Diversity and Pathogenicity of Six Diaporthe Species from Juglans regia in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation
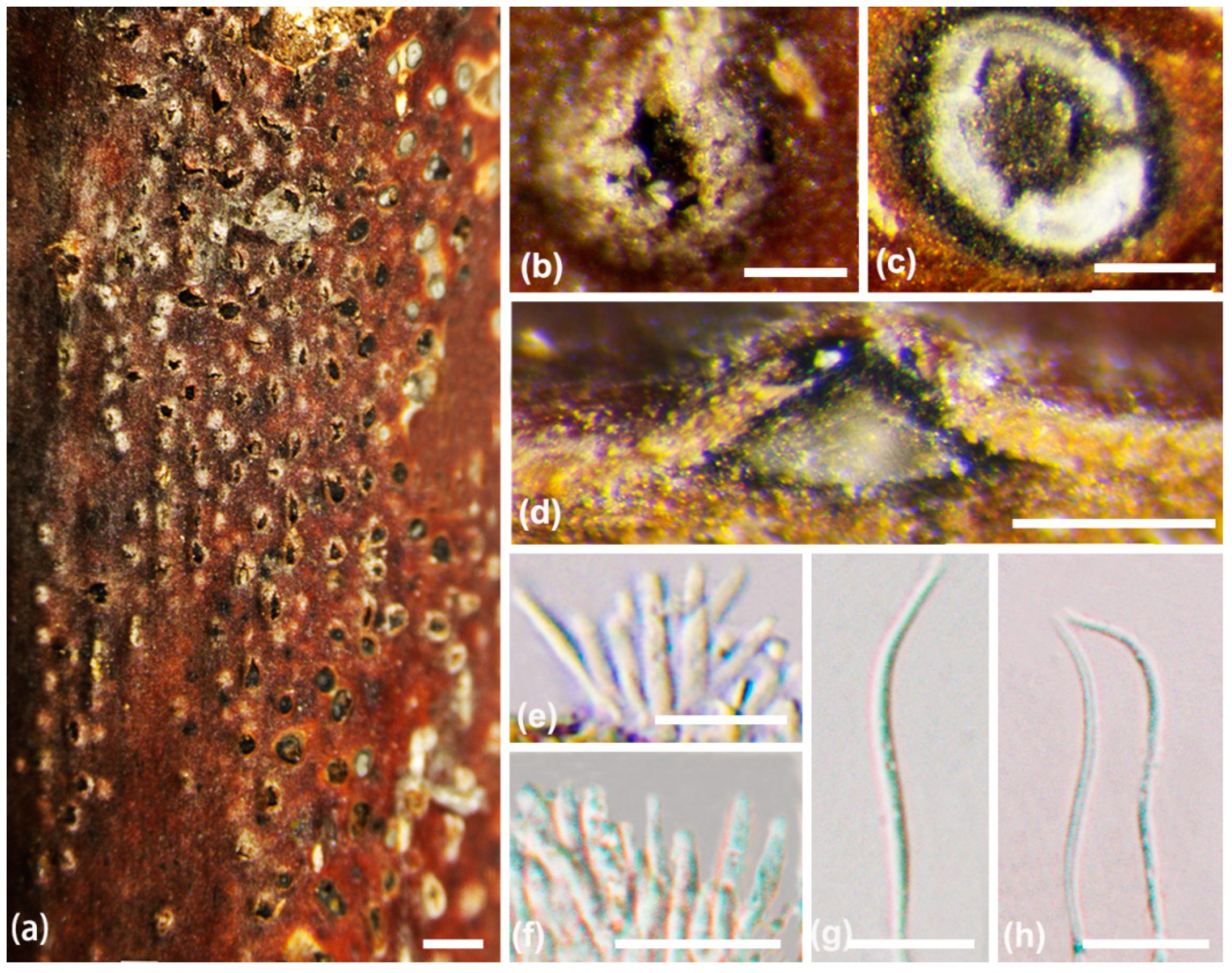
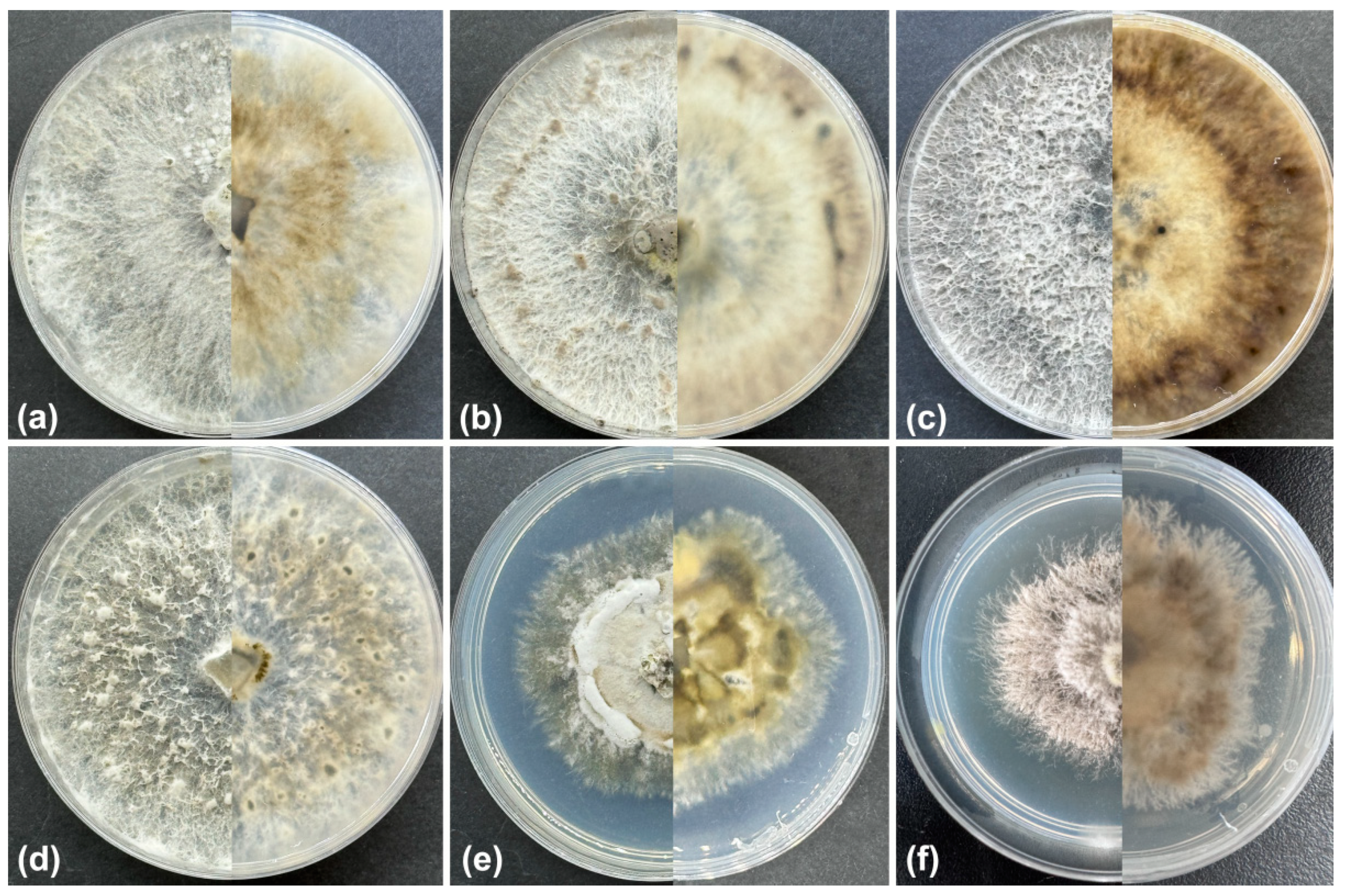
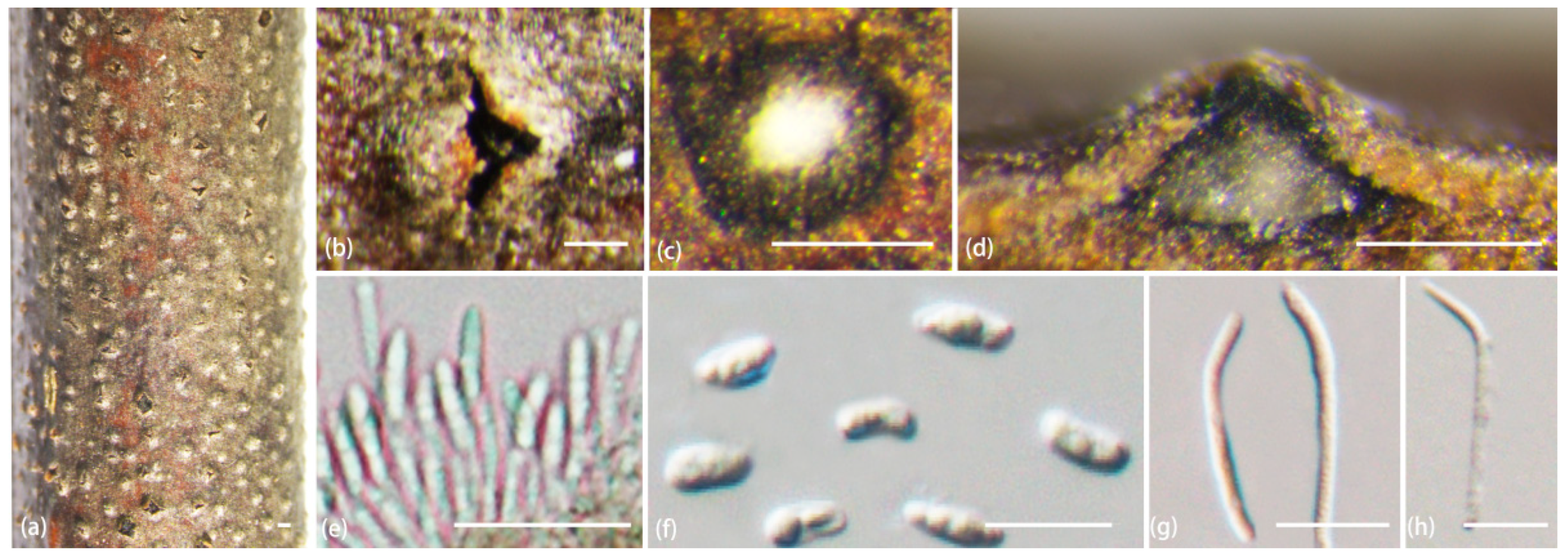
2.2. Morphological Analyses
2.3. DNA Extraction, PCR Amplification and Sequencing
2.4. Phylogenetic Analyses
2.5. Pathogenicity Test
| Locus | PCR Primers | PCR: Thermal Cycles: (Annealing Temp. in Bold) | References of Primers Used |
|---|---|---|---|
| ITS | ITS1 | (95 °C: 30 s, 51 °C: 30 s, 72 °C: 1 min) × 35 cycles | [45] |
| ITS4 | |||
| cal | CAL228F | (95 °C: 15 s, 54 °C: 20 s, 72 °C: 1 min) × 35 cycles | [46] |
| CAL737R | |||
| his3 | CYLH3F | (95 °C: 30 s, 58 °C: 30 s, 72 °C: 1 min) × 35 cycles | [47,48] |
| H3-1b | |||
| tef1-α | 728F | (95 °C: 15 s, 55 °C: 20 s, 72 °C: 1 min) × 35 cycles | [46] |
| 1567R | |||
| tub2 | T1 | (95 °C: 30 s, 55 °C: 30 s, 72 °C: 1min) × 35 cycles | [48] |
| Bt2b |
3. Results
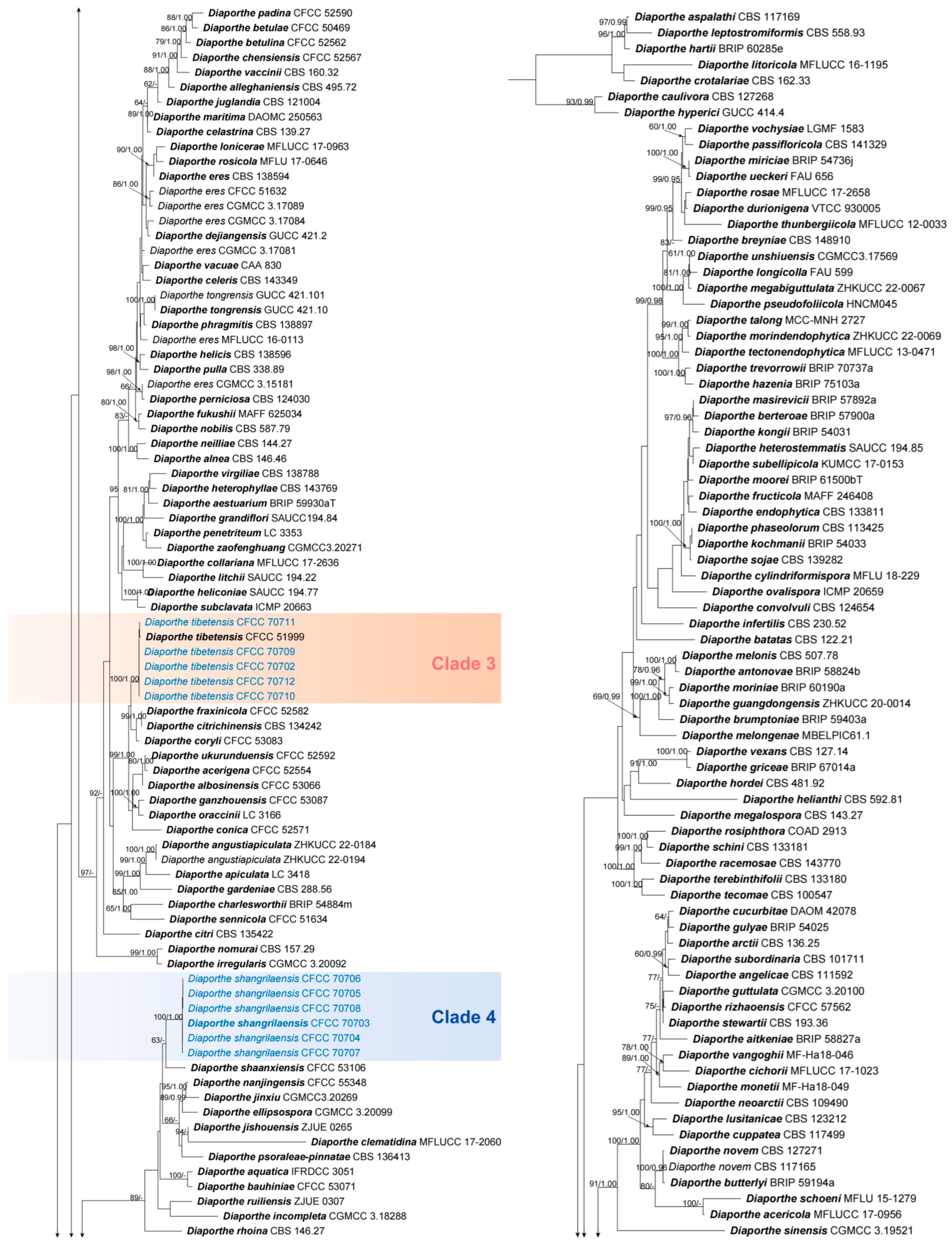
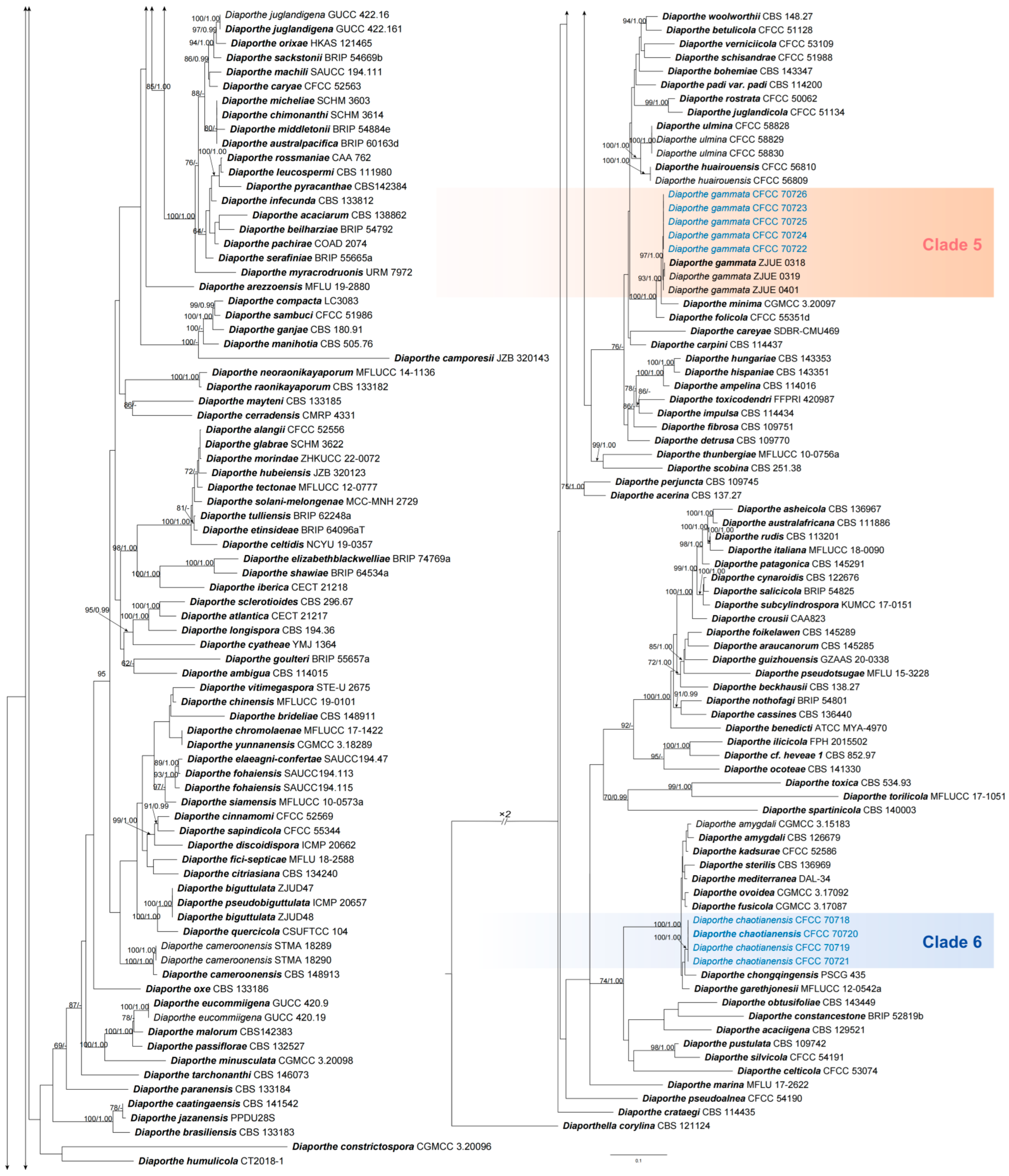
3.1. Phylogeny
3.2. Taxonomy
3.3. Analysis of Pathogenicity Test
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Larrinaga, F.; Mellado-Bermejo, E.; Valadares de Queirós, R.; Figueras-Lorenzo, M.; Gomes-Pires, J. Las nuevas plantaciones de nogal en Espana. Fruticultura 2017, 64, 45–59. [Google Scholar]
- Chen, S.F.; Moran, D.P.; Hasey, J.K.; Anderson, K.; Michailides, T.J. Phylogeny, morphology, distribution, and pathogenicity of Botryosphaeriaceae and Diaporthaceae from English walnut in California. Plant Dis. 2014, 98, 636–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Fan, K.; Li, D.W.; Han, C.M.; Qu, Y.Y.; Qi, Y.K.; Wu, X.Q. Identification, Virulence and Fungicide Sensitivity of Colletotrichum gloeosporioides s.s. Responsible for Walnut Anthracnose Disease in China. Plant Dis. 2020, 104, 1358–1368. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, Y.; Yan, A.H.; Wang, Z.G. The First Report of Juglans regia Leaf Spot Caused by Fusarium proliferatum in China. Plant Dis. 2022, 106, 2265. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Moral, J.; Felts, D.; Trapero, A.; Michailides, T.J. Interaction Between Diaporthe rhusicola and Neofusicoccum mediterraneum Causing Branch Dieback and Fruit Blight of English Walnut in California, and the Effect of Pruning Wounds on the Infection. Plant Dis. 2019, 103, 1196–1205. [Google Scholar] [CrossRef]
- Nitschke, T. Pyrenomycetes Germanici; Eduard Trewendt: Breslau, Germany, 1870. [Google Scholar]
- Udayanga, D.; Liu, X.Z.; McKenzie, E.H.C.; Chukeatirote, E.; Bahkali, A.H.A.; Hyde, K.D. The genus Phomopsis: Biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 2011, 50, 189–225. [Google Scholar] [CrossRef]
- Udayanga, D.; Liu, X.Z.; Crus, P.W.; McKenzie, E.H.C.; Chukeatirote, E.; Hyde, K.D. A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis). Fungal Divers. 2012, 56, 157–171. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. The Diaporthe sojae species complex, phylogenetic re-assessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biol. 2015, 119, 383–407. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 2013, 31, 1–41. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Camporesi, E.; Hyde, K.D.; Wei, Z.; Yan, J.Y.; Li, X.H. Molecular phylogenetic analysis reveals seven new Diaporthe species from ltaly. Mycosphere 2017, 8, 853–877. [Google Scholar] [CrossRef]
- Yang, Q.; Fan, X.L.; Guamaccia, V.; Tian, C.M. High diversity of Diaporthe species associated with dieback diseases in China, with twelve new species described. MycoKeys 2018, 39, 97–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiang, N.; Tian, C.M. Three new Diaporthe species from Shaanxi Province, China. MycoKeys 2020, 67, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiang, N.; Tian, C.M. New species and records of Diaporthe from Jiangxi Province, China. MycoKeys 2021, 77, 41–64. [Google Scholar] [CrossRef] [PubMed]
- Manawasinghe, I.S.; Zhang, W.; Li, X.; Zhao, W.; Chethana, K.W.T.; Xu, J.; Chen, Z.; Dissanayaka, A.J.; Mugnai, L.; Úrbez-Torres, J.R.; et al. Novel microsatellite markers reveal multiple origins of Botryosphaeria dothidea causing the Chinese grapevine trunk disease. Fungal Ecol. 2018, 33, 134–142. [Google Scholar] [CrossRef]
- Guo, Y.S.; Crous, P.W.; Bai, Q.; Fu, M.; Yang, M.M.; Wang, X.H.; Du, Y.M.; Hong, N.; Xu, W.X.; Wang, G.P. High diversity of Diaporthe species associated with pear shoot canker in China. Persoonia 2020, 45, 132–162. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.K.; Lin, L.; Pan, M.; Fan, X.L. Studies of Diaporthe (Diaporthaceae, Diaporthales) species associated with plant cankers in Beijing, China, with three new species described. Mycokeys 2023, 98, 59–86. [Google Scholar] [CrossRef] [PubMed]
- Wehmeyer, L.E. The Genus Diaporthe Nitschke and Its Segregates; Scientific Series; University of Michigan Studies: Ann Arbor, MI, USA, 1933. [Google Scholar]
- Uecker, F.A. A world list of Phomopsis names with notes on nomenclature, morphology and biology. Contributions from the U.S. Nationa Fungus Collection. Mycol. Mem. 1988, 13, 9–12. [Google Scholar]
- Rehner, S.A.; Uecker, F.A. Nuclear ribosomal internal transcribed spacer phylogeny and host diversity in the coelomycete Phomopsis. Can. J. Bot. 1994, 72, 1666–1674. [Google Scholar] [CrossRef]
- Mostert, L.; Crous, P.W.; Kang, J.C.; Phillips, A.J.L. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: Morphological, cultural, molecular and pathological characterization. Mycologia 2001, 93, 146–167. [Google Scholar] [CrossRef]
- Nitimargi, N.M. Studies in the genera Cytosporina, Phomopsis and Diaporthe. VII. Chemical factors influencing sporing characters. Ann. Bot. 1935, 49, 19–40. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Crous, P.W. Emerging citrus diseases in Europe caused by Diaporthe spp. IMA Fungus 2017, 8, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, V.; Groenewald, J.Z.; Woodhall, J.; Armengol, J.; Cinelli, T.; Eichmeier, A.; Ezra, D.; Fontaine, F.; Gramaje, D.; Gutierrez-Aguirregabiria, A.; et al. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia 2018, 40, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. Insights into the genus Diaporthe: Phylogenetic species delimitation in the D. eres species complex. Fungal Divers. 2014, 67, 203–229. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Hyde, K.D. Species limits in Diaporthe: Molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 2014, 32, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Pan, M.; Tian, C.M.; Fan, X.L. Cytospora and Diaporthe species associated with hazelnut canker and dieback in Beijing, China. Front. Cell. Infect. Microbiol. 2021, 11, 664366. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Voglmayr, H.; Piao, C.G.; Li, Y. Two new species of Diaporthe (Diaporthaceae, Diaporthales) associated with tree cankers in the Netherlands. MycoKeys 2021, 85, 31–56. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Chen, Y.Y.; Liu, J.K. Unravelling Diaporthe species associated with woody hosts from karst formations (Guizhou) in China. J. Fungi 2020, 6, 251. [Google Scholar] [CrossRef]
- Meng, L.; Yu, C.L.; Wang, C.X.; Li, G.F. First Report of Diaporthe amygdali Causing Walnut Twig Canker in Shandong Province of China. Plant Dis. 2018, 102, 1859. [Google Scholar] [CrossRef]
- Fan, X.L.; Yang, Q.; Bezerra, J.D.P.; Alvarez, L.V.; Tian, C.M. Diaporthe from walnut tree (Juglans regia) in China, with insight of the Diaporthe eres complex. Mycol. Pap. 2018, 17, 841–853. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Pan, M.; Bonthond, G.; Tian, C.M.; Fan, X.L. Diaporthalean fungi associated with canker and dieback of trees from Mount Dongling in Beijing, China. MycoKeys 2019, 59, 67–94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hou, C.L. Three new species of Diaporthe from China based on morphological characters and DNA sequence data analyses. Phytotaxa 2019, 422, 157–174. [Google Scholar] [CrossRef]
- Wang, S.Y.; McKenzie, E.H.C.; Phillips, A.J.L.; Li, Y.; Wang, Y. Taxonomy and Multigene Phylogeny of Diaporthales in Guizhou Province, China. J. Fungi 2022, 8, 1301. [Google Scholar] [CrossRef] [PubMed]
- Rayner, R.W. A Mycological Colour Chart; Commonwealth Mycological Institute: London, UK, 1970. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 3–15. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 304, 772–780. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Rannala, B.; Yang, Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996, 43, 304–311. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A. FigTree, version 1.3.1; Institute of Evolutionary Biology: Edinburgh, UK, 2010. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal, RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 3, 553–556. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Risède, J.M.; Simoneau, P.; Hywel-Jones, N.L. Calonectria species and their Cylindrocladium anamorphs: Species with sphaeropedunculate vesicles. Stud. Mycol. 2004, 50, 415–430. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Su, Y.; Sun, W.; Cai, L. Diaporthe species occurring on Lithocarpus glabra in China, with descriptions of five new species. Fungal Biol. 2015, 119, 295–309. [Google Scholar] [CrossRef] [PubMed]
- León, M.; Berbegal, M.; Rodríguez-Reina, J.M.; Elena, G.; Abad-campos, P.; Ramón-Albalat, A.; Olmo, D.; Vicent, A.; Luque, J.; Miarnau, X.; et al. Identification and Characterization of Diaporthe spp. Associated with Twig Cankers and Shoot Blight of Almonds in Spain. Agronomy 2020, 10, 1062. [Google Scholar] [CrossRef]
- Hyde, K.D.; Hongsanan, S.; Jeewon, R.; Bhat, D.J.; McKenzie, E.H.C.; Jones, E.B.G.; Phookamsak, R.; Ariyawansa, H.A.; Boonmee, S.; Zhao, Q.; et al. Fungal diversity notes 367–492, taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 80, 1–270. [Google Scholar] [CrossRef]
- Lombard, L.; Leeuwen, G.C.; Guarnaccia, V.; Polizzi, G.; Rijswick, P.C.; Rosendahl, K.C.; Gabler, J.; Crous, P.W. Diaporthe species associated with Vaccinium, with specific reference to Europe. Phytopathol. Mediterr. 2014, 53, 287–299. [Google Scholar]
- Xiao, X.E.; Liu, Y.D.; Zheng, F.; Xiong, T.; Zeng, Y.T.; Wang, W.; Zheng, X.L.; Wu, Q.; Xu, J.P.; Crous, P.W.; et al. High species diversity in Diaporthe associated with citrus diseases in China. Persoonia 2023, 51, 229–256. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Shivas, R.G.; Edwards, J.; Seifert, K.A.; Alfenas, A.C.; Alfenas, R.F.; Burgess, T.I.; Carnegie, A.J.; Hardy, G.E.; et al. Fungal Planet description sheets: 69–91. Persoonia 2011, 26, 108–156. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Dissanayake, A.J.; Li, X.; Liu, M.; Wanasinghe, D.N.; Xu, J.; Zhao, W.; Zhang, W.; Zhou, Y.; Hyde, K.D.; et al. High Genetic Diversity and Species Complexity of Diaporthe Associated with Grapevine Dieback in China. Front. Microbiol. 2019, 10, 1936. [Google Scholar] [CrossRef]
- Liu, H.Y.; Luo, D.; Huang, H.L.; Yang, Q. Two new species of Diaporthe (Diaporthaceae, Diaporthales) associated with Camellia oleifera leaf spot disease in Hainan Province, China. MycoKeys 2024, 102, 225–243. [Google Scholar] [CrossRef]
- Abeywickrama, P.; Qian, N.; Jayawardena, R.; Li, Y.; Zhang, W.; Guo, K.; Zhang, L.; Zhang, G.; Yan, J.; Li, X.Q.; et al. Endophytic fungi in green manure crops; friends or foe? Mycosphere 2023, 14, 1–106. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, F.; Cai, L. Unravelling Diaporthe species associated with Camellia. Syst. Biodivers. 2016, 14, 102–117. [Google Scholar] [CrossRef]
- Wan, Y.; Li, D.W.; Si, Y.Z.; Li, M.; Huang, L.; Zhu, L.H. Three New Species of Diaporthe Causing Leaf Blight on Acer palmatum in China. Plant Dis. 2023, 107, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Chiusa, G.; Arciuolo, R.; Somenzi, M.; Fontana, M.; Castello, G.; Spigolon, N. Diaporthe as the main cause of hazelnut defects in the Caucasus region. Phytopathol. Mediterr. 2018, 57, 320–333. [Google Scholar]
- Mondal, S.N.; Agostini, J.P.; Zhang, L.; Timmer, L.W. Factors affecting pycnidium production of Diaporthe citri on detached citrus twigs. Plant Dis. 2004, 88, 379–382. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. Diaporthe eres (Phomopsis oblonga) as a pathogen of butternut (Juglans cinerea) in Connecticut. Plant Dis. 2007, 91, 1198. [Google Scholar] [CrossRef] [PubMed]
- Bastide, F.; Serandat, I.; Gombert, J.; Laurent, E.; Morel, E.; Kolopp, J.; Guillermin, P.L.; Hamon, B.; Simoneau, P.; Bermyer, R.; et al. Characterization of fungal pathogens (Diaporthe angelicae and D. eres) responsible for umbel browning and stem necrosis on carrot in France. Plant Pathol. 2017, 66, 239–253. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Song, Q.; Liu, J.; Yang, Q.; Luan, F.; Li, D. First report of Diaporthe eres causing branch canker on Cinnamomum camphora (Camphor Tree) in Jiangxi Province. China. Plant Dis. 2021, 105, 1563. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Briscoe, W.E.; Techen, N.; Johnson, R.D.; Clausen, B.M.; Duke, S.O. Isolation of a phytotoxic isocoumarin from Diaporthe eres infected Hedera helix (English ivy) and synthesis of its phytotoxic analogs. Pest Manag. Sci. 2018, 74, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Wang, H.; Huang, S.X.; Zhang, Y.; Zhang, Z.H.; Liu, w.; Shi, N.X.; Zhu, F.; Ji, Z.L.; Chen, X.R. Identification and characterization of Diaporthe eres causing leaf blight disease on the medicinal herb Polygonatum sibiricum. J. Gen. Plant Pathol. 2020, 86, 468–476. [Google Scholar] [CrossRef]
- Du, Y.M.; Wang, X.H.; Guo, Y.S.; Xiao, F.; Peng, Y.H.; Hong, N.; Wang, G.P. Biological and molecular characterization of seven Diaporthe species associated with kiwifruit shoot blight and leaf spot in China. Phytopathol. Mediterr. 2021, 60, 177–198. [Google Scholar] [CrossRef]
- Smit, L.; Langenhoven, W.E.; Petersen, Y. Diaporthe species associated with dieback on Cyclopia (honeybush). Eur. J. Plant Pathol. 2021, 161, 565–578. [Google Scholar] [CrossRef]
- Zambelli, A.; Mancebo, M.F.; Bazzalo, M.E.; Reid, R.J.; Sanchez, M.C.; Kontz, B.J.; Mathew, F.M. Six species of Diaporthe associated with Phomopsis stem canker of sunflower in southern pampean region of Argentina. Plant Health Prog. 2021, 22, 136–142. [Google Scholar] [CrossRef]
- Zhao, X.; Li, K.; Zheng, S.; Yang, J.; Chen, C.; Zheng, X.; Wang, Y.; Ye, W. Diaporthe diversity and pathogenicity revealed from a broad survey of soybean stem blight in China. Plant Dis. 2022, 11, 2892–2903. [Google Scholar] [CrossRef]







| Species | Isolate | Disease Incidence (%) | Lesion Size (mm) |
|---|---|---|---|
| Diaporthe chaotianensis | CFCC 70718–70720 | 89 | 13.6 ± 0.8 d |
| Diaporthe gammata | CFCC 70722–70724 | 39 | 9.1 ± 0.6 e |
| Diaporthe olivacea | CFCC 70713, 70715, 70716 | 56 | 21.4 ± 1.1 b |
| Diaporthe shangluoensis | CFCC 70728, 70729, 70731 | 61 | 17.6 ± 1.7 c |
| Diaporthe shangrilaensis | CFCC 70703, 70705, 70706 | 94 | 31.3 ± 1.5 a |
| Diaporthe tibetensis | CFCC 70702, 70710, 70711 | 56 | 13.3 ± 0.5 d |
| Control | Noncolonized potato dextrose agar plug | 0 | 6.5 ± 0.2 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, A.; Lin, L.; Li, Y.; Fan, X. Diversity and Pathogenicity of Six Diaporthe Species from Juglans regia in China. J. Fungi 2024, 10, 583. https://doi.org/10.3390/jof10080583
Jia A, Lin L, Li Y, Fan X. Diversity and Pathogenicity of Six Diaporthe Species from Juglans regia in China. Journal of Fungi. 2024; 10(8):583. https://doi.org/10.3390/jof10080583
Chicago/Turabian StyleJia, Aoli, Lu Lin, Yixuan Li, and Xinlei Fan. 2024. "Diversity and Pathogenicity of Six Diaporthe Species from Juglans regia in China" Journal of Fungi 10, no. 8: 583. https://doi.org/10.3390/jof10080583






