Development of a Multiplex Real-Time PCR Assay for the Simultaneous Detection of Two Fungal Pathogens Causing Pneumonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primer/Probe Design
2.2. Storage of Clinical Specimens
2.3. Nucleic Acid Extraction
2.4. Aspergillus spp. Screening and Aspergillus fumigatus—Specific PCR and Sanger Sequencing
2.5. RF2 mRT-PCR and Control Isolates
2.6. Analytical Performance
2.7. Clinical Performance of RF2 mRT-PCR Assay
2.8. Statistical Analysis
3. Results
3.1. Analytical Specificity of the RF2 mRT-PCR Assay
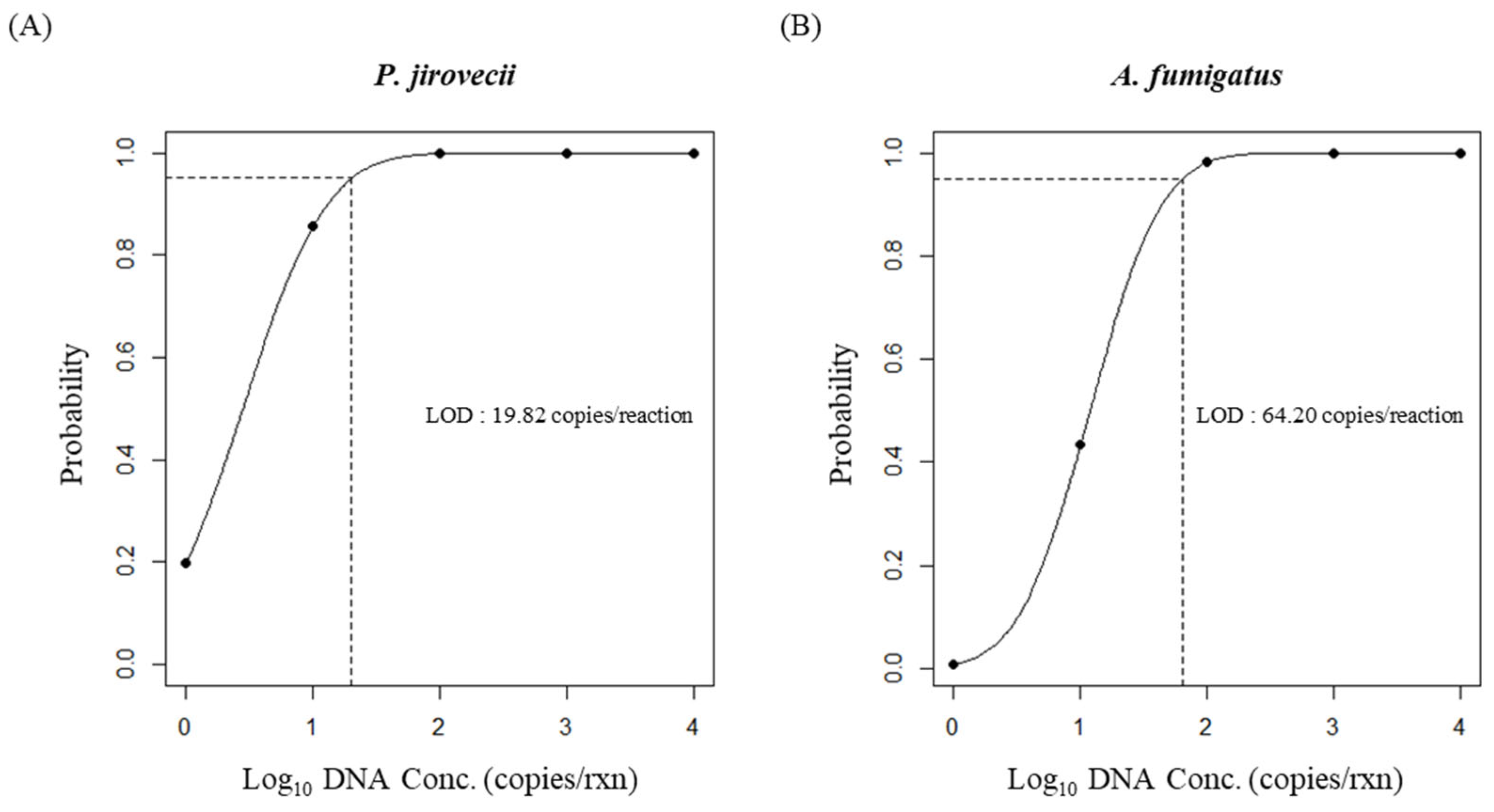
3.2. The Analytical Sensitivity and LOD of the RF2 mRT-PCR Assay
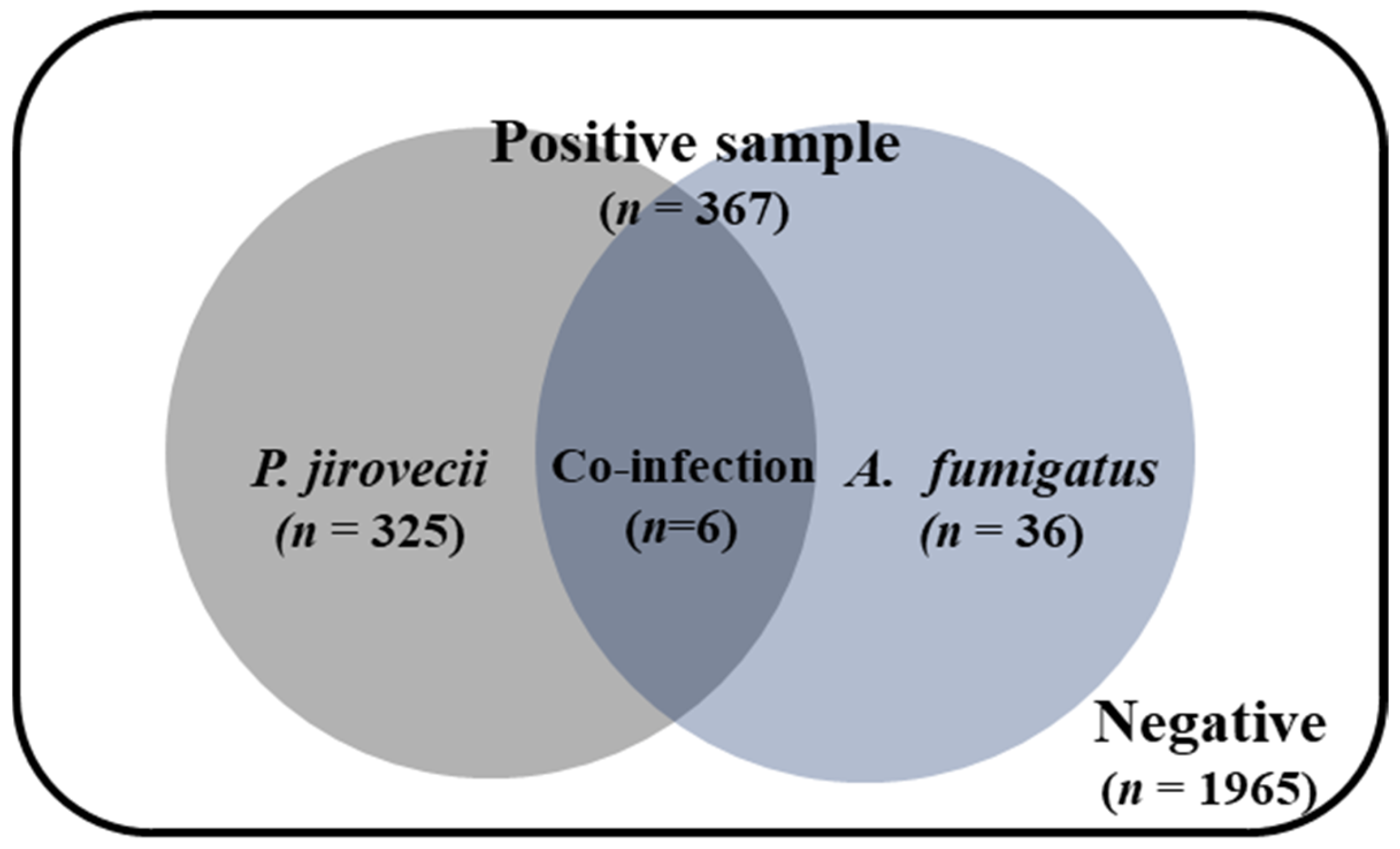
3.3. Aspergillus and P. jirovecii Co-Infection Rates in Clinical Samples
3.4. Comparison of RF2 mRT-PCR with the Reference Assays Using Clinical Specimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Report. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. 2022. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 4 June 2024).
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef]
- Kousha, M.; Tadi, R.; Soubani, A.O. Pulmonary aspergillosis: A clinical review. Eur. Respir. Rev. 2011, 20, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Balajee, S.A.; Park, B.J. Pneumocystis Jirovecii Pneumonia: Current Knowledge and Outstanding Public Health Issues. Curr. Fungal Infect. Rep. 2010, 4, 229–237. [Google Scholar] [CrossRef]
- Li, Z.; Lu, G.; Meng, G. Pathogenic Fungal Infection in the Lung. Front. Immunol. 2019, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.H.; Rodriguez-Barradas, M.C.; Whitaker, M.; Jackson, B.R.; Kim, L.; Surie, D.; Cikesh, B.; Lindsley, M.D.; McCotter, O.Z.; Berkow, E.L.; et al. Fungal Pathogens as Causes of Acute Respiratory Illness in Hospitalized Veterans: Frequency of Fungal Positive Test Results Using Rapid Immunodiagnostic Assays. J. Fungi 2023, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention Explore Topics. Clinical Overview of Aspergillosis. Available online: https://www.cdc.gov/aspergillosis (accessed on 24 April 2024).
- Lamoth, F.; Calandra, T. Pulmonary aspergillosis: Diagnosis and treatment. Eur. Respir. Rev. 2022, 31, 220114. [Google Scholar] [CrossRef]
- Stemler, J.; Tobben, C.; Lass-Florl, C.; Steinmann, J.; Ackermann, K.; Rath, P.M.; Simon, M.; Cornely, O.A.; Koehler, P. Diagnosis and Treatment of Invasive Aspergillosis Caused by Non-fumigatus Aspergillus spp. J. Fungi 2023, 9, 500. [Google Scholar] [CrossRef]
- Bateman, M.; Oladele, R.; Kolls, J.K. Diagnosing Pneumocystis jirovecii pneumonia: A review of current methods and novel approaches. Med. Mycol. 2020, 58, 1015–1028. [Google Scholar] [CrossRef]
- Douglas, A.P.; Smibert, O.C.; Bajel, A.; Halliday, C.L.; Lavee, O.; McMullan, B.; Yong, M.K.; van Hal, S.J.; Chen, S.C.; Australasian Antifungal Guidelines Steering, C. Consensus guidelines for the diagnosis and management of invasive aspergillosis, 2021. Intern. Med. J. 2021, 51 (Suppl. S7), 143–176. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. S1), e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Russell, L.; Van de Louw, A.; Metaxa, V.; Bauer, P.; Povoa, P.; Montero, J.G.; Loeches, I.M.; Mehta, S.; Puxty, K.; et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. 2020, 46, 298–314. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, L.; Zhou, Y.; Zhao, Y.; Huang, J.; Tang, W.; Liang, Y. Rapid and precise diagnosis of pneumonia coinfected by Pneumocystis jirovecii and Aspergillus fumigatus assisted by next-generation sequencing in a patient with systemic lupus erythematosus: A case report. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ji, T.; Qin, D.; Cheng, D. Clinical characteristics and risk factors of in-hospital mortality in patients coinfected with Pneumocystis jirovecii and Aspergillus. J. Mycol. Med. 2023, 33, 101330. [Google Scholar] [CrossRef]
- Markantonatou, A.M.; Ioakimidou, A.; Arvaniti, K.; Manou, E.; Papadopoulos, V.; Kiriklidou, P.; Samaras, K.; Kioumi, A.; Vyzantiadis, T.A. Pulmonary co-infections by Pneumocystis jirovecii and Aspergillus fumigatus in non-HIV patients: A report of two cases and literature review. Mycoses 2017, 60, 626–633. [Google Scholar] [CrossRef]
- Kaira, K.; Shinomiya, Y.; Takahashi, Y.; Iida, T.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Ishizuka, T.; Mori, M. Coinfection of invasive pulmonary aspergillosis and pneumocystis jiroveci pneumonia in a non-HIV patient. Acta Med. Okayama 2007, 61, 235–238. [Google Scholar] [CrossRef]
- Lee, P.H.; Fu, P.K. Coinfection of pneumocystis jiroveci pneumonia and pulmonary aspergillosis in a non-HIV-infected patient. J. Microbiol. Immunol. Infect. 2018, 51, 860–861. [Google Scholar] [CrossRef]
- Alshahrani, M.Y.; Alfaifi, M.; Ahmad, I.; Alkhathami, A.G.; Hakami, A.R.; Ahmad, H.; Alshehri, O.M.; Dhakad, M.S. Pneumocystis Jirovecii detection and comparison of multiple diagnostic methods with quantitative real-time PCR in patients with respiratory symptoms. Saudi J. Biol. Sci. 2020, 27, 1423–1427. [Google Scholar] [CrossRef]
- Scharmann, U.; Kirchhoff, L.; Hain, A.; Buer, J.; Koldehoff, M.; Steinmann, J.; Rath, P.M. Evaluation of Three Commercial PCR Assays for the Detection of Azole-Resistant Aspergillus fumigatus from Respiratory Samples of Immunocompromised Patients. J. Fungi 2021, 7, 132. [Google Scholar] [CrossRef]
- White, P.L.; Posso, R.B.; Barnes, R.A. Analytical and Clinical Evaluation of the PathoNostics AsperGenius Assay for Detection of Invasive Aspergillosis and Resistance to Azole Antifungal Drugs during Testing of Serum Samples. J. Clin. Microbiol. 2015, 53, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, I.; Delforge, M.L.; Ajjaham, F.; Brancart, F.; Hites, M.; Jacobs, F.; Denis, O. Evaluation of a new commercial real-time PCR assay for diagnosis of Pneumocystis jirovecii pneumonia and identification of dihydropteroate synthase (DHPS) mutations. Diagn. Microbiol. Infect. Dis. 2017, 87, 32–36. [Google Scholar] [CrossRef]
- Nijhuis, R.H.T.; Godschalk, P.C.R.; Smink, J.H.I.; van der Zee, C.; van Hannen, E.J. Comparison of the PneumoGenius® and RealStar® Pneumocystis jirovecii PCR CE-IVD assays with a lab developed test for the detection of Pneumocystis jirovecii. Med. Mycol. 2022, 60, myab080. [Google Scholar] [CrossRef] [PubMed]
- Khot, P.D.; Ko, D.L.; Fredricks, D.N. Sequencing and analysis of fungal rRNA operons for development of broad-range fungal PCR assays. Appl. Environ. Microbiol. 2009, 75, 1559–1565. [Google Scholar] [CrossRef]
- Dannaoui, E.; Gabriel, F.; Gaboyard, M.; Lagardere, G.; Audebert, L.; Quesne, G.; Godichaud, S.; Verweij, P.E.; Accoceberry, I.; Bougnoux, M.E. Molecular Diagnosis of Invasive Aspergillosis and Detection of Azole Resistance by a Newly Commercialized PCR Kit. J. Clin. Microbiol. 2017, 55, 3210–3218. [Google Scholar] [CrossRef] [PubMed]
- Denis, J.; Forouzanfar, F.; Herbrecht, R.; Toussaint, E.; Kessler, R.; Sabou, M.; Candolfi, E.; Letsher-Bru, V. Evaluation of Two Commercial Real-Time PCR Kits for Aspergillus DNA Detection in Bronchoalveolar Lavage Fluid in Patients with Invasive Pulmonary Aspergillosis. J. Mol. Diagn. 2018, 20, 298–306. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, S.; Ponce, C.A.; Pesantes, N.; Bustamante, R.; Gatti, G.; San Martin, V.; Gutierrez, M.; Borquez, P.; Vargas, S.L.; Magne, F.; et al. A Real-Time PCR Assay for Detection of Low Pneumocystis jirovecii Levels. Front. Microbiol. 2021, 12, 787554. [Google Scholar] [CrossRef]
- Yang, S.L.; Wen, Y.H.; Wu, Y.S.; Wang, M.C.; Chang, P.Y.; Yang, S.; Lu, J.J. Diagnosis of Pneumocystis pneumonia by real-time PCR in patients with various underlying diseases. J. Microbiol. Immunol. Infect. 2020, 53, 785–790. [Google Scholar] [CrossRef]
- Chun, J.Y.; Kim, K.J.; Hwang, I.T.; Kim, Y.J.; Lee, D.H.; Lee, I.K.; Kim, J.K. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 2007, 35, e40. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Yao, G.; Guo, Q.; Wang, J.; Liu, G. A TaqMan-probe-based multiplex real-time RT-qPCR for simultaneous detection of porcine enteric coronaviruses. Appl. Microbiol. Biotechnol. 2019, 103, 4943–4952. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Houbraken, J.; Lombardi, L.; Garcia-Rubio, R.; Jenks, J.D.; Rivero-Menendez, O.; Aljohani, R.; Jacobsen, I.D.; Berman, J.; et al. Aspergillus fumigatus and aspergillosis: From basics to clinics. Stud. Mycol. 2021, 100, 100115. [Google Scholar] [CrossRef]
- Sugui, J.A.; Kwon-Chung, K.J.; Juvvadi, P.R.; Latge, J.P.; Steinbach, W.J. Aspergillus fumigatus and related species. Cold Spring Harb. Perspect. Med. 2014, 5, a019786. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Kang, E.R.; Park, M.Y.; Kim, B.K.; Kim, M.J.; Jung, S.; Roh, K.H.; Sung, N.; Yang, J.H.; Lee, M.W.; et al. Development of a multiplex real-time PCR assay for the simultaneous detection of four bacterial pathogens causing pneumonia. PLoS ONE 2021, 16, e0253402. [Google Scholar] [CrossRef]
- Lim, H.J.; Jung, H.S.; Park, M.Y.; Baek, Y.H.; Kannappan, B.; Park, J.Y.; Yang, J.H.; Seol, J.H.; Lee, M.W.; Jung, S.K.; et al. Evaluation of Three Automated Extraction Systems for the Detection of SARS-CoV-2 from Clinical Respiratory Specimens. Life 2022, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Sugita, C.; Makimura, K.; Uchida, K.; Yamaguchi, H.; Nagai, A. PCR identification system for the genus Aspergillus and three major pathogenic species: Aspergillus fumigatus, Aspergillus flavus and Aspergillus niger. Med. Mycol. 2004, 42, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Wissel, M.C.; Grantham, K.J.; Petraitiene, R.; Petraitis, V.; Kasai, M.; Francesconi, A.; Cotton, M.P.; Hughes, J.E.; Greene, L.; et al. Molecular detection and species-specific identification of medically important Aspergillus species by real-time PCR in experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 2011, 49, 4150–4157. [Google Scholar] [CrossRef]
- Lim, H.J.; Park, M.Y.; Jung, H.S.; Kwon, Y.; Kim, I.; Kim, D.K.; Yu, N.; Sung, N.; Lee, S.H.; Park, J.E.; et al. Development of an efficient Sanger sequencing-based assay for detecting SARS-CoV-2 spike mutations. PLoS ONE 2021, 16, e0260850. [Google Scholar] [CrossRef]
- Stokdyk, J.P.; Firnstahl, A.D.; Spencer, S.K.; Burch, T.R.; Borchardt, M.A. Determining the 95% limit of detection for waterborne pathogen analyses from primary concentration to qPCR. Water Res. 2016, 96, 105–113. [Google Scholar] [CrossRef]
- Henke, E.; Zoch, M.; Peng, Y.; Reinecke, I.; Sedlmayr, M.; Bathelt, F. Conceptual design of a generic data harmonization process for OMOP common data model. BMC Med. Inform. Decis. Mak. 2024, 24, 58. [Google Scholar] [CrossRef]
- Barratt, R.; Shaban, R.Z.; Gilbert, G.L. Clinician perceptions of respiratory infection risk; a rationale for research into mask use in routine practice. Infect. Dis. Health 2019, 24, 169–176. [Google Scholar] [CrossRef]
- Procop, G.W. Molecular diagnostics for invasive fungal infections: A call for refinement and implementation. J. Mol. Diagn. 2010, 12, 17–19. [Google Scholar] [CrossRef] [PubMed]
- AlMaghrabi, R.S.; Al-Musawi, T.; Albaksami, O.; Subhi, A.L.; Fakih, R.E.; Stone, N.R. Challenges in the Management of Invasive Fungal Infections in the Middle East: Expert Opinion to Optimize Management Using a Multidisciplinary Approach. Cureus 2023, 15, e44356. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, M.; Xu, Y.; Wang, F.; Wang, J.; Wang, H.; Xu, X.; Wang, Y.; Sun, H. Evaluation of the Performance of a Multiplex Real-Time PCR Assay for the Identification of Aspergillus, Cryptococcus neoformans, and Pneumocystis jirovecii Simultaneously from Sputum in Multicenter. Infect. Drug Resist. 2022, 15, 6009–6017. [Google Scholar] [CrossRef]
- Godoy, M.C.B.; Ferreira Dalla Pria, H.R.; Truong, M.T.; Shroff, G.S.; Marom, E.M. Invasive Fungal Pneumonia in Immunocompromised Patients. Radiol. Clin. N. Am. 2022, 60, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.d.S.e.S.; Nascimento, A.F.d.; Silva, L.D.; Lira, N.d.A.; Passamani, F.R.F.; Batista, L.R.; Matteoli, F.P. Occurrence of filamentous fungi isolated from matured blue cheese. Braz. J. Food Technol. 2020, 23, e2019074. [Google Scholar] [CrossRef]
- Karageorgopoulos, D.E.; Qu, J.M.; Korbila, I.P.; Zhu, Y.G.; Vasileiou, V.A.; Falagas, M.E. Accuracy of beta-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: A meta-analysis. Clin. Microbiol. Infect. 2013, 19, 39–49. [Google Scholar] [CrossRef]
- Chen, L.; Tao, Y.; Hu, X. Utility of Intraocular Fluid beta-D-glucan Testing in Fungal Endophthalmitis: A Series of 5 Cases. Am. J. Case Rep. 2020, 21, e921188. [Google Scholar] [CrossRef]
- Egger, M.; Jenks, J.D.; Hoenigl, M.; Prattes, J. Blood Aspergillus PCR: The Good, the Bad, and the Ugly. J. Fungi 2020, 6, 18. [Google Scholar] [CrossRef]
- Wright, W.F.; Simner, P.J.; Carroll, K.C.; Auwaerter, P.G. Progress Report: Next-Generation Sequencing, Multiplex Polymerase Chain Reaction, and Broad-Range Molecular Assays as Diagnostic Tools for Fever of Unknown Origin Investigations in Adults. Clin. Infect. Dis. 2022, 74, 924–932. [Google Scholar] [CrossRef]
- Hong, K.H.; Kim, G.J.; Roh, K.H.; Sung, H.; Lee, J.; Kim, S.Y.; Kim, T.S.; Park, J.S.; Huh, H.J.; Park, Y.; et al. Update of Guidelines for Laboratory Diagnosis of COVID-19 in Korea. Ann. Lab. Med. 2022, 42, 391–397. [Google Scholar] [CrossRef]
- Park, M.; Won, J.; Choi, B.Y.; Lee, C.J. Optimization of primer sets and detection protocols for SARS-CoV-2 of coronavirus disease 2019 (COVID-19) using PCR and real-time PCR. Exp. Mol. Med. 2020, 52, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Pryce, T.M.; Haygarth, E.J.; Boan, P.A. Quantitative Pneumocystis jirovecii real-time PCR to differentiate disease from colonisation. Pathology 2021, 53, 896–901. [Google Scholar] [CrossRef] [PubMed]
| Pathogen | Target Gene | Primer | Sequences (5′–3′) | Tm (°C) |
|---|---|---|---|---|
| P. jirovecii | mtLSU-rRNA | Fwd. | CTAGGATATAGCTGGTTTTCTGCGIIIIITGTTTTGGCA | 60 |
| Rev. | AGCTTTAATTACTGTTCTGGGCTGIIIIICTTTCGACTA | 59.5 | ||
| Probe | Cal Red 610-TAGGTATAGCACTGAATATCTCGAGGGA | 64 | ||
| A. fumigatus | 28S rRNA | Fwd. | GGGGTTCAGCCGGCATTIIIIICGGTGTACTT | 59.7 |
| Rev. | GTTCCTCGGTCCAGGCAGGIIIIITTGCACCCTC | 61.2 | ||
| Probe | FAM-CCTCGGAATGTATCACCTCTCGG | 64.2 | ||
| Internal control | HBB | Fwd. | GGCATAAAAGTCAGGGCAGAIIIIICTATTGCT | 56.9 |
| Rev. | CCAACTTCATCCACGTTCACCIIIIICCACAGGG | 59.0 | ||
| Probe | HEX-CCTGAGGAGAAGTCTGCCGTTACTGC | 68.8 |
| Group | Organism | Source | Catalog No. | P. jirovecii | A. fumigatus |
|---|---|---|---|---|---|
| Fungi | Pneumocystis jirovecii | Synthetic DNA | - | positive | negative |
| Aspergillus fumigatus | Zeptometrix | Z014 | negative | positive | |
| Aspergillus flavus | Zeptometrix | Z013 | negative | negative | |
| Aspergillus niger | Zeptometrix | Z105 | negative | negative | |
| Aspergillus terreus | Zeptometrix | Z016 | negative | negative | |
| Aspergillus nidulans | ATCC | 38163 | negative | negative | |
| Aspergillus versicolor | ATCC | 11730 | negative | negative | |
| Penicillium chrysogenum | KCCM | 11609 | negative | negative | |
| Talaromyces marneffei | KCCM | 60287 | negative | negative | |
| Bacteria | Streptococcus pneumoniae | ATCC | 49619 | negative | negative |
| Legionella pneumophila | KCTC | 12009 | negative | negative | |
| Bordetella pertussis | KCCM | 42710 | negative | negative | |
| Bordetella parapertussis | ATCC | 15311 | negative | negative | |
| Mycoplasma pneumoniae | ATCC | 29342 | negative | negative | |
| Chlamydophila pneumoniae | ATCC | 53592 | negative | negative | |
| Haemophilus influenzae | ATCC | 9007 | negative | negative | |
| Pseudomonas aeruginosa | KCCM | 11266 | negative | negative | |
| Staphylococcus aureus | KCCM | 32395 | negative | negative | |
| Klebsiella pneumoniae | KCCM | 42750 | negative | negative | |
| Moraxella catarrhalis | KCCM | 42706 | negative | negative | |
| Streptococcus pyogenes | ATCC | 19615 | negative | negative | |
| Virus | Respiratory syncytial virus A | ATCC | VR-1803 | negative | negative |
| Respiratory syncytial virus B | ATCC | VR-955 | negative | negative | |
| Influenza A virus | ATCC | VR-810 | negative | negative | |
| Influenza B virus | Zeptometrix | 0810255CF | negative | negative | |
| Parainfluenza type 1 virus | ATCC | VR-1380 | negative | negative | |
| Parainfluenza type 2 virus | ATCC | VR-92 | negative | negative | |
| Parainfluenza type 3 virus | ATCC | VR-93 | negative | negative | |
| Parainfluenza type 4 virus | Zeptometrix | 0810060CF | negative | negative | |
| Enterovirus A | KBPV | VR-10 | negative | negative | |
| Adenovirus type 3 | Zeptometrix | 0810062CF | negative | negative | |
| Metapneumovirus 27 | Zeptometrix | 0810164CF | negative | negative | |
| SARS-CoV-2 | NCCP | 43330 | negative | negative | |
| Coronavirus NL63 | Zeptometrix | 0810228CFHI | negative | negative | |
| Coronavirus 229E | ATCC | VR-740 | negative | negative | |
| Coronavirus OC43 | ATCC | VR-1558 | negative | negative | |
| Rhinovirus | Zeptometrix | 0810285CF | negative | negative | |
| Dengue virus type 2 | ATCC | VR-1584 | negative | negative | |
| Dengue virus type 4 | ATCC | VR-1257CAF | negative | negative | |
| Echovirus 30 | KNRRC | 45 | negative | negative |
| Assay | RealStar P. jirovecii PCR Kit 1.0 | Sensitivity (%) | Specificity (%) | κ | p-Value | ||
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| RF2 mRT-PCR | Positive | 331 | 0 | 100 | 100 | 1.0 | <0.001 |
| Negative | 0 | 400 | |||||
| Assay | Sanger Sequencing for A. fumigatus | ||
|---|---|---|---|
| Positive | Negative (Aspergillus spp.) | Negative (non-Aspergillus spp.) | |
| RF2 mRT-PCR | |||
| Positive (n) | 42 | 0 | 0 |
| Negative (n) | 0 | 23 | 666 |
| Sensitivity (%) | 100 | ||
| Specificity (%) | 100 | ||
| κ | 1.0 | ||
| p-value | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-J.; Ahn, S.; No, J.-H.; Park, M.-Y.; Kim, M.-J.; Sohn, Y.-H.; Shin, K.-S.; Park, J.-E.; Yang, Y.-J. Development of a Multiplex Real-Time PCR Assay for the Simultaneous Detection of Two Fungal Pathogens Causing Pneumonia. J. Fungi 2024, 10, 619. https://doi.org/10.3390/jof10090619
Lim H-J, Ahn S, No J-H, Park M-Y, Kim M-J, Sohn Y-H, Shin K-S, Park J-E, Yang Y-J. Development of a Multiplex Real-Time PCR Assay for the Simultaneous Detection of Two Fungal Pathogens Causing Pneumonia. Journal of Fungi. 2024; 10(9):619. https://doi.org/10.3390/jof10090619
Chicago/Turabian StyleLim, Ho-Jae, Seojin Ahn, Jee-Hyun No, Min-Young Park, Min-Jin Kim, Yong-Hak Sohn, Kwang-Soo Shin, Jung-Eun Park, and Yong-Jin Yang. 2024. "Development of a Multiplex Real-Time PCR Assay for the Simultaneous Detection of Two Fungal Pathogens Causing Pneumonia" Journal of Fungi 10, no. 9: 619. https://doi.org/10.3390/jof10090619







