A Fusarium verticillioides MAT1-2 Strain near Isogenic to the Sequenced FGSC7600 Strain for Producing Homozygous Multigene Mutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Growth Conditions, and Media
2.2. Genome Sequencing
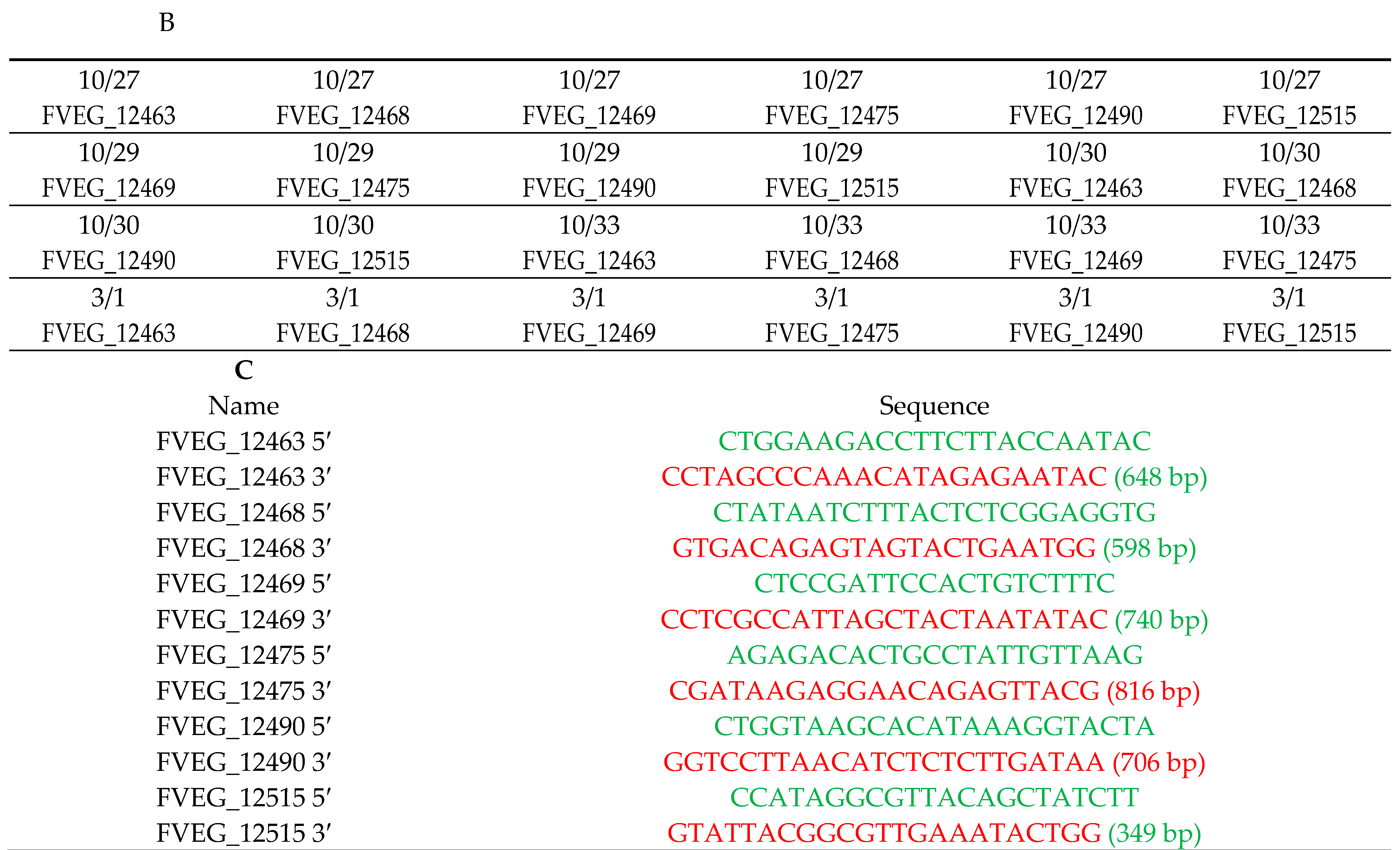
2.3. Methods for Fusarium verticillioides Variant Calling
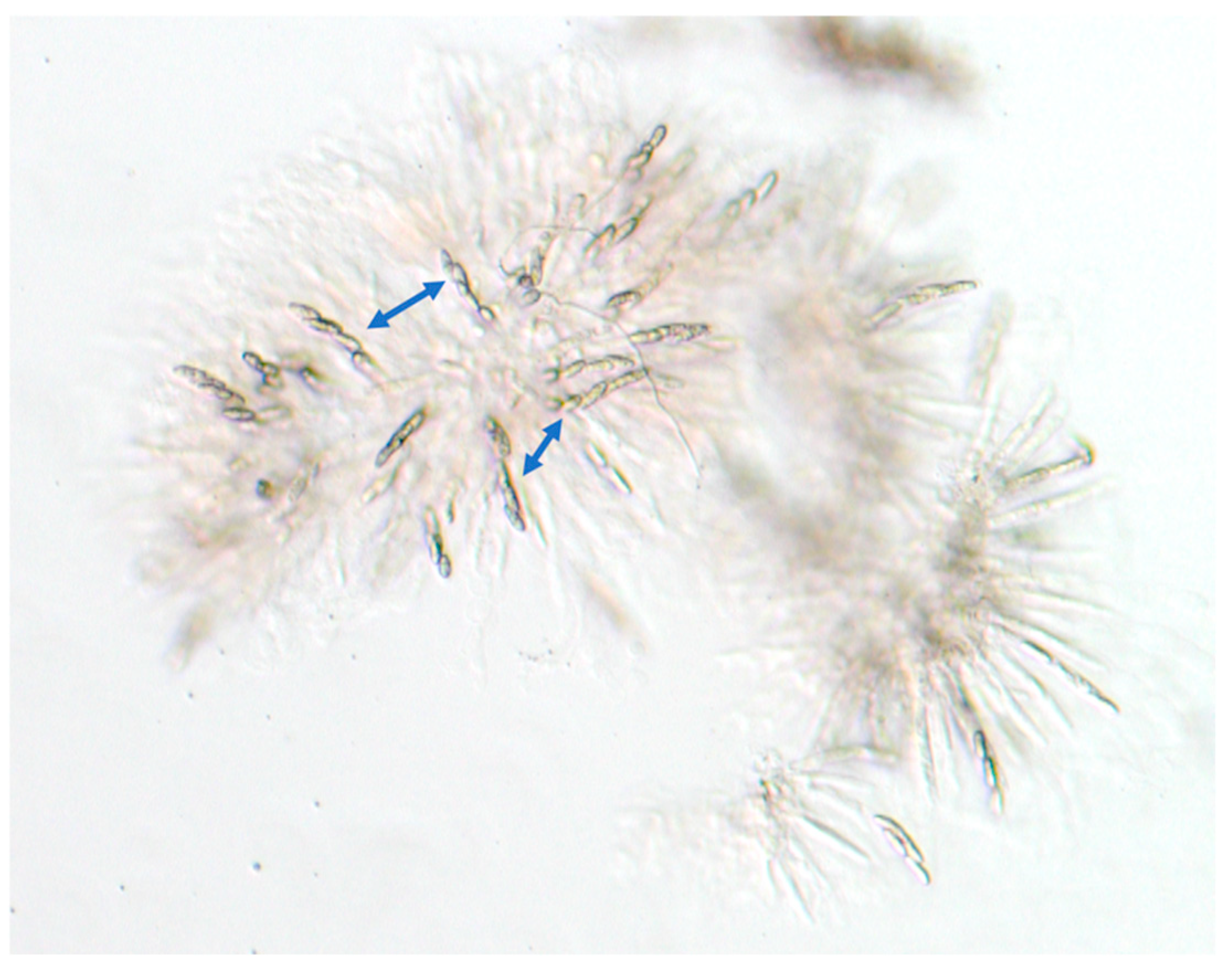
3. Results
3.1. Production of Progeny
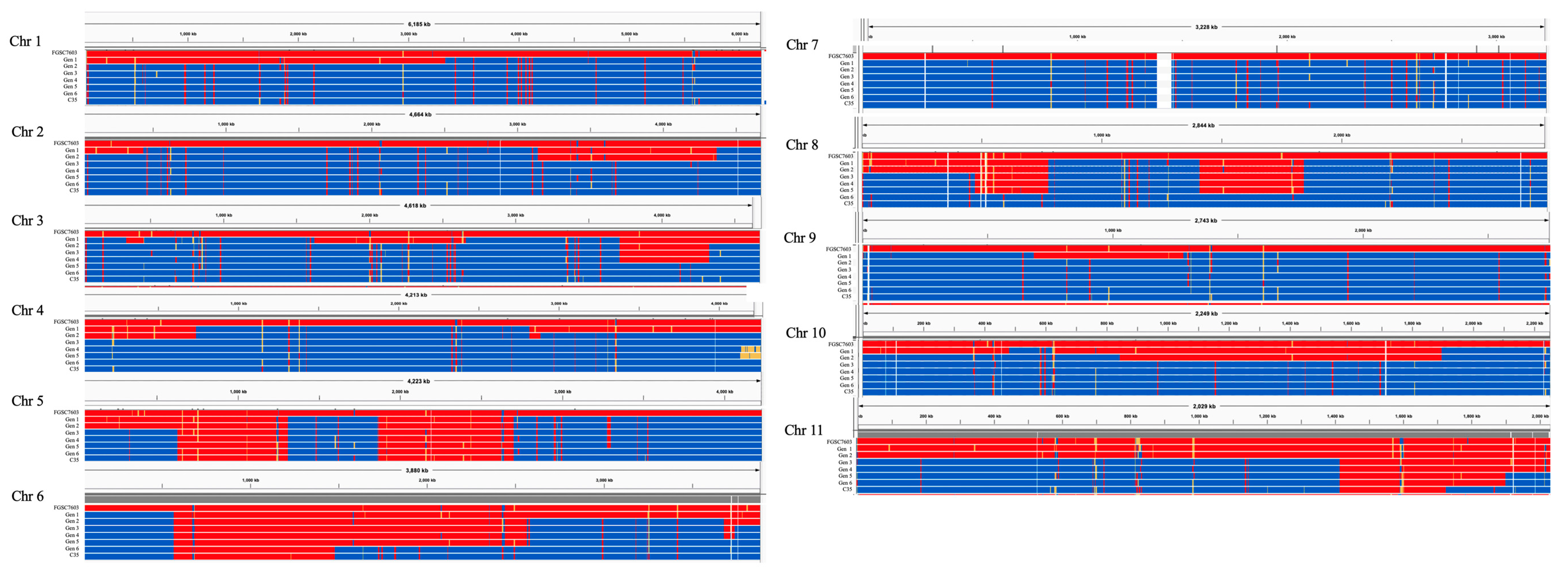
3.2. Genome Mapping through the Generations
3.3. Genotype of BC6 near Isogenic MAT1-2 Strain TMRU10/35
3.4. Increase in Fertility of Final Product
3.5. Nonessential Genes at Right Arm of Chromosome 4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marasas, W.F. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001, 109, 239–243. [Google Scholar] [PubMed]
- Pokoo-Aikins, A.; McDonough, C.; Mitchell, T.R.; Hawkins, J.A.; Adams, L.F.; Read, Q.D.; Li, X.; Shanmugasundaram, R.; Rodewald, E.; Acharya, P.; et al. Mycotoxin contamination and its effects on the nutritional content of corn targeted for animal feed. J. Poult. Sci. 2024. [Google Scholar]
- Marasas, W.F.; Kellerman, T.S.; Pienaar, J.G.; Naude, T.W. Leukoencephalomalacia: A mycotoxicosis of Equidae caused by Fusarium moniliforme Sheldon. Onderstepoort J. Vet. Res. 1976, 43, 113–122. [Google Scholar] [PubMed]
- Kriek, N.P.; Kellerman, T.S.; Marasas, W.F. A comparative study of the toxicity of Fusarium verticillioides (=F. moniliforme) to horses, primates, pigs, sheep and rats. Onderstepoort J. Vet. Res. 1981, 48, 129–131. [Google Scholar] [PubMed]
- Gelineau-van Waes, J.; Voss, K.A.; Stevens, V.L.; Speer, M.C.; Riley, R.T. Maternal fumonisin exposure as a risk factor for neural tube defects. Adv. Food Nutr. Res. 2009, 56, 145–181. [Google Scholar] [PubMed]
- Gao, S.; Gold, S.E.; Glenn, A.E. Characterization of two catalase-peroxidase-encoding genes in Fusarium verticillioides reveals differential responses to in vitro versus in planta oxidative challenges. Mol. Plant Pathol. 2018, 19, 1127–1139. [Google Scholar] [PubMed]
- Chaisrisook, C.; Leslie, J. A maternally expressed nuclear gene controlling perithecial pigmentation in Gibberella fujikuroi (Fusarium moniliforme). J. Hered. 1990, 81, 189–192. [Google Scholar]
- Kathariou, S.; Spieth, P.T. Spore killer polymorphism in Fusarium moniliforme. Genetics 1982, 102, 19–24. [Google Scholar] [PubMed]
- Glenn, A.E.; Gold, S.E.; Bacon, C.W. Fdb1 and Fdb2, Fusarium verticillioides loci necessary for detoxification of preformed antimicrobials from corn. Mol. Plant-Microbe Interact. 2002, 15, 91–101. [Google Scholar] [PubMed]
- Leslie, J.F. Mating populations of Gibberella fujikuroi (Fusarium section Liseola). Phytopathology 1991, 81, 1058–1060. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006; p. 388. [Google Scholar]
- Klittich, C.; Leslie, J.F. Nitrate reduction mutants of Fusarium moniliforme (Gibberella fujikuroi). Genetics 1988, 118, 417–423. [Google Scholar] [PubMed]
- Stajich, J.E.; Harris, T.; Brunk, B.P.; Brestelli, J.; Fischer, S.; Harb, O.S.; Kissinger, J.C.; Li, W.; Nayak, V.; Pinney, D.F.; et al. FungiDB: An integrated functional genomics database for fungi. Nucleic Acids Res. 2012, 40, D675–D681. [Google Scholar] [PubMed]
- Pyle, J.; Patel, T.; Merrill, B.; Nsokoshi, C.; McCall, M.; Proctor, R.H.; Brown, D.W.; Hammond, T.M. A meiotic drive element in the maize pathogen Fusarium verticillioides is located within a 102 kb region of chromosome V. Genes/Genomes/Genet. 2016, 6, 2543–2552. [Google Scholar]
- Lohmar, J.M.; Rhoades, N.A.; Patel, T.N.; Proctor, R.H.; Hammond, T.M.; Brown, D.W. A-to-I mRNA editing controls spore death induced by a fungal meiotic drive gene in homologous and heterologous expression systems. Genetics 2022, 221, iyac029. [Google Scholar] [PubMed]
- Raju, N.B. Ascomycete spore killers: Chromosomal elements that distort genetic ratios among the products of meiosis. Mycologia 1994, 86, 461–473. [Google Scholar]
- Xu, J.R.; Leslie, J.F. A genetic map of Gibberella fujikuroi mating population A (Fusarium moniliforme). Genetics 1996, 143, 175–189. [Google Scholar] [PubMed]
- Lindholm, A.K.; Dyer, K.A.; Firman, R.C.; Fishman, L.; Forstmeier, W.; Holman, L.; Johannesson, H.; Knief, U.; Kokko, H.; Larracuente, A.M.; et al. The ecology and evolutionary dynamics of meiotic drive. Trends Ecol. Evol. 2016, 31, 315–326. [Google Scholar] [PubMed]
- Torvi, J.R.; Wong, J.; Serwas, D.; Moayed, A.; Drubin, D.G.; Barnes, G. Reconstitution of kinetochore motility and microtubule dynamics reveals a role for a kinesin-8 in establishing end-on attachments. Elife 2022, 11, e78450. [Google Scholar] [PubMed]

| Cross | Male Recurrent Parent | Female Parent | Expected FGSC7603 Genotype of Female | Actual Observed % @ | NRRL Product Stock Strain Designation |
|---|---|---|---|---|---|
| 1 (parental) | FGSC7600 (TMRU3/1, NRRL 20956) | FGSC7603 (TMRU3/8, NRRL 20984) | 100% | 98% | |
| 2 | FGSC7600 | F1 (10/24) Gen1 | 50% | 54% | 64803 |
| 3 | FGSC7600 | BC1 (10/25) Gen2 | 25% | 32% | 64804 |
| 4 | FGSC7600 | BC2 (10/27) Gen3 | 12.5% | 12% | 64805 |
| 5 | FGSC7600 | BC3 * (10/29) Gen4 | 6.25% | 12% | 64806 |
| 6 | FGSC7600 | BC4 (10/30) Gen5 | 3.12% | 10% | 64807 |
| 7 | FGSC7600 | BC5 (10/33) Gen6 | 1.56% | 8% | 64808 |
| Product | BC6 (TMRU10/35; FGSC27326) Gen7 | 0.78% | 7% | 64809 | |
| Chromosome | SNPs | Generation:Crossover(s) | Maximum % FGSC7600 Generation |
|---|---|---|---|
| 1 | 25,874 | 1:1, 2:1 * | 100% generation 2 |
| 2 | 21,797 | 1:3, 2:1, 3:2 * | 100% generation 3 |
| 3 | 19,070 | 1:5, 5:2 * | 100% generation 5 |
| 4 | 28,516 | 1:2, 2:1, 3:3 * | 100% generation 3 |
| 5 | 22,138 | 1:5, 6:2 | 65% generation 6 |
| 6 | 23,264 | 1:1, 2:2, 3:1, 5:1, 6:1 | 77% generation 6 |
| 7 | 18,434 | 1:0 * | 100% generation 1 |
| 8 | 26,083 | 1:3, 3:1, 6:4 * | 100% generation 6 |
| 9 | 17,656 | 1:2, 2:2 * | 100% generation 2 |
| 10 | 20,639 | 1:3, 2:1, 3:2 * | 100% generation 3 |
| 11 | 19,698 | 3:1, 5:1, 7:1 | 85% generation 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gold, S.E.; Brown, D.W.; Williams, F.N.; Nadon, B.D.; Vo, V.T.; Miller, C.E. A Fusarium verticillioides MAT1-2 Strain near Isogenic to the Sequenced FGSC7600 Strain for Producing Homozygous Multigene Mutants. J. Fungi 2024, 10, 592. https://doi.org/10.3390/jof10080592
Gold SE, Brown DW, Williams FN, Nadon BD, Vo VT, Miller CE. A Fusarium verticillioides MAT1-2 Strain near Isogenic to the Sequenced FGSC7600 Strain for Producing Homozygous Multigene Mutants. Journal of Fungi. 2024; 10(8):592. https://doi.org/10.3390/jof10080592
Chicago/Turabian StyleGold, Scott E., Daren W. Brown, Felicia N. Williams, Brian D. Nadon, Vivian T. Vo, and Christine E. Miller. 2024. "A Fusarium verticillioides MAT1-2 Strain near Isogenic to the Sequenced FGSC7600 Strain for Producing Homozygous Multigene Mutants" Journal of Fungi 10, no. 8: 592. https://doi.org/10.3390/jof10080592






