Integrated Transcriptomics–Proteomics Analysis Reveals the Response Mechanism of Morchella sextelata to Pseudodiploöspora longispora Infection
Abstract
1. Introduction
2. Materials and Methods
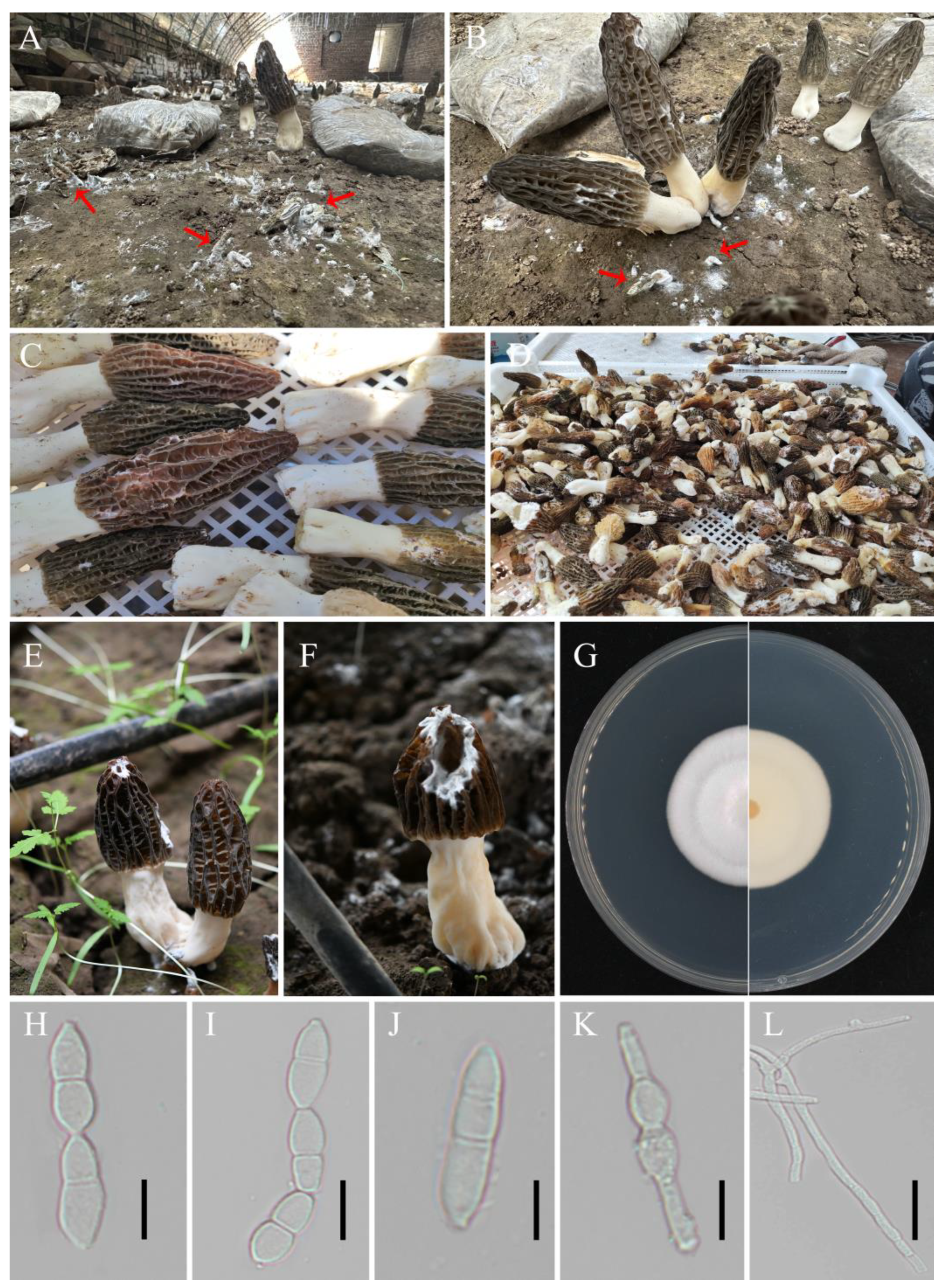
2.1. Collection of Diseased White Mold M. sextelata and Isolation of the Pathogenic Fungus
2.2. Pathogenicity Test and Microscopic Observation
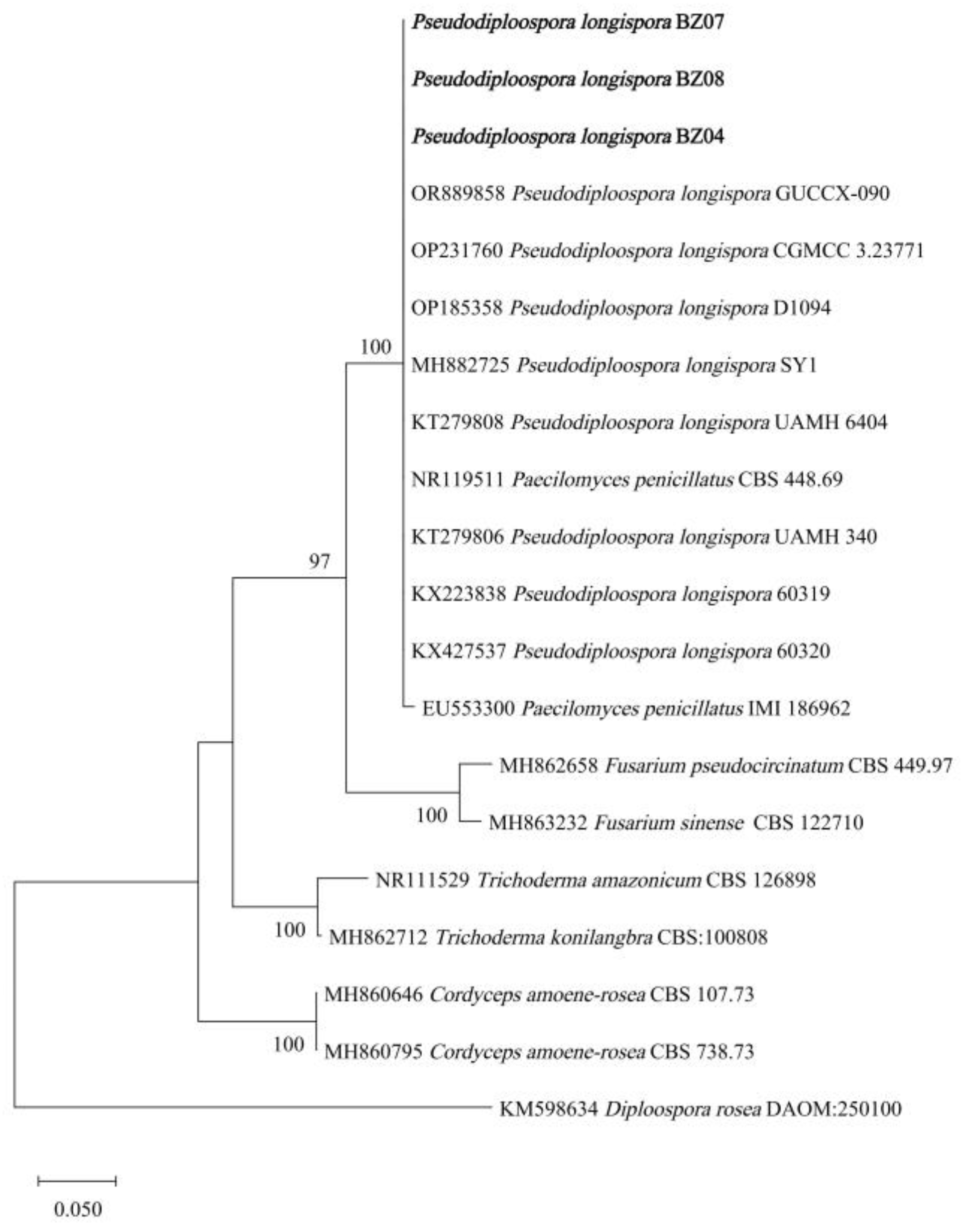
2.3. Identification of Pathogens Causing White Mold Disease in M. sextelata
2.4. Inhibitory Effect of the Pathogenic Fungus on M. sextelata on Plates
2.5. Effect of Nutrients from M. sextelata on Pathogenic Fungi
2.6. Transcriptome Sequencing
2.7. Proteomic Sequencing
2.8. Quantitative PCR (qPCR) Analysis
2.9. Statistical Analysis and Data Availability
3. Results
3.1. Pathogen Isolation and Identification
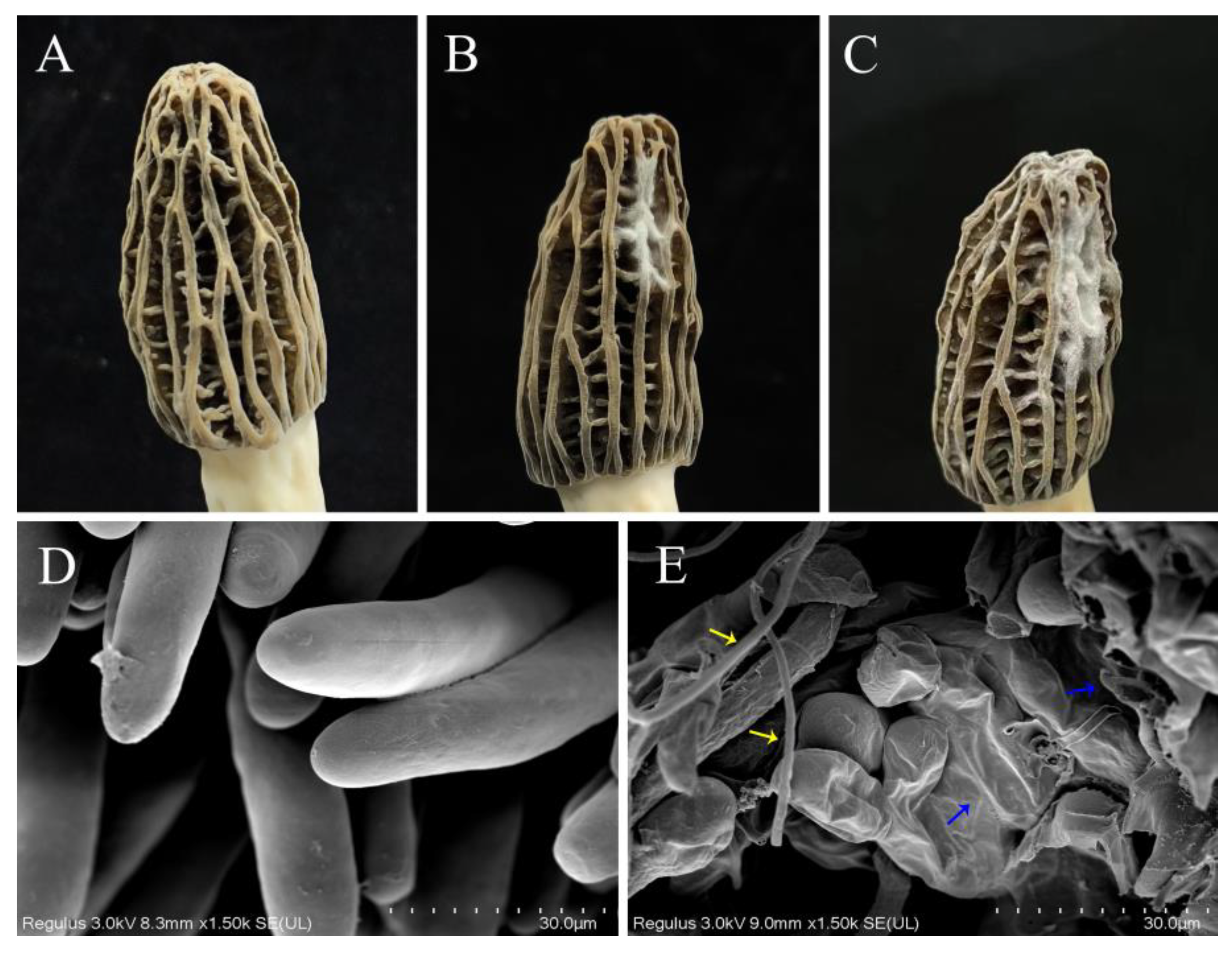
3.2. Pathogenicity Testing and Effect of P. longispora Infestation on the Micromorphology of M. sextelata
3.3. The Response of M. sextelata to P. longispora Infection
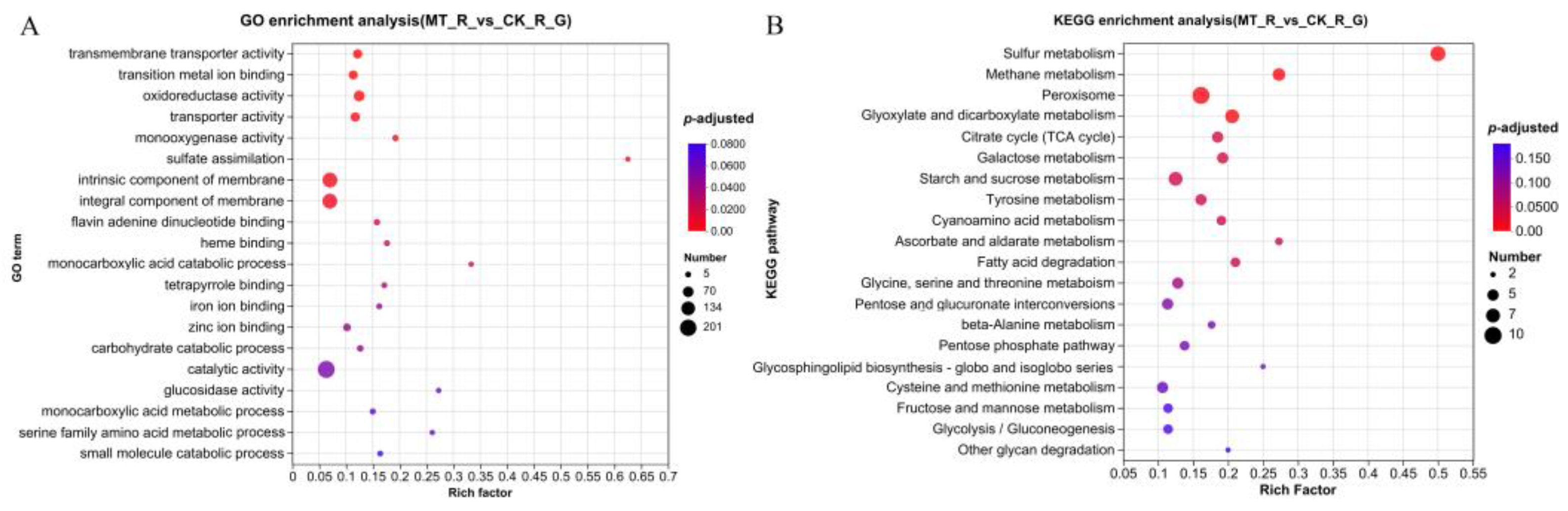
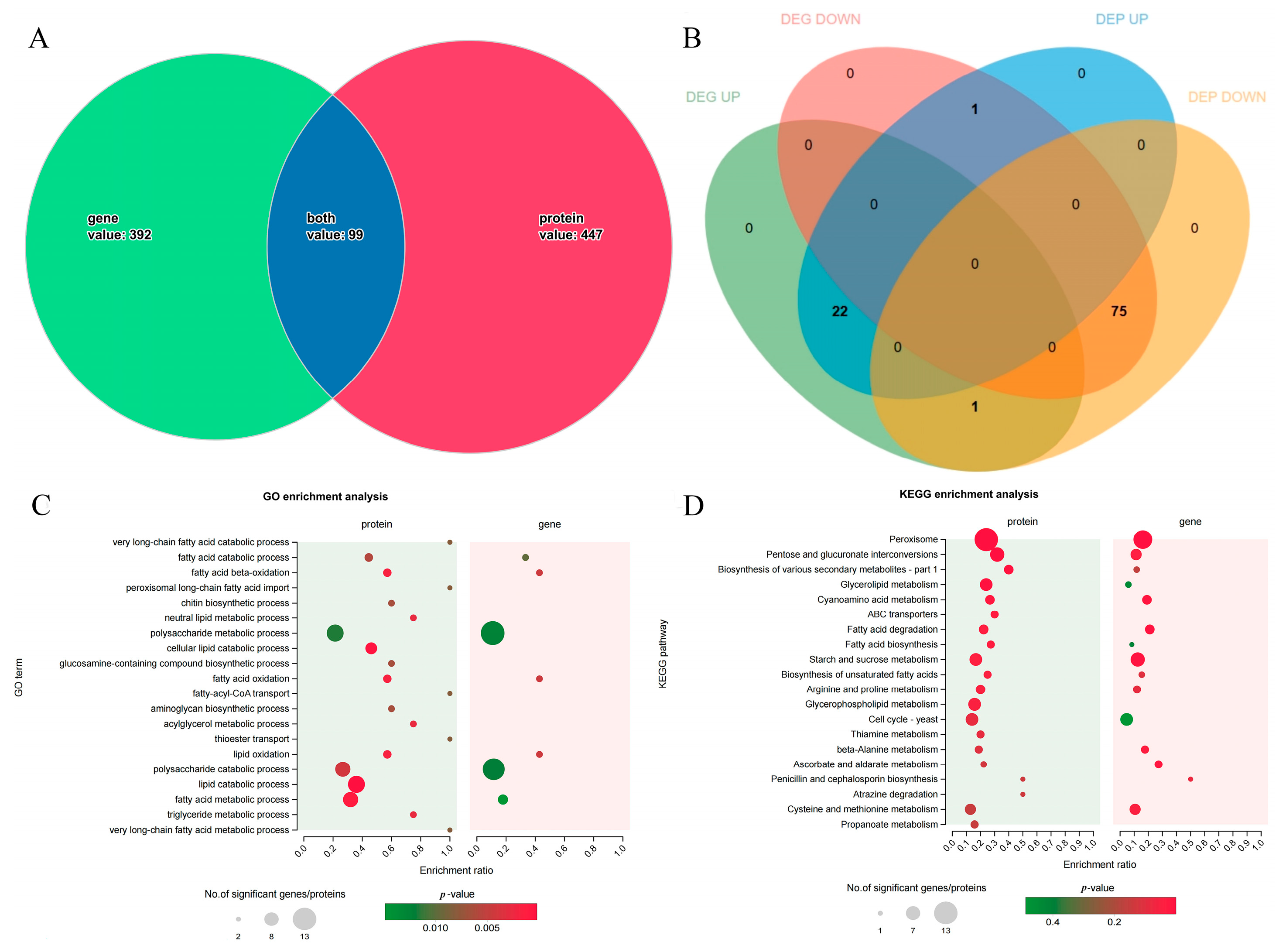
3.4. Functional Enrichment Analysis of DEGs
3.5. Functional Annotation and Enrichment Analysis of DEPs
3.6. Combined Transcriptomics and Proteomics Analysis
3.7. Validation of Candidate Genes by qPCR Analysis
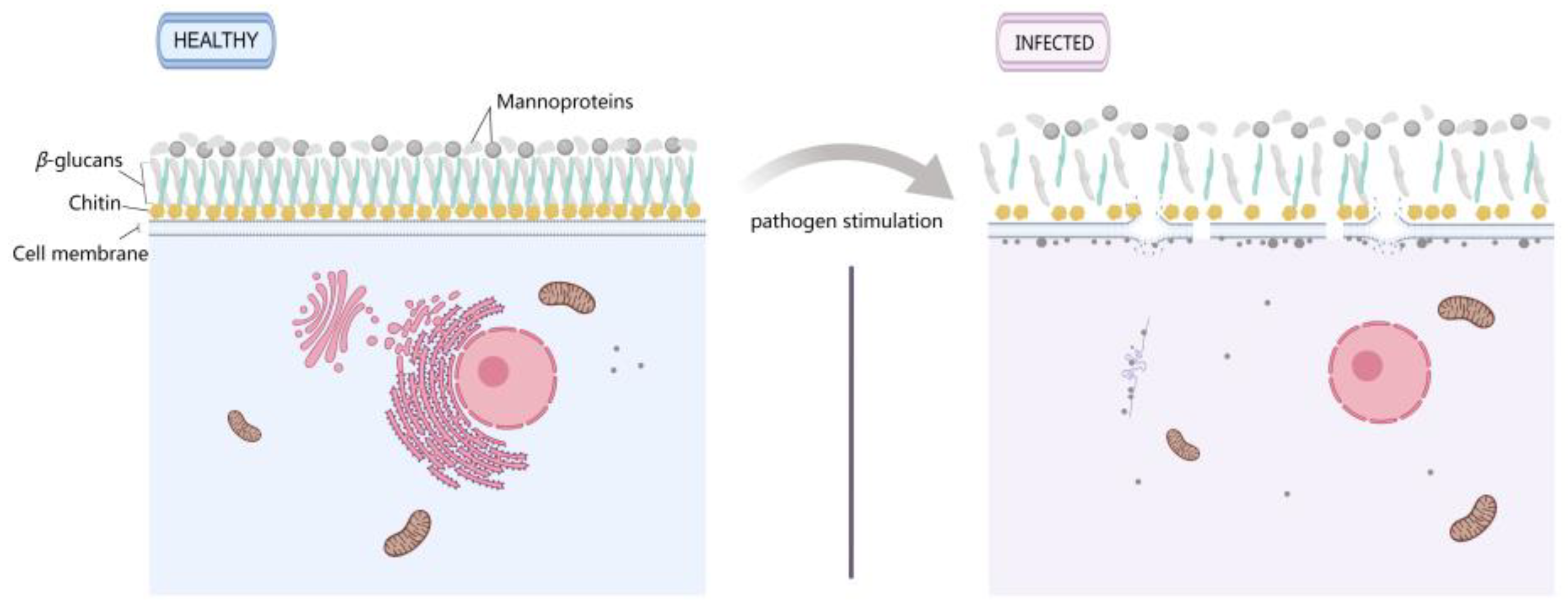
3.8. Changes in the Cell Membrane and Wall of M. sextelata Are Markers in Response to P. longispora Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, X.H.; Wang, H.; Sun, J.; Xiong, L.; Yu, J. Hybridization, Characterization and Transferability of SSRs in the Genus Morchella. Fungal Biol. 2019, 123, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.; Zhang, X. Cultivation, Nutritional Value, Bioactive Compounds of Morels, and Their Health Benefits: A Systematic Review. Front. Nutr. 2023, 10, 1159029. [Google Scholar] [CrossRef]
- Ower, R. Notes on the Development of the Morel Ascocarp: Morchella esculenta. Mycologia 1982, 74, 142–144. [Google Scholar] [CrossRef]
- Masaphy, S. Biotechnology of Morel Mushrooms: Successful Fruiting Body Formation and Development in A Soilless System. Biotechnol. Lett. 2010, 32, 1523–1527. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial Cultivation of True Morels: Current State, Issues and Perspectives. Crit. Rev. Biotechnol. 2017, 38, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Tang, J.; He, X.; Chen, Y.; Tan, H. Status Analysis of Morel Artificial Cultivation in Sichuan. Edible Med. Mushrooms 2016, 24, 145–150. [Google Scholar]
- He, P.; Li, C.; Cai, Y.; Zhang, Y.; Bian, Y.; Liu, W. First Report of Pileus Rot Disease on Cultivated Morchella importuna Caused by Diploöspora longispora in China. J. Gen. Plant Pathol. 2017, 84, 65–69. [Google Scholar] [CrossRef]
- He, X.L.; Peng, W.H.; Miao, R.Y.; Tang, J.; Chen, Y.; Liu, L.X.; Wang, D.; Gan, B.C. White Mold on Cultivated Morels Caused by Paecilomyces penicillatus. FEMS Microbiol Lett. 2017, 364, fnx037. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.P.; Chen, K.; Wang, G.Z.; Bian, Y.B. First report of stipe rot disease on Morchella importuna caused by Fusarium incarnatum—F. equiseti species complex in China. Plant Dis. 2016, 100, 2530. [Google Scholar] [CrossRef]
- Lan, Y.F.; Cong, Q.Q.; Wang, Q.W.; Tang, L.N.; Li, X.M.; Yu, Q.W.; Cui, X.; An, X.R.; Yu, C.X.; Kong, F.H. First Report of Cladobotryum protrusum Causing Cobweb Disease on Cultivated Morchella importuna. Plant Dis. 2019, 104, 977. [Google Scholar] [CrossRef]
- Liu, Z.H.; Cong, Y.L.; Sossah, F.L.; Lu, Y.Z.; Kang, J.C.; Li, Y. Characterization and Genome Analysis of Cladobotryum mycophilum, the Causal Agent of Cobweb Disease of Morchella sextelata in China. J. Fungi 2023, 9, 411. [Google Scholar] [CrossRef]
- Yu, M.; Yin, Q.; He, P.X. Isolation and Identification of Pathogen of Morel White Rot. N. Hortic. 2020, 7, 142–145. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, H.; Liu, T.; Liu, L.; Tang, J.; Peng, W. Dual RNA-Seq Analysis of The Interaction Between Edible Fungus Morchella sextelata and Its Pathogenic Fungus Paecilomyces penicillatus Uncovers the Candidate Defense and Pathogenic Factors. Front. Microbiol. 2021, 12, 760444. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, X.; Wu, H.; Li, L.; Wang, J.; Sun, Y.; Gu, L.; Yu, Q. First Report of Clonostachys Rosea Causing Rot of Morchella sextelata in Anhui Province, China. Plant Dis. 2023, 107, 1623. [Google Scholar] [CrossRef]
- Lv, B.; Yu, S.; Chen, Y.; Yu, H.; Mo, Q. First Report of Lecanicillium aphanocladii Causing Rot of Morchella sextelata in China. Plant Dis. 2022, 106, 3202. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wu, H.; Wang, S.; Yu, Q.; Tian, D.; Xu, X. First Report of Trichoderma atroviride Causing Rot of Morchella sextelata in Anhui Province, China. Crop Prot. 2023, 168, 106206. [Google Scholar] [CrossRef]
- Shi, X.F.; Liu, D.; He, X.; Liu, W.; Yu, F. Epidemic Identification of Fungal Diseases in Morchella Cultivation Across China. J. Fungi 2022, 8, 1107. [Google Scholar] [CrossRef]
- Garajova, S.; Mathieu, Y.; Beccia, M.R.; Bennati-Granier, C.; Biaso, F.; Fanuel, M.; Ropartz, D.; Guigliarelli, B.; Record, E.; Rogniaux, H.; et al. Single-domain Flavoenzymes Trigger Lytic Polysaccharide Monooxygenases for 0xidative Degradation of Cellulose. Sci. Rep. 2016, 6, 28276. [Google Scholar] [CrossRef]
- Dong, W.; Chen, B.S.; Zhang, R.; Dai, H.Q.; Han, J.; Lu, Y.; Zhao, Q.; Liu, X.; Liu, H.; Sun, J. Identification and Characterization of Peptaibols As the Causing Agents of Pseudodiploöspora longispora Infecting the Edible Mushroom Morchella. J. Agric. Food Chem. 2023, 71, 18385–18394. [Google Scholar] [CrossRef]
- Xie, J.T.; Liu, X.; Qin, Z.L.; Mei, S.H.; Tarafder, E.; Li, C.; Zeng, X.Y.; Tian, F.H. Evolution and Related Pathogenic Genes of Pseudodiploöspora longispora on Morchella Based on Genomic Characterization and Comparative Genomic Analysis. Sci. Rep. 2024, 14, 18588. [Google Scholar] [CrossRef]
- Han, M.; Wang, Q.S.; Baiyintala; Wuhanqimuge. The Whole-genome Sequence Analysis of Morchella sextelata. Sci. Rep. 2019, 9, 15376. [Google Scholar] [CrossRef]
- Tan, H.; Kohler, A.; Miao, R.; Liu, T.; Zhang, Q.; Zhang, B.; Jiang, L.; Wang, Y.; Xie, L.; Tang, J.; et al. Multi-omic Analyses of Exogenous Nutrient Bag Decomposition by the Black Morel Morchella importuna Reveal Sustained Carbon Acquisition and Transferring. Environ. Microbiol. 2019, 21, 3909–3926. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, J.; Sun, L.; Bonito, G.; Guo, Y.; Li, Y.; Fu, Y. Genome Sequencing of Paecilomyces penicillatus Provides Insights into Its Phylogenetic Placement and Mycoparasitism Mechanisms on Morel Mushrooms. Pathogens 2020, 9, 834. [Google Scholar] [CrossRef]
- Jia, L.J.; Tang, H.Y.; Wang, W.Q.; Yuan, T.L.; Wei, W.Q.; Pang, B.; Gong, X.M.; Wang, S.F.; Li, Y.J.; Zhang, D.; et al. A Linear Nonribosomal Octapeptide from Fusarium graminearum Facilitates cell-to-cell Invasion of Wheat. Nat. Commun. 2019, 10, 922. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Li, X.M.; Sun, Z.C.; Meng, L.H.; Wang, B.G. Secondary Metabolites with Agricultural Antagonistic Potentials from Beauveria felina, a Marine-Derived Entomopathogenic Fungus. J. Agric. Food Chem. 2020, 68, 14824–14831. [Google Scholar] [CrossRef]
- Li, B.; Yuan, B.; Duan, J.; Qin, Y.; Shen, H.; Ren, J.; Francis, F.; Chen, M.; Li, G. Identification of Fcl-29 as an Effective Antifungal Natural Product against Fusarium graminearum and Combinatorial Engineering Strategy for Improving Its Yield. J. Agric. Food Chem. 2023, 71, 5554–5564. [Google Scholar] [CrossRef]
- Ni, Y.Y.; Mu, J.Y.; Sang, W.J. Identification of Aniseed Leaf Spot Pathogen with Toxicity Evaluation of Five Fungicides. J. Mt. Agric. Biol. 2010, 29, 360–363. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. True Morels (Morchella)—Nutritional and Phytochemical Composition, Health Benefits and Flavor: A Review. Crit. Rev. Food Sci. Nutr. 2017, 58, 1888–1901. [Google Scholar] [CrossRef]
- Zheng, Z.K.; Kong, L.; Dai, M.; Chen, Y.D.; Chen, Y.H. ADSC-Exos Outperform BMSC-Exos in Alleviating Hydrostatic Pressure-Induced Injury to Retinal Ganglion Cells by Upregulating Nerve Growth Factors. World J. Stem Cells 2023, 15, 1077–1092. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.Y.; Zheng, H.; Cai, Q.Y.; Luo, J.J.; Luo, K.; Li, S.J.; Yang, Y.L. Identification and Cultural Characterization of Diplospora Longispora Associated with Pileus Rot Disease on Cultivated Morel. Plant Prot. 2022, 48, 66–72. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Zhao, D.H.; Zhuang, F.Y.; Ou, C.G.; Yao, X.C.; Liang, C.; Zhang, Y.F.; Liu, X. First Report of Black Rot of Carrot Caused by Alternaria carotiincultae in China. Plant Dis. 2023, 108, 223. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.T.; Ma, K.L.; Sun, M.Y.; Zhang, J.M.; Liu, L.J.; Niu, S.Q. Isolation and Ddentification of Pathogens of Morchella sextelata Bacterial Disease. Front. Microbiol. 2023, 14, 1231353. [Google Scholar] [CrossRef]
- Samson, R.A. Paecilomyces and Some Allied Hyphomycetes. Stud. Mycol. 1974, 6, 1–119. [Google Scholar]
- Rocha, M.C.; de Godoy, K.F.; Bannitz-Fernandes, R.; Fabri, J.H.T.M.; Barbosa, M.M.F.; de Castro, P.A.; Almeida, F.; Goldman, G.H.; da Cunha, A.F.; Netto, L.E.S.; et al. Analyses of the three 1-Cys Peroxiredoxins from Aspergillus fumigatus reveal that cytosolic Prx1 is central to H2O2 metabolism and virulence. Sci. Rep. 2018, 8, 12314. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, L.F.; Ciosek, T.; Grogan, H.; McKeown, P.C.; Spillane, C.; Brychkova, G. Unravelling the Transcriptional Response of Agaricus Bisporus under Lecanicillium Fungicola Infection. Int. J. Mol. Sci. 2024, 25, 1283. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Coluccio, A.; Rolli, E.; Rodriguez-Peña, M.J.; Colasante, G.; Arroyo, J.; Neiman, A.M.; Popolo, L. GAS2 and GAS4, A Pair of Developmentally Regulated Genes Required for Spore Wall Assembly in Saccharomyces cerevisiae. Eukaryot. Cell 2007, 6, 302–316. [Google Scholar] [CrossRef]
- Zheng, Z.T.; Liu, H.Q.; Luo, X.; Liu, R.Z.; Joe, D.A.; Li, H.L.; Sun, H.Y.; Lin, Y.L.; Li, Y.Z.; Wang, Y.P. Comparative Transcriptome Analysis Provides Insights into the Resistance Regulation Mechanism and Inhibitory Effect of Fungicide Phenamacril in Fusarium Asiaticum. Pestic. Biochem. Physiol. 2024, 201, 105848. [Google Scholar] [CrossRef]
- Droce, A.; Sørensen, J.L.; Sondergaard, T.E.; Rasmussen, J.J.; Lysøe, E.; Giese, H. PTR2 Peptide Transporters in Fusarium Graminearum Influence Secondary Metabolite Production and Sexual Development. Fungal. Biol. 2017, 121, 515–527. [Google Scholar] [CrossRef]
- Coton, E.; Coton, M.; Hymery, N.; Mounier, J.; Jany, J.L. Penicillium Roqueforti: An Overview of Its Genetics, Physiology, Metabolism and Biotechnological Applications. Fungal Biol. Rev. 2020, 34, 59–73. [Google Scholar] [CrossRef]
- Schuler, D.; Wahl, R.; Wippel, K.; Vranes, M.; Münsterkötter, M.; Sauer, N.; Kämper, J. Hxt1, A Monosaccharide Transporter and Sensor Required for Virulence of the Maize Pathogen Ustilago Maydis. New Phytol. 2015, 206, 1086–1100. [Google Scholar] [CrossRef]
- Li, G.L.; Xu, Y.; Xing, P.J.; Gao, T.T.; Ji, R.Q. Pathogenicity of Trichoderma spp. to Lentinula Edodes and the Inhibitory Effects of Biocontrol Agents. Mol. Plant Breed. 2019, 17, 6530–6534. [Google Scholar] [CrossRef]
- Cantu, D.; Vicente, A.R.; Labavitch, J.M.; Bennett, A.B.; Powell, A.L. Strangers in the Matrix: Plant Cell Walls and Pathogen Susceptibility. Trends Plant Sci. 2008, 13, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Arana, D.M.; Prieto, D.; Roman, E.; Nombela, C.; Alonso-Monge, R.; Pla, J. The Role of the Cell Wall in Fungal Pathogenesis. Microb. Biotechnol. 2009, 2, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell Wall Integrity Maintenance During Plant Development and Interaction with the Environment. Nat. Plants. 2019, 5, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Hamann, T. Plant Cell Wall Integrity Maintenance in Model Plants and Crop Species-Relevant Cell Wall Components and Underlying Guiding Principles. Cell. Mol. Life Sci. 2020, 77, 2049–2077. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Li, D.Y.; Zhang, H.J.; Song, F.M. Cell Wall-Mediated Disease Resistance and its Molecular Mechanism in Plants. Plant Physiol. J. 2011, 47, 661–668. [Google Scholar] [CrossRef]
- Liang, Y.L.; Zhao, J.; Liu, L.; Yang, J.; Li, C.Y. The Role of the Plant Cell Wall in Plant Pathogen Interactions. Mol. Plant Breed. 2016, 14, 1255–1261. [Google Scholar] [CrossRef]
- Chi, L.X.; Xie, Z.P.; Feng, R.; Du, X.Q.; Lu, X.Y.; Fan, W.Q.; Gao, H.; Yue, S.J.; Zheng, R. Comparative Transcriptome Analysis of Lycium Barbarum Response to Fusarium Solani Stress. Mol. Plant Breed. 2024, in press.
- Hu, J.J. Study on the Identification and Infection Mechanism of Pleurotus eryngii Pathogenic Bacteria. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2018. [Google Scholar]
- Liu, J.B.; Wang, L.H.; Zuo, X.L. Cell Membranes Functionalization Based on DNA. Prog. Chem. 2019, 31, 1067–1074. [Google Scholar] [CrossRef]
- Kang, Z.T.; Jiang, L.M.; Luo, Y.Y.; Liu, C.J.; Li, X.R. The Research Advances of Mechanism of Pathogenicity of Alternaria Phytopathogenic Fungi. Chin. Bull. Life Sci. 2013, 25, 908–914. [Google Scholar] [CrossRef]
- Wiczew, D.; Szulc, N.; Tarek, M. Molecular Dynamics Simulations of the Effects of Lipid Oxidation on the Permeability of Cell Membranes. Bioelectrochemistry 2021, 141, 107869. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, Y.; Iida, A.; Kambara, T.; Asami, K.; Tachikawa, E.; Fujita, T. Role of Proline Residue in the Channel-Forming and Catecholamine-Releasing Activities of the Peptaibol, Trichosporin-B-VIa. Biochim. Biophys. Act. 1996, 1, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lubkowitz, M. The Oligopeptide Transporters: A Small Gene Family with A Diverse Group of Substrates and Functions? Mol. Plant 2011, 4, 407–415. [Google Scholar] [CrossRef]
- Frederickson Matika, D.E.; Loake, G.J. Redox Regulation in Plant Immune Function. Free Radic. Biol. Med. 2014, 21, 1373–1388. [Google Scholar] [CrossRef]


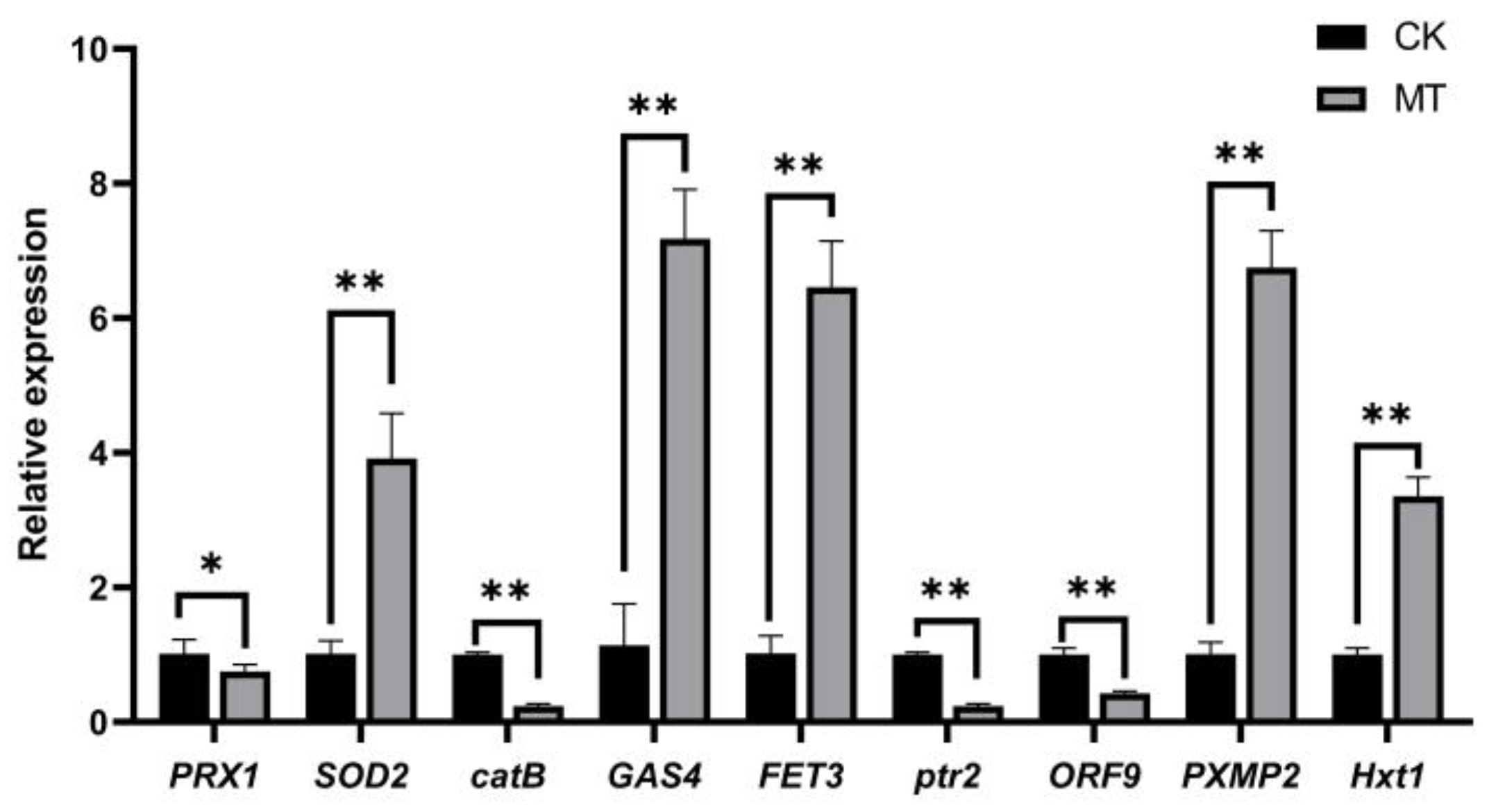

| Gene ID | Description | Gene Name | Function | Organism |
|---|---|---|---|---|
| gene-H6S33_012182 | Mitochondrial peroxiredoxin | PRX1 | reduces hydrogen peroxide and alkyl hydroperoxides | Aspergillus fumigatus [36] |
| gene-H6S33_004573 | Manganese superoxide dismutase | SOD2 | neutralizes superoxide anion radicals and protects cells from oxidative stress | Agaricus bisporus [37] |
| gene-H6S33_004442 | Catalase B | catB | serves to protect cells from the toxic effects of hydrogen peroxide | |
| gene-H6S33_008738 | 1,3-beta-glucanosyltransferase | GAS4 | involved with Gas2p in spore wall assembly | Saccharomyces cerevisiae [38] |
| gene-H6S33_005983 | Iron transport multicopper oxidase | FET3 | multicopper oxidase that oxidizes ferrous (Fe2+) to ferric iron (Fe3+) for subsequent cellular uptake by transmembrane permease Ftr1p | Fusarium graminearum [39] |
| gene-H6S33_000069 | Probable peptide transporter | ptr2 | transport nitrogen-containing substrates | Fusarium graminearum [40] |
| gene-H6S33_000010 | Cytochrome P450 monooxygenase | ORF9 | involved in the metabolism of various endogenous substrates, including fatty acids, steroid hormones, and vitamins | Penicillium roqueforti [41] |
| gene-H6S33_005547 | Peroxisomal membrane protein 2 | PXMP2 | involved in pore-forming activity and may contribute to the unspecific permeability of the peroxisomal membrane | NO |
| gene-H6S33_006216 | Hexose transporter | Hxt1 | monosaccharide transporter and sensor | Ustilago maydis [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, J.; Wang, T.; Li, T.; Xu, L.; Cheng, Y.; Chang, M.; Meng, J.; Hou, L. Integrated Transcriptomics–Proteomics Analysis Reveals the Response Mechanism of Morchella sextelata to Pseudodiploöspora longispora Infection. J. Fungi 2024, 10, 604. https://doi.org/10.3390/jof10090604
Wang S, Wang J, Wang T, Li T, Xu L, Cheng Y, Chang M, Meng J, Hou L. Integrated Transcriptomics–Proteomics Analysis Reveals the Response Mechanism of Morchella sextelata to Pseudodiploöspora longispora Infection. Journal of Fungi. 2024; 10(9):604. https://doi.org/10.3390/jof10090604
Chicago/Turabian StyleWang, Shurong, Jingyi Wang, Tengyun Wang, Tonglou Li, Lijing Xu, Yanfen Cheng, Mingchang Chang, Junlong Meng, and Ludan Hou. 2024. "Integrated Transcriptomics–Proteomics Analysis Reveals the Response Mechanism of Morchella sextelata to Pseudodiploöspora longispora Infection" Journal of Fungi 10, no. 9: 604. https://doi.org/10.3390/jof10090604
APA StyleWang, S., Wang, J., Wang, T., Li, T., Xu, L., Cheng, Y., Chang, M., Meng, J., & Hou, L. (2024). Integrated Transcriptomics–Proteomics Analysis Reveals the Response Mechanism of Morchella sextelata to Pseudodiploöspora longispora Infection. Journal of Fungi, 10(9), 604. https://doi.org/10.3390/jof10090604






