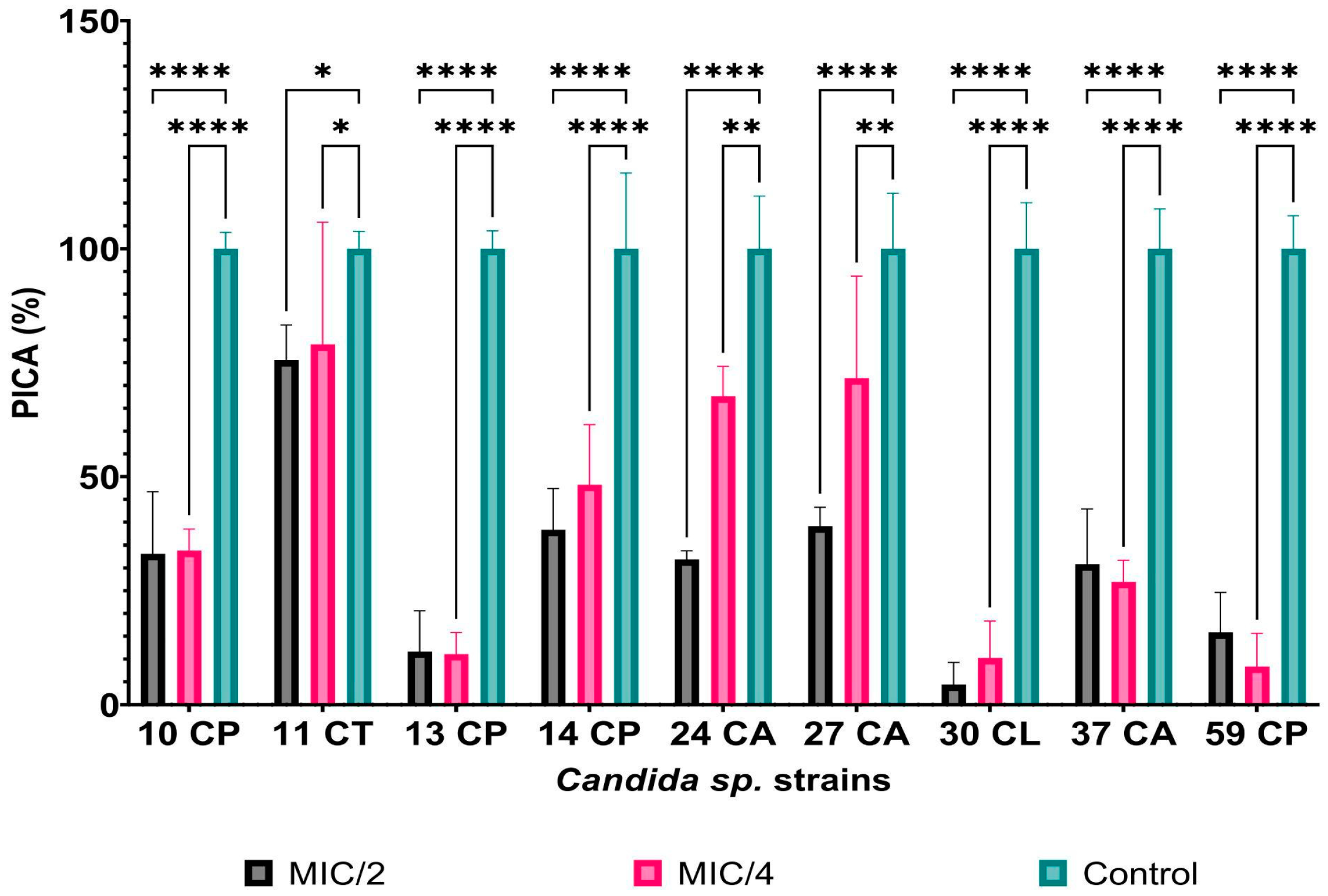
| Strain | MIC/2 (μg/mL) | PICV% | p-Value | MIC/4 (μg/mL) | PICV% | p-Value |
|---|---|---|---|---|---|---|
| 10 C. parapsilosis | 3.90 | 38.98 ± 0.51% | <0.0001 | 1.95 | 45.88 ± 1.37% | <0.0001 |
| 11 C. tropicalis | 2.60 | 18.72 ± 0.88% | <0.0001 | 1.30 | 75.26 ± 2.15% | <0.0001 |
| 13 C. parapsilosis | 3.90 | 44.95 ± 5.67% | <0.0001 | 3.90 | 65.23 ± 2.49% | <0.0001 |
| 14 C. parapsilosis | 3.90 | 6.43 ± 0.23% | <0.0001 | 1.95 | 54.80 ± 1.51% | <0.0001 |
| 24 C. albicans | 1.95 | 10.84 ± 0.34% | <0.0001 | 0.97 | 13.95 ± 0.28% | <0.0001 |
| 27 C. albicans | 3.90 | 0.10 ± 0.02% | <0.0001 | 1.95 | 46.82 ± 2.50% | <0.0001 |
| 30 C. lusitaniae | 6.51 | 0.39 ± 0.03% | <0.0001 | 3.25 | 66.69 ± 8.88% | <0.0001 |
| 37 C. albicans | 3.90 | 0.36 ± 0.07% | <0.0001 | 1.95 | 2.98 ± 0.07% | <0.0001 |
| 59 C. parapsilosis | 7.81 | 22.53 ± 2.30% | <0.0001 | 3.90 | 43.02 ± 0.81% | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbu, V.M.; Georgescu, A.-M.; Marinas, I.C.; Pericleanu, R.; Mogos, D.V.; Dumbravă, A.Ș.; Marinescu, L.; Pecete, I.; Vassu-Dimov, T.; Czobor Barbu, I.; et al. Phenotypic and Genotypic Characterization of Resistance and Virulence Markers in Candida spp. Isolated from Community-Acquired Infections in Bucharest, and the Impact of AgNPs on the Highly Resistant Isolates. J. Fungi 2024, 10, 563. https://doi.org/10.3390/jof10080563
Corbu VM, Georgescu A-M, Marinas IC, Pericleanu R, Mogos DV, Dumbravă AȘ, Marinescu L, Pecete I, Vassu-Dimov T, Czobor Barbu I, et al. Phenotypic and Genotypic Characterization of Resistance and Virulence Markers in Candida spp. Isolated from Community-Acquired Infections in Bucharest, and the Impact of AgNPs on the Highly Resistant Isolates. Journal of Fungi. 2024; 10(8):563. https://doi.org/10.3390/jof10080563
Chicago/Turabian StyleCorbu, Viorica Maria, Ana-Maria Georgescu, Ioana Cristina Marinas, Radu Pericleanu, Denisa Vasilica Mogos, Andreea Ștefania Dumbravă, Liliana Marinescu, Ionut Pecete, Tatiana Vassu-Dimov, Ilda Czobor Barbu, and et al. 2024. "Phenotypic and Genotypic Characterization of Resistance and Virulence Markers in Candida spp. Isolated from Community-Acquired Infections in Bucharest, and the Impact of AgNPs on the Highly Resistant Isolates" Journal of Fungi 10, no. 8: 563. https://doi.org/10.3390/jof10080563
APA StyleCorbu, V. M., Georgescu, A.-M., Marinas, I. C., Pericleanu, R., Mogos, D. V., Dumbravă, A. Ș., Marinescu, L., Pecete, I., Vassu-Dimov, T., Czobor Barbu, I., Csutak, O., Ficai, D., & Gheorghe-Barbu, I. (2024). Phenotypic and Genotypic Characterization of Resistance and Virulence Markers in Candida spp. Isolated from Community-Acquired Infections in Bucharest, and the Impact of AgNPs on the Highly Resistant Isolates. Journal of Fungi, 10(8), 563. https://doi.org/10.3390/jof10080563







