Study on Enzyme Activity and Metabolomics during Culture of Liquid Spawn of Floccularia luteovirens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Strains
2.2. Main Instruments and Equipment
2.3. Culture Medium Preparation and Liquid Spawn Culture
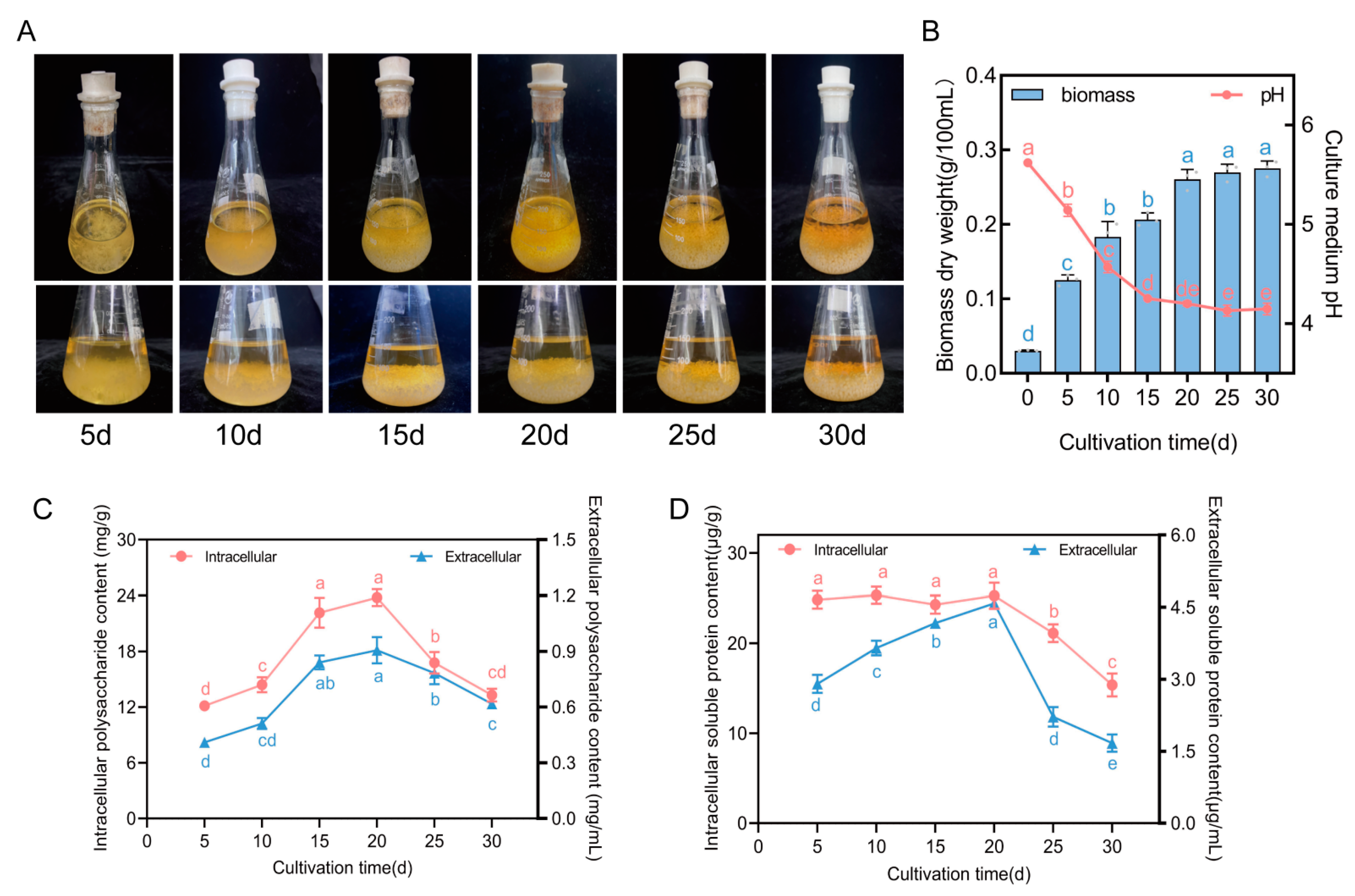
2.4. Sample Collection and Physiological Index Measurement
2.5. Non-Targeted Metabolomics Analysis by GC-MS and LC-MS Coupling
2.5.1. Sample Pretreatment
2.5.2. LC-MS Conditions
2.5.3. GC-MS Conditions
2.5.4. Data Preprocessing and Statistical Analysis
3. Results
3.1. Changes in Four Physiological Indexes of F. luteovirens Liquid Spawn
3.2. Activity Changes in Seven Enzymes in F. luteovirens Liquid Spawn
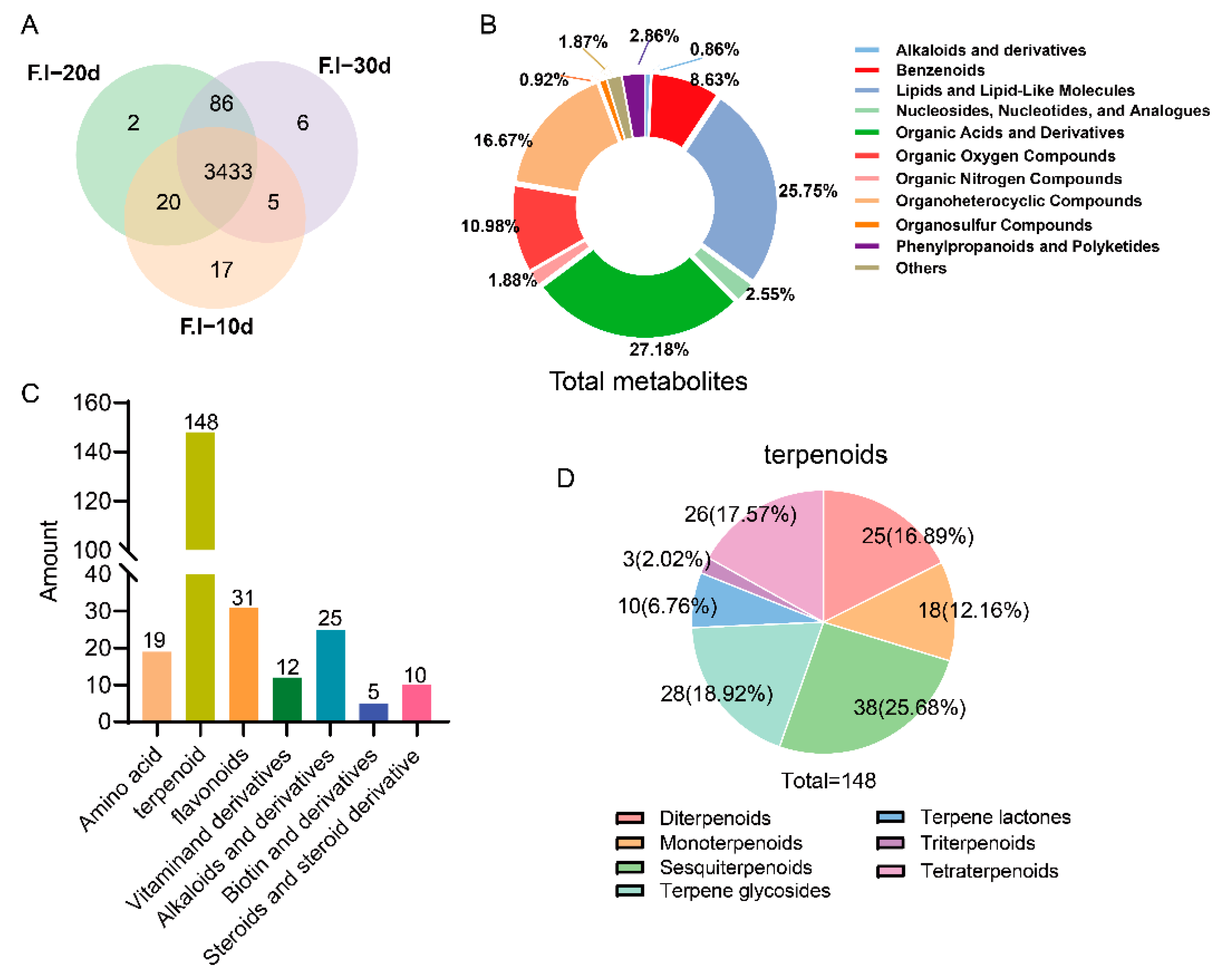
3.3. Comprehensive Analysis of Primary Metabolites of F. luteovirens Liquid Spawn
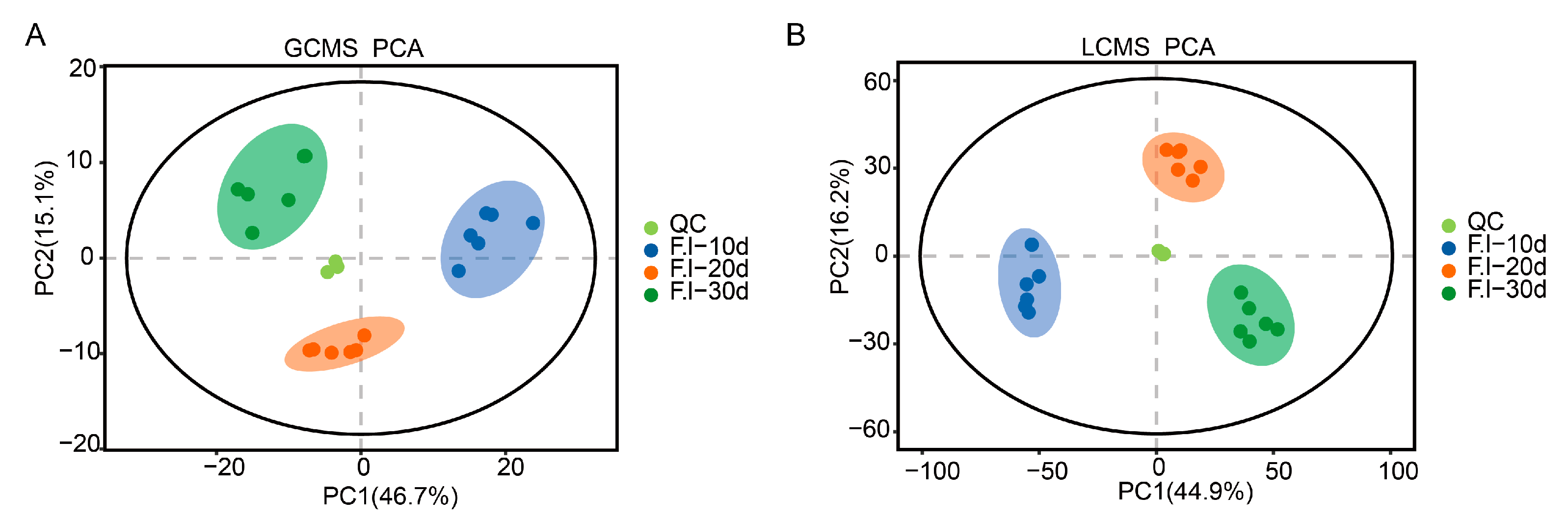
3.4. Principal Component Analysis (PCA) of Mycelium at Different Culture Stages
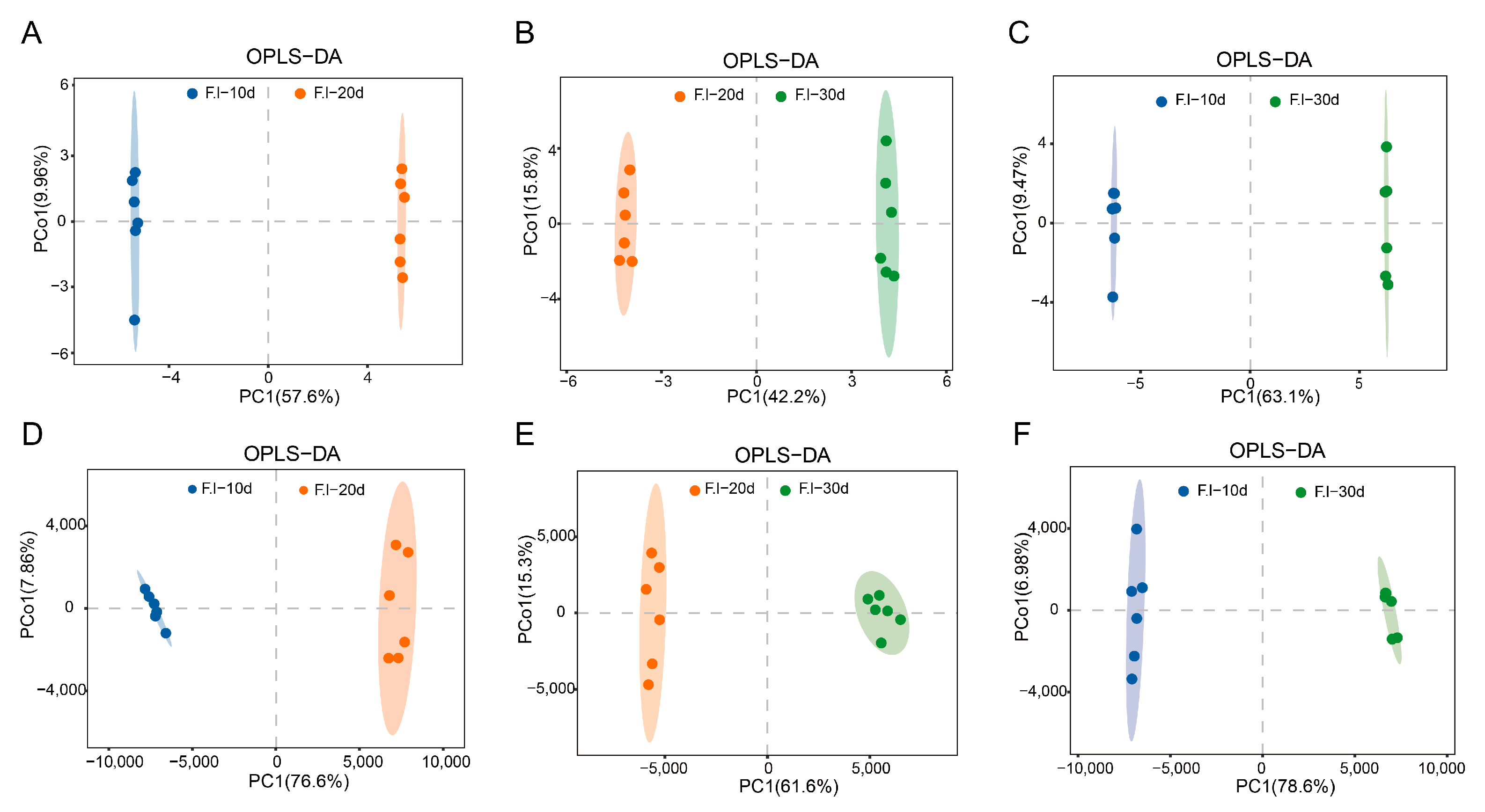
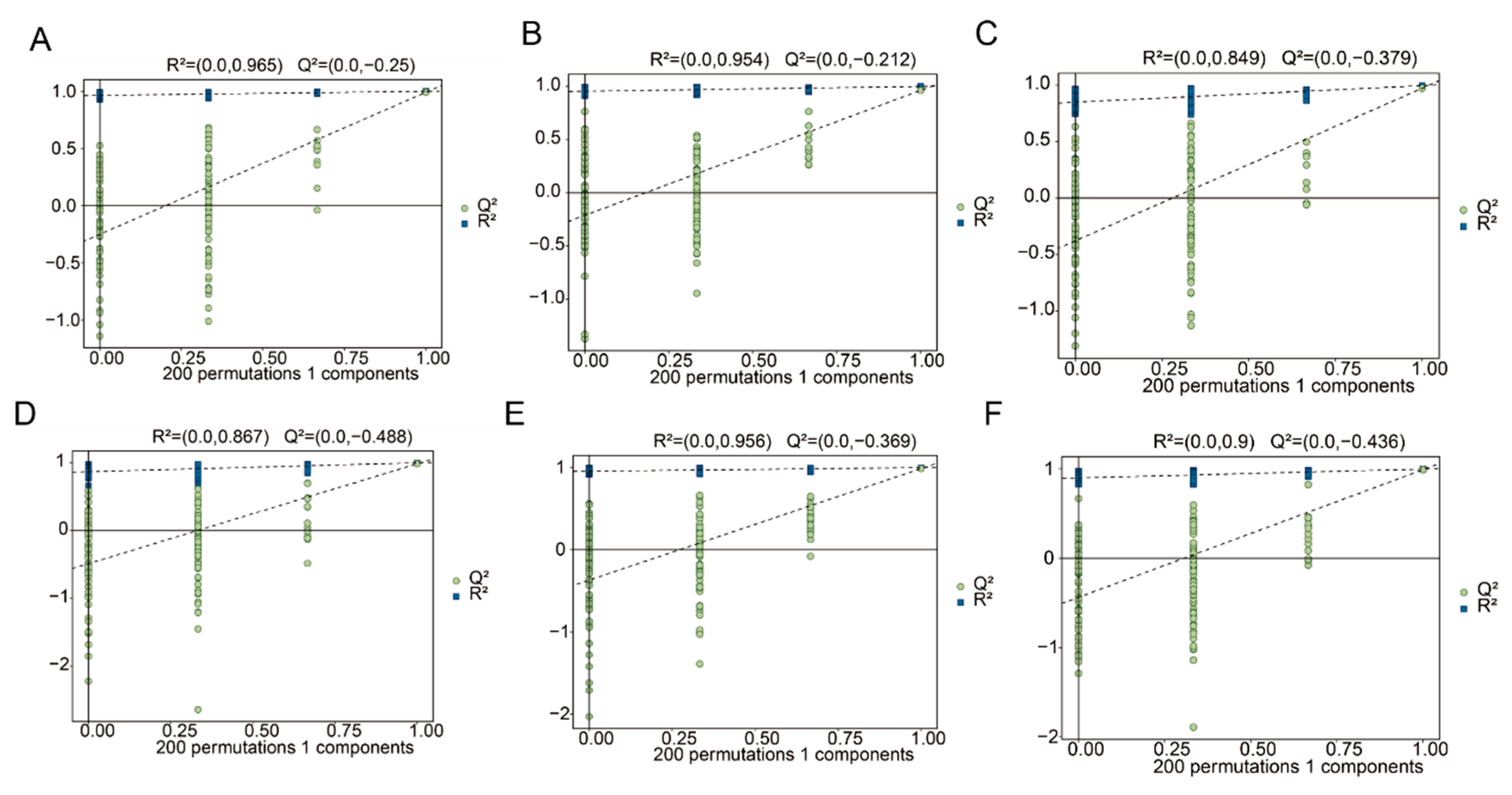
3.5. Orthogonal Partial Least Squares (OPLS-DA) Analysis
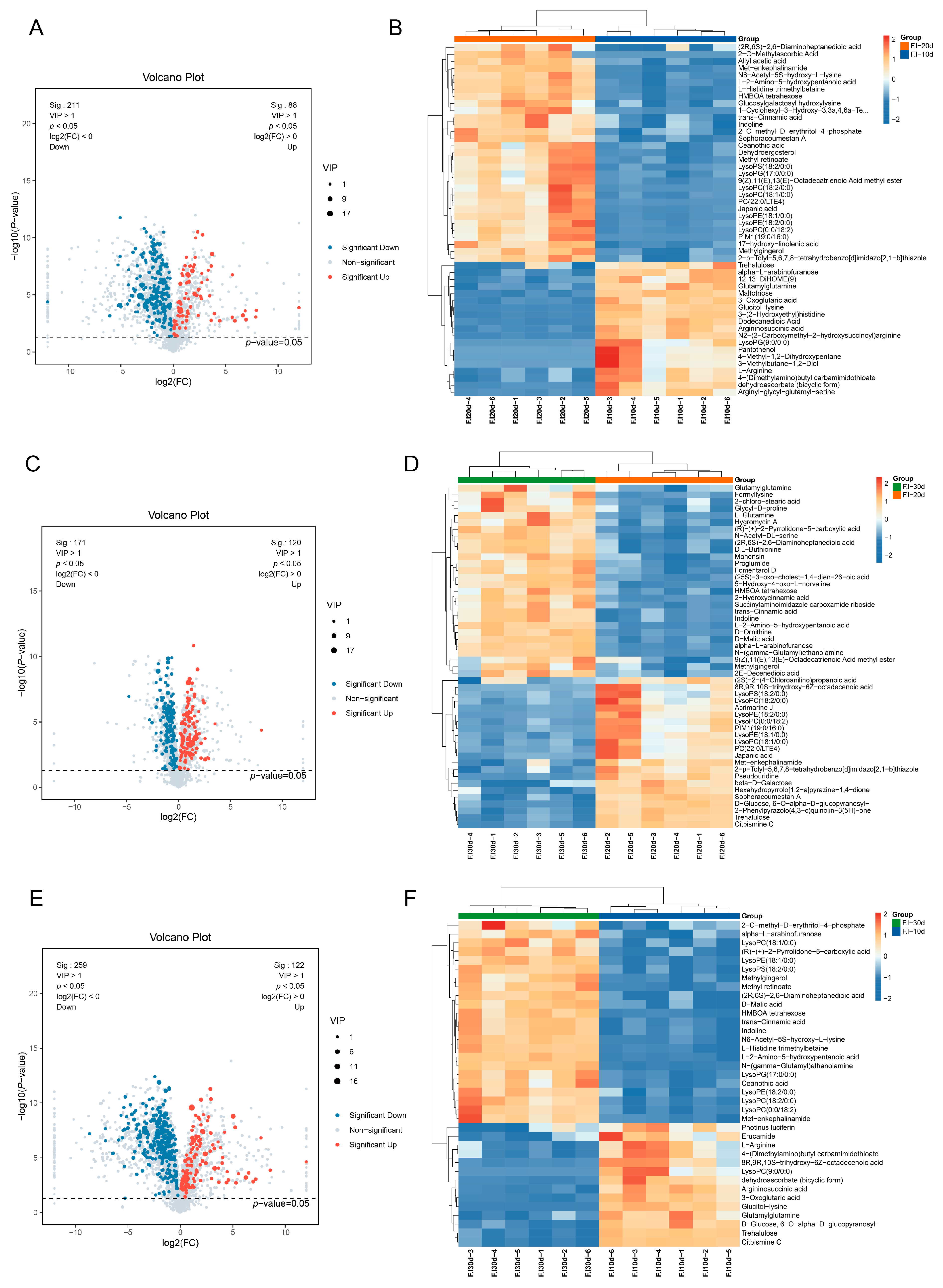
3.6. Statistical Analysis of Differential Metabolites
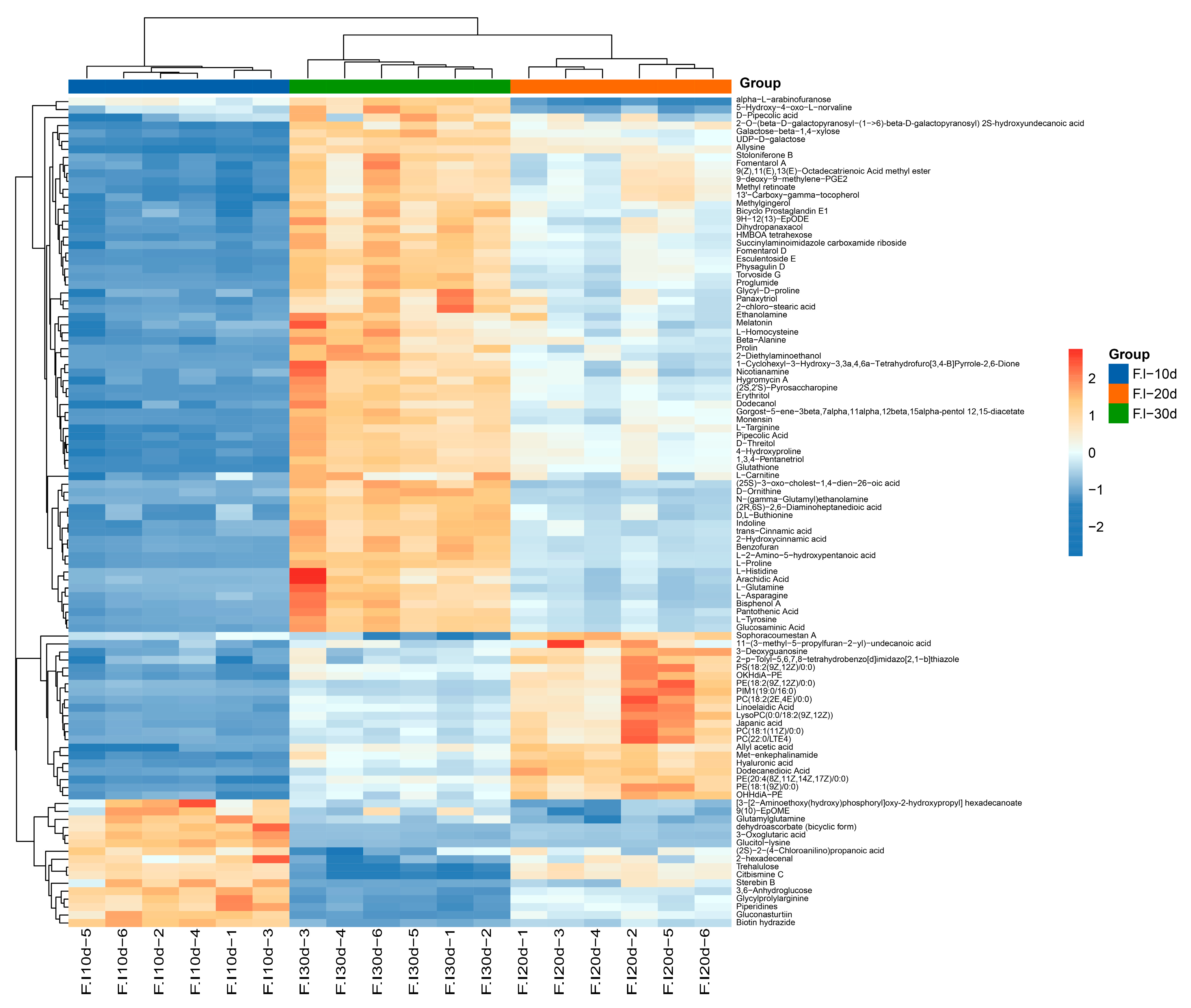
3.7. Comparative Analysis of Differential Metabolites
3.8. KEGG Pathway Enrichment Analysis of Differential Metabolites
4. Discussion and Conclusions
4.1. Analysis of Changes in Enzyme Activity of F. luteovirens Liquid Spawn
4.2. Analysis of Metabolic Products of F. luteovirens Mycelium
4.3. Differential Metabolic Pathway Analysis
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, R.; Gao, Q.; Zhang, F.; Fu, P.; Wang, J.; Yan, H.; Chen, S. Genetic variation and phylogenetic relationships of the ectomycorrhizal Floccularia luteovirens on the Qinghai-Tibet Plateau. J. Microbiol. 2017, 55, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Tao, Y.; Wang, W.; Mei, L.; Shao, Y.; Wang, Q.; Dang, J. Chemical Constituents of Fruit Body of Armillaria luteo-virens. Chem. Nat. Compd. 2019, 55, 373–375. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Cao, L.; Li, W.; Zhang, Q.; Feng, R.; Zhao, Z.; Zhao, X. The Research Status and Prospects of Floccularia luteovirens: A Mycorrhizal Fungus with Edible Fruiting Bodies. J. Fungi 2023, 9, 1071. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Zhang, W.-R.; Liu, S.-R.; Kuang, Y.-B.; Zheng, S.-Z. Development of a Novel Spawn (Block Spawn) of an Edible Mushroom, Pleurotus ostreatus, in Liquid Culture and its Cultivation Evaluation. Mycobiology 2019, 47, 97–104. [Google Scholar] [CrossRef]
- Kawai, G.; Kobayashi, H.; Fukushima, Y.; Ohsaki, K. Effect of liquid mycelial culture used as a spawn on sawdust cultivation of shiitake (Lentinula edodes). Mycoscience 1996, 37, 201–207. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.H.; Li, F.; Wang, D.J.; Bu, W.L.; Ding, X.X.; Shang, Y.L. Optimization of semi-continuous fermentation conditions and preservation of Flammulina velutipes liquid strains. J. Food Process Pres. 2019, 43, e14010. [Google Scholar] [CrossRef]
- Xv, W.; Zheng, Q.; Ye, Z.-W.; Wei, T.; Guo, L.-Q.; Lin, J.-F.; Zou, Y. Submerged Culture of Edible and Medicinal Mushroom Mycelia and Their Applications in Food Products: A Review. Int. J. Med. Mushrooms 2024, 26, 1–13. [Google Scholar] [CrossRef]
- Qin, P.; Li, T.; Liu, C.; Liang, Y.; Sun, H.; Chai, Y.; Yang, T.; Gong, X.; Wu, Z. Extraction and utilization of active substances from edible fungi substrate and residue: A review. Food Chem. 2023, 398, 133872. [Google Scholar] [CrossRef]
- Harris, E.D. Regulation of antioxidant enzymes1. FASEB J. 1992, 6, 2675–2683. [Google Scholar] [CrossRef]
- Kang, C.; Zeng, W.; Zheng, X.; Yang, L.; Liu, Z.; Wang, W. Changes of TTC-Dehydrogenase and Extra-cellular Enzyme Activities for Morchella sextelata Mycelium. North. Hortic. 2022, 5, 119–124. (In Chinese) [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Xiang, Q. A Fast Text Means of Auricularia auricular-judae Hypha Activity in Laboratory. North. Hortic. 2016, 24, 136–139. (In Chinese) [Google Scholar] [CrossRef]
- Berger, R.G.; Bordewick, S.; Krahe, N.-K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Men, Y.; Zhang, J.; Liu, H.; Sun, Y. Structural characterization and immunomodulatory activity of exopolysaccharides from submerged culture of Auricularia auricula-judae. Int. J. Biol. Macromol. 2018, 115, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Liu, Y.; Tang, C. Recent Advances in the Preparation, Structure, and Biological Activities of β-Glucan from Ganoderma Species: A Review. Foods 2023, 12, 2975. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Lei, J.-Y.; Li, S.-L.; Guo, L.-Q.; Lin, J.-F.; Wu, G.-H.; Lu, J.; Ye, Z.-W. Progress in biological activities and biosynthesis of edible fungi terpenoids. Crit. Rev. Food Sci. 2023, 63, 7288–7310. [Google Scholar] [CrossRef]
- Panaccione, D.G. Derivation of the multiply-branched ergot alkaloid pathway of fungi. Microb. Biotechnol. 2023, 16, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Kapri, M.; Tiwari, A.; Sharma, S. Medicinal importance of mushroom mycelium: Mechanisms and applications. J. Funct. Foods 2019, 56, 182–193. [Google Scholar] [CrossRef]
- Goodacre, R. Metabolic profiling: Pathways in discovery. Drug Discov. Today 2004, 9, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Ren, R.; Tang, S.; Zhou, Y.; Cai, M.; Zhao, W.; He, Y.; Xu, J. Transcriptomics combined with metabolomics unveiled the key genes and metabolites of mycelium growth in Morchella importuna. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hengchao, E.; Dong, H.; Zhang, Y.; Qiu, J.; Qian, Y.; Zhou, C. Combination of untargeted metabolomics approach and molecular networking analysis to identify unique natural components in wild Morchella sp. by UPLC-Q-TOF-MS. Food Chem. 2022, 366, 130642. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, Q.; Miao, R.; Lin, J.; Feng, R.; Ni, Y.; Li, W.; Yang, D.; Zhao, X. Application of omics technology in the research on edible fungi. Curr. Res. Food Sci. 2023, 6, 100430. [Google Scholar] [CrossRef]
- dos Santos, F.A.; Sousa, I.P.; Furtado, N.A.J.C.; Da Costa, F.B. Combined OPLS-DA and decision tree as a strategy to identify antimicrobial biomarkers of volatile oils analyzed by gas chromatography–mass spectrometry. Rev. Bras. Farmacogn. 2018, 28, 647–653. [Google Scholar] [CrossRef]
- Song, J.; Yuan, W.; Zhou, Z.; Wang, W.; Lu, N.; Cheng, J.; Yan, J. Activities of extracellular enzymes and polysaccha-rides in liquid culture of Sanghuangporus. Mycosystema 2020, 39, 352–361. (In Chinese) [Google Scholar] [CrossRef]
- Yang, X.; Zhao, F.; Deng, B.; Xie, X. Extracellular Enzyme Metabolism of Tremella aurantialba. Food Res. Dev. 2021, 42, 165–170. (In Chinese) [Google Scholar] [CrossRef]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine, recent developments. Redox Biol. 2021, 42, 101868. [Google Scholar] [CrossRef]
- Xiong, K.; Xue, S.; Guo, H.; Dai, Y.; Ji, C.; Dong, L.; Zhang, S. Ergothioneine: New functional factor in fermented foods. Crit. Rev. Food Sci. 2023, 64, 7505–7516. [Google Scholar] [CrossRef]
- Huang, T.; Tang, L.; Zheng, T.; Wan, J.; Zou, G.; Bao, D.; Li, F.; Gong, M. Effects of arginine on the mycelial growth of Hypsizygus marmoreus. Mycosystema 2023, 42, 609–619. (In Chinese) [Google Scholar] [CrossRef]
- Chang, T.; Zhao, Y.; Yang, H.; Song, X.; Dong, Q.; Chen, M. Optimization of compound amino acid medium for Lentinula edodes and the effect on mycelial growth and physiology. Microbiol. China 2022, 49, 545–555. (In Chinese) [Google Scholar] [CrossRef]
- Gabius, H.-J. The sugar code: Why glycans are so important. Biosystems 2018, 164, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Yu, M.-H.; Xu, L.-J.; Niu, L.-X.; Huang, C.-Y.; Xu, H.; Yang, P.-M.; Hu, X. Diels–Alder type adducts with potent alpha-glucosidase inhibitory activity from Morus macroura. Phytochem. Lett. 2018, 26, 149–153. [Google Scholar] [CrossRef]
- Oshima, Y.; Saito, J.; Hikino, H. Sterebins A, B, C and D, bisnorditerpenoids of leaves. Tetrahedron 1986, 42, 6443–6446. [Google Scholar] [CrossRef]
- Cayetano-Salazar, L.; Olea-Flores, M.; Zuñiga-Eulogio, M.D.; Weinstein-Oppenheimer, C.; Fernández-Tilapa, G.; Mendoza-Catalán, M.A.; Zacapala-Gómez, A.E.; Ortiz-Ortiz, J.; Ortuño-Pineda, C.; Navarro-Tito, N. Natural isoflavonoids in invasive cancer therapy: From bench to bedside. Phytother. Res. 2021, 35, 4092–4110. [Google Scholar] [CrossRef]
- Murata, T.; Kohno, S.; Ogawa, K.; Ito, C.; Itoigawa, M.; Ito, M.; Hikita, K.; Kaneda, N. Cytotoxic activity of dimeric acridone alkaloids derived from Citrus plants towards human leukaemia HL-60 cells. J. Pharm. Pharmacol. 2020, 72, 1445–1457. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Santarelli, F.; Paul, S.; Dumeignil, F.; Cavani, F.; Gonçalves, R.V. Recent developments in maleic acid synthesis from bio-based chemicals. Sustain. Chem. Process. 2015, 3, 9. [Google Scholar] [CrossRef]
- Gai, Y.; Li, L.; Ma, H.; Riely, B.K.; Liu, B.; Li, H. Critical Role of MetR/MetB/MetC/MetX in Cysteine and Methionine Metabolism, Fungal Development, and Virulence of Alternaria alternata. Appl. Environ. Microbiol. 2021, 87, e01911-20. [Google Scholar] [CrossRef]
- Lu, H.; Wang, S.; Gu, T.; Sun, L.; Wang, Y. Roles of the Fungal-Specific Lysine Biosynthetic Pathway in the Nematode-Trapping Fungus Arthrobotrys oligospora Identified through Metabolomics Analyses. J. Fungi 2023, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tian, L.; Qiu, H.; Qin, C.; Ling, S.; Xu, J. Metabolomics Analysis of Sporulation-Associated Metabolites of Metarhizium anisopliae Based on Gas Chromatography-Mass Spectrometry. J. Fungi 2023, 9, 1011. [Google Scholar] [CrossRef]
- Miyamoto, T.; Homma, H. D-Amino acid metabolism in bacteria. J. Biochem. 2021, 170, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bastings, J.J.A.J.; van Eijk, H.M.; Olde Damink, S.W.; Rensen, S.S. d-amino Acids in Health and Disease: A Focus on Cancer. Nutrients 2019, 11, 2205. [Google Scholar] [CrossRef] [PubMed]
- Cunin, R.; Glansdorff, N.; Piérard, A.; Stalon, V. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 1986, 50, 314–352. [Google Scholar] [CrossRef]
- Charlier, D.; Bervoets, I. Regulation of arginine biosynthesis, catabolism and transport in Escherichia coli. Amino Acids 2019, 51, 1103–1127. [Google Scholar] [CrossRef]
- Milner, J.L.; Vink, B.; Wood, J.M. Transmembrane Amino Acid Flux in Bacterial Cells. Crit. Rev. Biotechnol. 1987, 5, 1–47. [Google Scholar] [CrossRef]
- Lan, J.; Zhang, L.; Gao, J.; He, R. TrLys9 participates in fungal development and lysine biosynthesis in Trichoderma reesei. J. Gen. Appl. Microbiol. 2023, 69, 159–166. [Google Scholar] [CrossRef]


| Types of Metabolites | F.l-10d vs. F.l-20d | F.l-20d vs. F.l-30d | F.l-10d vs. F.l-30d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | UP | Down | Total | UP | Down | Total | UP | Down | |
| Azacyclic compounds | 1 | 0 | 1 | 5 | 2 | 3 | 6 | 2 | 4 |
| Furans | 5 | 1 | 4 | 7 | 4 | 3 | 7 | 4 | 3 |
| Nucleotides and derivatives | 8 | 1 | 7 | 11 | 6 | 5 | 11 | 3 | 8 |
| Flavonoids | 3 | 2 | 1 | 1 | 1 | 0 | 2 | 2 | 0 |
| Harmala alkaloids | 3 | 1 | 2 | 3 | 3 | 0 | 3 | 2 | 1 |
| Pteridines and derivatives | 5 | 2 | 3 | 1 | 0 | 1 | 5 | 2 | 3 |
| Glycerophospholipids | 32 | 10 | 22 | 27 | 19 | 8 | 29 | 8 | 21 |
| Lactones | 4 | 2 | 2 | 1 | 0 | 1 | 4 | 2 | 2 |
| Organic phosphoric acids and derivatives | 3 | 1 | 2 | 3 | 2 | 1 | 3 | 1 | 2 |
| Phenols | 3 | 1 | 2 | 1 | 0 | 1 | 5 | 2 | 3 |
| Benzene and substituted derivatives | 11 | 4 | 7 | 9 | 4 | 5 | 13 | 6 | 7 |
| Biotin and derivatives | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| Carboxylic acids and derivatives | 69 | 22 | 47 | 85 | 21 | 64 | 95 | 28 | 67 |
| Cinnamic acids and derivatives | 3 | 0 | 3 | 3 | 1 | 2 | 2 | 0 | 2 |
| Benzodiazepines | 4 | 1 | 3 | 2 | 1 | 1 | 5 | 2 | 3 |
| Fatty acyls | 43 | 6 | 37 | 43 | 21 | 22 | 52 | 12 | 40 |
| Hydroxy acids and derivatives | 4 | 0 | 4 | 4 | 2 | 2 | 7 | 1 | 6 |
| Indoles and their derivatives | 3 | 0 | 3 | 4 | 1 | 3 | 3 | 0 | 3 |
| Keto acids and derivatives | 3 | 1 | 2 | 2 | 1 | 1 | 4 | 2 | 2 |
| Organonitrogen compounds | 6 | 1 | 5 | 7 | 2 | 5 | 7 | 2 | 5 |
| Organooxygen compounds | 42 | 18 | 24 | 38 | 17 | 21 | 53 | 22 | 31 |
| Prenol lipids | 12 | 1 | 11 | 9 | 4 | 5 | 17 | 3 | 14 |
| Steroids and steroid derivatives | 14 | 2 | 12 | 13 | 1 | 12 | 25 | 2 | 23 |
| Other | 17 | 10 | 17 | 11 | 6 | 5 | 22 | 13 | 9 |
| Total | 299 | 88 | 221 | 291 | 120 | 171 | 381 | 122 | 259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Y.; Liao, Q.; Gou, S.; Shi, T.; Li, W.; Feng, R.; Zhao, Z.; Zhao, X. Study on Enzyme Activity and Metabolomics during Culture of Liquid Spawn of Floccularia luteovirens. J. Fungi 2024, 10, 618. https://doi.org/10.3390/jof10090618
Ni Y, Liao Q, Gou S, Shi T, Li W, Feng R, Zhao Z, Zhao X. Study on Enzyme Activity and Metabolomics during Culture of Liquid Spawn of Floccularia luteovirens. Journal of Fungi. 2024; 10(9):618. https://doi.org/10.3390/jof10090618
Chicago/Turabian StyleNi, Yanqing, Qiuhong Liao, Siyuan Gou, Tongjia Shi, Wensheng Li, Rencai Feng, Zhiqiang Zhao, and Xu Zhao. 2024. "Study on Enzyme Activity and Metabolomics during Culture of Liquid Spawn of Floccularia luteovirens" Journal of Fungi 10, no. 9: 618. https://doi.org/10.3390/jof10090618






