A Review of Laboratory Requirements to Culture Lichen Mycobiont Species
Abstract
:1. Introduction
2. Materials and Methods
3. Overview of Mycobiont Cultures
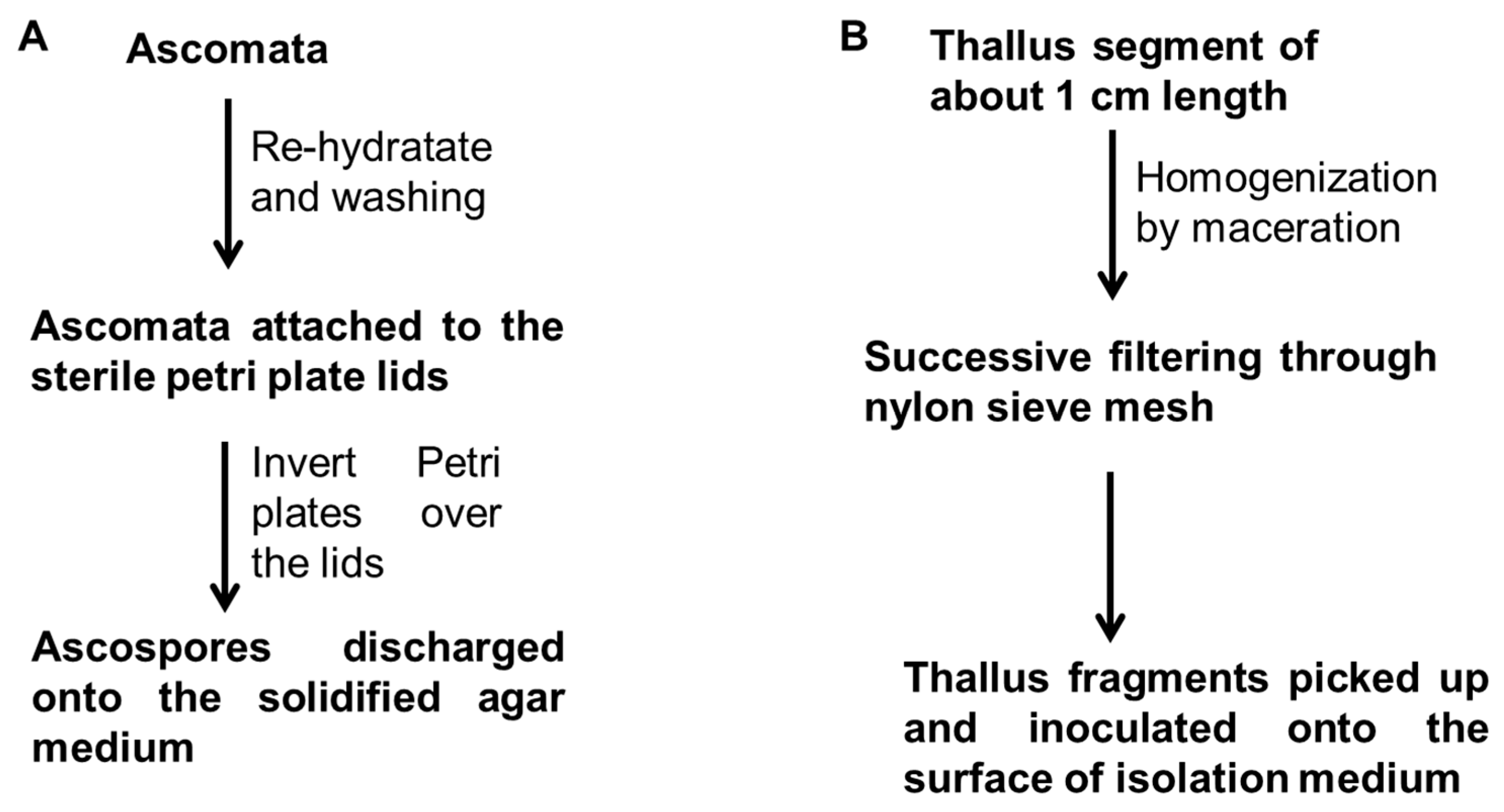
4. Mycobiont Isolation
5. Isolation Media
6. Culture Media
7. Physicochemical Conditions for Culture of Mycobionts
8. Secondary Metabolites Production of Mycobiont Cultures
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Species | Class/Order | Family | Mycobiont Isolation | Isolation Media | Culture Media | Physicchemical Parameters | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Acarospora contigua H. Magn. | Lecanoromycetes/Acarosporales | Acarosporaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] + |
| 2 | Acarospora socialis H. Magn. | Lecano-romycetes/Acarosporales | Acarosporaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 3 | Acarospora strigata (Nyl.) Jatta | Lecanoromycetes/Acarosporales | Acarosporaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 4 | Anaptychia ciliaris (L.) Flot. | Lecanoromycetes/Caliciales | Physciaceae | Ascospore | BBM, BBM 1% glucose | BBM 2% glucose, LBM 3% glucose, MY0.2% glucose, CMA | 18–20 °C in dark | [61] + |
| Ascospore | Agar | MY | 18 °C in dark | [54] * | ||||
| 5 | Arctoparmelia centrifuga (L.) Hale | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | BBM | MY 2% glucose, BBM 2% glucose, LBM 3% glusose, CMA | 20 °C in dark | [77] |
| Ascospore Thallus fragment | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 6 | Arthonia cinnabarina (DC.) Wallr. | Arthoniomycetes/Arthoniales | Arthoniaceae | Ascospore | Agar, LBA, LBG, MY | LBA | 15,20, 25 °C in dark | [58] |
| 7 | Astrothelium galbineum Kremp. | Dothideomycetes/Trypetheliales | Trypetheliaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 8 | Buellia sp. | Lecanoromycetes/Caliciales | Caliciaceae | Ascospore | MY10 | DNR | 18 °C, pH 6.8 | [81] |
| 9 | Buellia stellulata (Taylor) Mudd | Lecanoromycetes/Caliciales | Caliciaceae | Ascospore | Agar, LBA, LBG, MY | DNR | 15, 20, 25 °C in dark, pH 5, 6, 7 | [58] |
| Ascospore | MY 4% sucrose | MY, MY10, MY 20, MY 30 EG1.8, EG3.6, EG5.4 MYA3, MYA5 N1, N3, N5 | 18 °C in dark, pH 6.8, RH: 50–70% k | [84] | ||||
| 10 | Circinaria contorta (Hoffm.) A. Nordin, Savić & Tibell | Lecanoromycetes/Pertusariales | Megasporaceae | Ascospore Thallus fragment | MBB, BBM, LBM, Agar | DNR | 14, 22, 28 °C in dark | [53] + |
| 11 | Cladonia cristatella Tuck. | Lecanoromycetes/Lecanorales | Cladoniaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 12 | Cladonia fimbriata (L.) Fr. | Lecanoromycetes/Lecanorales | Cladoniaceae | Soredia | DNR | MY, BBM/SAB/TOM 1% glucose, 1% manitol, 1% ribitol | 16.5 °C; Photoperiod: 12 h/12 h, Light intensity: 18 μmol photons m2 | [55] + |
| Soredia | MBB, BBM, LB, Agar | DNR | 14, 22, 28 °C in dark | [53] | ||||
| 13 | Cladonia grayi G. Merr. ex Sandst. | Lecanoromycetes/Lecanorales | Cladoniaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 14 | Cladonia macilenta Hoffm | Lecanoromycetes/Lecanorales | Cladoniaceae | Soredia | DNR | MBB, BBM, LB, Agar | 14, 22, 28 °C in dark | [53] |
| 15 | Cladonia metacorallifera Asahina | Lecanoromycetes/Lecanorales | Cladoniaceae | Soredia | DNR | MBB, BBM, LB, Agar | 15 °C in dark, 150 rpm | [74] + |
| 16 | Cladonia peziziformis (With.) J.R. Laundon | Lecanoromycetes/Lecanorales | Cladoniaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 17 | Cladonia rangiferina (L.) Weber | Lecanoromycetes/Lecanorales | Cladoniaceae | Ascospore | Agar | MYA | 20 °C in dark, 120 rpm | [85] + |
| Ascospore Thallus fragment | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 18 | Cladonia rei Schaer. | Lecanoromycetes/Lecanorales | Cladoniaceae | Soredia | DNR | MY, BBM/SAB/TOM 1% glucose, 1% manitol, 1% ribitol | 16.5 °C; Photoperiod: 12 h/12 h, Light intensity: 18 μmol photons m2 s−1 | [55] |
| 19 | Cladonia submultiformis Asahina | Lecanoromycetes/Lecanorales | Cladoniaceae | Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] |
| Ascospore | MYA | MYA, PDA, CMA, Oatmeal, CDA | Room temperature | [60] * | ||||
| 20 | Dibaeis absoluta (Tuck.) Kalb & Gierl | Lecanoromycetes/Pertusariales | Icmadophilaceae | Ascospore | Agar, LBG, MY | DNR | DNR | [58] |
| 21 | Diorygma soozanum (Zahlbr.) M. Nakan. & Kashiw. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | Agar, LBA, LBG | LBA | 15, 20 °C in dark | [58] |
| 22 | Diplotomma sp1 | Lecanoromycetes/Caliciales | Caliciaceae | Ascospore | BBM | LBM 4% glucose | 18–20 °C in dark | [86] |
| 23 | Diplotomma sp2 | Lecanoromycetes/Caliciales | Caliciaceae | Ascospore | BBM | LBM 4% glucose | 18–20 °C in dark | [86] |
| 24 | Dirinaria applanata (Fée) D.D. Awasthi | Lecanoromycetes/Caliciales | Caliciaceae | Soredia | MY | MY 4% sucrose, MBBM 4% sucrose, MS 4% sucrose | 22–28 °C, Photoperiod: 12 h/12 h, pH 6.6 Light intensity: 50–100 µE/m2s, RH: 70–80% | [67] |
| Ascospore Thallus fragment | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 25 | Flavoparmelia exornata (Zahlbr.) Hale | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | MY 4% sucrose | MY10 | 18–22 °C in dark | [87] |
| 26 | Graphis afzelii Ach. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] |
| Ascospore | MYA | MYA, PDA, CMA, Oatmeal, CDA | Room temperature | [60] | ||||
| 27 | Graphis cervina Müll. Arg. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | Agar, LBA, LBG, MY | LBA | 15, 20, 25 °C in dark, pH 5, 6, 7, 8 | [58] |
| 28 | Graphis cognata Müll. Arg. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | MY10 | DNR | 18 °C, pH 6.8 | [81] |
| 29 | Graphis elegans (Borrer ex Sm.) Ach. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] |
| Ascospore | MYA | MYA, PDA, CMA, Oatmeal, CDA | Room temperature | [60] | ||||
| 30 | Graphis librata C. Knight | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] |
| Ascospore | MYA | MYA, PDA, CMA, Oatmeal, CDA | Room temperature | [60] | ||||
| 31 | Graphis handelii Zahlbr. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | MY10 | DNR | 18 °C, pH 6.8 | [81] |
| Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] | ||||
| 32 | Graphis prunicola Vain. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | MY10 | DNR | 18 °C, pH 6.8 | [81] |
| 33 | Graphis scripta (L.) Ach. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | MY10 | DNR | 18 °C, pH 6.8 | [81] |
| Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] | ||||
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 34 | Graphis sp | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 35 | Graphis tenella Ach. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | MY10 | DNR | 18 °C, pH 6.8 | [81] |
| Ascospore | Agar, LBA, LBG, MY | LBA | DNR | [58] | ||||
| 36 | Gymnoderma insulare Yoshim. & Sharp | Lecanoromycetes/Lecanorales | Cladoniaceae | Ascospore | Agar | DNR | DNR | [58] |
| 37 | Haematomma puniceum (Ach.) A. Massal. | Lecanoromycetes/Lecanorales | Haematommataceae | Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] |
| Ascospore | MYA | MYA, PDA, CMA, Oatmeal, CDA | Room temperature | [60] | ||||
| 38 | Heterodea muelleri (Hampe) Nyl. | Lecanoromycetes/Lecanorales | Cladoniaceae | Ascospore | Saboureaud 2% glucose | Saboureaud 2% glucose | Photoperiod: 12 h/27 °C, L:12 h/24 °C, D, Light intensity 50–100 μmol m−2 s−1 | [88] + |
| 39 | Hypogymnia physodes (L.) Nyl. | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | Agar, LBA, LBG, MY | LBA | 15, 20, 25 °C in dark | [58] |
| 40 | Ionaspis alba Lutzoni | Lecanoromycetes/Lecanorales | Hymeneliaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 41 | Lecanora cinereofusca H. Magn. | Lecanoromycetes/Lecanorales | Lecanoraceae | Ascospore | MY10 | DNR | 18° C, pH 6.8 | [81] |
| 42 | Lecanora hybocarpa (Tuck.) Brodo | Lecanoromycetes/Lecanorales | Lecanoraceae | Ascospore | MY10 | DNR | 18° C, pH 6.8 | [81] |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18° C in dark | [54] | ||||
| 43 | Lecanora megalocheila (Hue) H. Miyaw. | Lecanoromycetes/Lecanorales | Lecanoraceae | Ascospore | Agar, LBA, LBG | DNR | DNR | [58] |
| 44 | Lecanora mikuraensis H. Miyaw. | Lecanoromycetes/Lecanorales | Lecanoraceae | Ascospore | MY10 | DNR | 18° C, pH 6.8 | [81] |
| 45 | Lecanora nipponica H. Miyaw. | Lecanoromycetes/Lecanorales | Lecanoraceae | Ascospore | MY10 | DNR | 18° C, pH 6.8 | [81] |
| 46 | Lecanora polytropa (Hoffm.) Rabenh. | Lecanoromycetes/Lecanorales | Lecanoraceae | Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] |
| Ascospore | MYA | MYA, PDA, CMA, Oatmeal, CDA | Room temperature | [60] | ||||
| 47 | Lecanora rupícola (L.) Zahlbr. | Lecanoromycetes/Lecanorales | Lecanoraceae | Thallus fragment | LBM 4% glucose | MS, LBM, Saboureaud 2% glucose, MY | 20° C, Photoperiod: 14 h/10 h | [68] |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 48 | Lecanora subimmergens Vain. | Lecanoromycetes/Lecanorales | Lecanoraceae | Ascospore | Agar, LBA, LBG, MY | LBA | 15, 20, 25 °C in dark, pH 5, 6, 7 | [58] |
| 49 | Lecidea sp. | Lecanoromycetes/Lecideales | Lecideaceae | Ascospore | Agar, LBA, LBG | LBA | 15, 20, 25 °C in dark | [58] |
| 50 | Lecidella elaeocroma (Ach.) M. Choisy | Lecanoromycetes/Lecanorales | Lecanoraceae | Ascospore | MY10 | DNR | 18 °C, pH 6.8 | [81] |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 51 | Leiorreuma sericeum (Eschw.) Staiger | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20° C | [83] |
| 52 | Letharia columbiana (Nutt.) J.W. Thomson | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | LBG | DNR | DNR | [58] |
| 53 | Lobaria adscripturiens (Nyl.) Hue | Lecanoromycetes/Peltigerales | Lobariaceae | Ascospore | LBA | DNR | DNR | [58] |
| 54 | Lobaria oregana (Tuck.) Müll. Arg. | Lecanoromycetes/Peltigerales | Lobariaceae | Ascospore | Cornmeal agar + BSA -Cornmeal agar + cyclodextrin -Agar + cyclodextrin + sucrose -Water agar + cyclodextrin + dextrose -Water agar + cyclodextrin + ribitol | DNR | DNR | [59] |
| 55 | Lobaria pulmonaria (L.) Hoffm. | Lecanoromycetes/Peltigerales | Lobariaceae | Ascospore | Cornmeal agar + BSA -Cornmeal agar + cyclodextrin -Agar + cyclodextrin + sucrose -Agar + cyclodextrin + dextrose -Agar + cyclodextrin + ribitol | DNR | DNR | [59] |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 56 | Loxospora ochrophaea (Tuck.) R.C. Harris | Lecanoromycetes/Lecanorales | Sarrameanaceae | Ascospore | Agar, MY | LBA | DNR | [58] |
| 57 | Megalospora tuberculosa (Fée) Sipman | Lecanoromycetes/Teloschistales | Megalosporaceae | Ascospore | Agar, LBA, MY | DNR | pH 5, 6, 8 in dark | [58] |
| 58 | Myelochroa entotheiochroa (Hue) Elix & Hale | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | Agar, LBA, MY | LBA | 15, 20, 25 °C in dark, pH 5, 6 | [58] |
| 59 | Nephroma helveticum Ach. | Lecanoromycetes/Peltigerales | Nephromataceae | Ascospore | Agar, LBA | DNR | DNR | [58] |
| 60 | Nephroma tropicum (Müll. Arg.) Zahlbr. | Lecanoromycetes/Peltigerales | Nephromataceae | Ascospore | Agar | DNR | DNR | [58] |
| 61 | Ochrolechia parellula (Müll. Arg.) Zahlbr. | Lecanoromycetes/Pertusariales | Ochrolechiaceae | Ascospore | Agar, LBA, LBG, MY | LBA | 15, 20, 25 °C in dark, pH 6, 7 | [58] |
| 62 | Parmelia laevior Nyl. | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | Agar, LBA, LBG, MY | LBA | 15, 20, 25 °C in dark, pH 5, 6 | [58] |
| 63 | Parmelia saxatilis (L.) Ach. | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | BBM | MY 2% glucose, BBM 2% glucose, LBM 3% glusose, CMA | 20 °C in dark | [77] |
| Ascospore | BBM | LBM 4% glucose | 18–20 °C in dark | [86] | ||||
| Ascospore Thallus fragment | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 64 | Parmelia sulcata Taylor | Lecanoromycetes/Lecanorales | Parmeliaceae | Soredia Thallus fragment | MBB, BBM, LB, Agar | DNR | 14, 22, 28 °C in dark | [53] |
| 65 | Parmelina carporrhizans (Taylor) Hale | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | BBM | LBM 3% glusose, MY 0.2% glucose, CMA | 20 °C in dark | [89] + |
| 66 | Parmelina quercina (Willd.) Hale | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | BBM | LBM 3% glusose, MY 0.2% glucose, CMA | 20 °C in dark | [89] |
| 67 | Parmelina tiliácea (Hoffm.) Hale | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | BBM | MY 2% glucose, BBM 2% glucose, LBM 3% glucose, CMF | 20 °C in dark | [77] |
| 68 | Parmotrema eciliatum (Nyl.) Hale | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | MY 4% sucrose | MY10 | 18–22 °C in dark | [87] |
| 69 | Parmotrema reticulatum (Taylor) M. Choisy | Lecanoromycetes/Lecanorales | Parmeliaceae | Thallus fragment | DNR | MY, BBM, MY10, LB | 23 °C in continuous light | |
| 70 | Peltigera aphthosa (L.) Willd. | Lecanoromycetes/Peltigerales | Peltigeraceae | Ascospore | XAD2 | DNR | DNR | [58] |
| 71 | Peltigera dactyla (With.) J.R. Laundon | Lecanoromycetes/Peltigerales | Peltigeraceae | Soredia | DNR | Sterile soil | 19 °C, Photoperiod: 14 h/10 h, Light intensity 15–22 W/m2 (60–90 µE/m2s, 500–750 Lx), RH 50–60%, | [90] |
| Thallus fragment | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 72 | Peltigera praetextata (Flörke ex Sommerf.) Zopf | Lecanoromycetes/Peltigerales | Peltigeraceae | Ascospore | Agar | DNR | DNR- | [58] |
| 72 | Peltigera rufescens (Weiss) Humb. | Lecanoromycetes/Peltigerales | Peltigeraceae | Ascospore | XAD2 | DNR | DNR | [58] |
| 74 | Phaeographis elliptica Müll. Arg. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 75 | Phaeographina quassiicola (Fée) Müll. Arg. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] |
| Ascospore | MYA | MYA, PDA, CMA, Oatmeal, CDA | Room temperature | [60] | ||||
| 76 | Physconia distorta (With.) J.R. Laundon | Lecanoromycetes/Lecanorales | Physciaceae | Ascospore | DNR | LBM 4% glucose | 18 °C, pH 5.3 | [82] |
| Ascospore | BBM | LBM 4% glucose | 18–20 °C in dark | [86] | ||||
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 77 | Platismatia glauca (L.) WL Culb. & CF Culb. | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | BBM | MY 2% glucose, BBM 2% glucose, LBM 3% glucose, CMF | 20 °C in dark | [77] |
| Thallus fragment | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 78 | Platygramme sp. | Lecanoromycetes/Ostropales | Graphidaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 79 | Polymeridium subcinereum (Nyl.) R.C. Harris | Dothideomycetes/Trypetheliales | Trypetheliaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 80 | Porpidia albocaerulescens (Wulfen) Hertel & Knoph | Lecanoromycetes/Lecideales | Lecideaceae | Ascospore | Agar, LBA, LBG, MY | LBA | pH 5, 6, 7; 15, 20, 25 °C in dark | [58] |
| 81 | Protoparmeliopsis muralis (Schreb.) M. Choisy | Lecanoromycetes/Lecanorales | Lecanoraceae | Ascospore Thallus fragment | MBB, BBM, LB, Agar | DNR | 14, 22, 28 °C in dark | [53] |
| 82 | Pyrenula cruenta (Mont.) Vain. | Eurotiomycetes/Pyrenulales | Pyrenulaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 83 | Pyrenula japónica Kurok. | Eurotiomycetes/Pyrenulales | Pyrenulaceae | Ascospore | MY10 | DNR | 18 °C, pH 6.8 | [81] |
| 84 | Pyrenula ochraceoflava (Nyl.) R.C. Harris | Eurotiomycetes/Pyrenulales | Pyrenulaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 85 | Pyrenula pseudobufonia (Rehm) R.C. Harris | Eurotiomycetes/Pyrenulales | Pyrenulaceae | Ascospore | MY10 | DNR | 18 °C, pH 6.8 | [81] |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 86 | Pyxine endochrysina Nyl. | Lecanoromycetes/Caliciales | Caliciaceae | Ascospore | Agar, LBA, LBG | LBA | 15, 20, 25 °C in dark, pH 5, 6 | [58] |
| 87 | Ramalina crassa (Delise ex Nyl.) Motyka | Lecanoromycetes/Lecanorales | Ramalinaceae | Ascospore | Agar, LBA, LBG, MY | LBA | 15, 20, 25 °C in dark, pH 5, 6, 7, 8 * | [58] |
| 88 | Ramalina celastri (Spreng.) A. Massal. | Lecanoromycetes/Lecanorales | Ramalinaceae | Ascospore Thallus fragment | BBM | LBM 4% glucose, MY, Sabouraud 4%glucose-agar | Photoperiod-Temperature: 12 h-24/12 h-27 °C | [68] |
| Ascospore | Agar | MEYE medium | 23 °C, Photoperiod: Continuous light | [91] | ||||
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 89 | Ramalina complanata (Sw.) Ach. | Lecanoromycetes/Lecanorales | Ramalinaceae | Ascospore Thallus fragment | BBM 2% Agar | LBM 4% glucose, MY, Saboureaud 4%glucose-agar | Photoperiod-Temperature: 12 h-24/12 h-27 °C | [68] |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 90 | Ramalina dendriscoides Nyl. | Lecanoromycetes/Lecanorales | Ramalinaceae | Ascospore Thallus fragment | Saboureaud 2%glucose-agar, Saboureaud 4%glucose-agar, LBM 4% glucose, MY, MIX, MS, SSA | DNR | Photoperiod-Temperature: 12 h-24/12 h-27 °C | [68] |
| 91 | Ramalina dilacerata (Hoffm.) Hoffm. | Lecanoromycetes/Lecanorales | Ramalinaceae | Ascospore | Glucose agar, malt agar, MY, malt glucose agar, MY glucose agar | DNR | 15 °C, pH 7-8-9, 16 h/8 h, Light intensity: 13–14 µE/m2s | [75] + |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 92 | Ramalina farinacea (L.) Ach. | Lecanoromycetes/Lecanorales | Ramalinaceae | Thallus fragment | LBM + bark extract | LBM 4% sucrose, LBM 2% mannitol | 21 °C, pH 6, Photoperiod: 14 h:10 h, Light intensity: 200 µE/m2s, dessication periods | [72] + |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 93 | Ramalina gracilis (Pers.) Nyl. | Lecanoromycetes/Lecanorales | Ramalinaceae | Ascospore Thallus fragment | Saboureaud 2%glucose-agar, LBM 4% glucose, MY, MIX MS, SSA, MYSM | DNR | Photoperiod-Temperature: 12 h-24/12 h-27 °C | [68] |
| 94 | Ramalina peruviana Ach. | Lecanoromycetes/Lecanorales | Ramalinaceae | Ascospore Thallus fragment | BBM | LBM 4% glucose, MY | Photoperiod-Temperature: 12 h-24/12 h-27 °C | [68] |
| 95 | Ramalina sprengelii Krog & Swinscow | Lecanoromycetes/Lecanorales | Ramalinaceae | Ascospore Thallus fragment | BBM 2% Agar | DNR | Photoperiod-Temperature: 12 h-24/12 h-27 °C | [68] |
| 96 | Stereocaulon commixtum (Asahina) Asahina | Lecanoromycetes/Lecanorales | Stereocaulaceae | Ascospore | Agar, LBA, LBG, MY | LBA | 15, 20, 25 °C in dark, pH 5, 6, 7, 8 * | [58] |
| 97 | Stereocaulon sorediiferum Hue | Lecanoromycetes/Lecanorales | Stereocaulaceae | Ascospore | Agar, LBA, LBG, MY | LBA | 15, 20, 25 °C in dark | [58] |
| 98 | Teloschistes chrysophthalmus (L.) Norman ex Tuck. | Lecanoromycetes/Teloschistales | Teloschistaceae | Ascospore | Agar | MEYE | 23 °C, Photoperiod: Continuous light | [91] |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 99 | Trypethelium aeneum (Eschw.) Zahlbr. | Dothideomycetes/Trypetheliales | Trypetheliaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 100 | Trypethelium eluteriae Spreng. | Dothideomycetes/Trypetheliales | Trypetheliaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| Ascospore | Agar | MYA | - | [60] | ||||
| 101 | Trypethelium virens Tuck. ex Michener | Dothideomycetes/Trypetheliales | Trypetheliaceae | Ascospore Tthallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| 102 | Usnea complanata (Müll. Arg.) Motyka | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] |
| Ascospore | MYA | MYA, PDA, CMA, Oatmeal, CDA | Room temperature | [60] | ||||
| 103 | Usnea ghattensis G. Awasthi | Lecanoromycetes/Lecanorales | Parmeliaceae | Thallus fragment | MY, Saboureaud-Dextrosa-Agar | Agar, MY with diferentes amounts of carbon and nitrogen sources | 18 °C, Photoperiod: 8 h/16 h, Light intensity: 400 lux, RH: 80% | [73] |
| Ascospore | BBM | MY 2% glucose, BBM 2% glucose, LBM 3% glusose, CMA | 20 °C in dark | [77] | ||||
| 104 | Usnea orientalis Motyka | Lecanoromycetes/Lecanorales | Parmeliaceae | Thallus fragment | MY | MY 2% mannitol, MY 2% sorbitol | 18 °C, pH 5.8 | [78] |
| 105 | Usnea rubescens Stirt. | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | Agar, LBA, LBG | DNR | - | [58] |
| 106 | Usnea strigosa (Ach.) Pers. | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore Thallus fragment | YES, MY, NMBBM, Oatmeal, Potato-Carrot | MY, NMBBM | 20 °C | [83] |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18° C in dark | [54] | ||||
| 107 | Xanthoparmelia tinctina (Maheu & A. Gillet) Hale | Lecanoromycetes/Lecanorales | Parmeliaceae | Ascospore | BBM | MY 2% glucose, BBM 2% glucose, LBM 3% glucose, CMF | 20 °C in dark | [77] |
| 108 | Xanthoria elegans (Link) Th. Fr. | Lecanoromycetes/Teloschistales | Teloschistaceae | Thallus fragment | LBM, Saboureaud 2% glucose | MS, LBM, Saboureaud 2% glucose, MY | 20 °C, Photoperiod: 14 h/10 h | [71] + |
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| 109 | Xanthoria parietina (L.) Th. Fr. | Lecanoromycetes/Teloschistales | Teloschistaceae | Ascospore | LBM 2% sucrose | LBM 1–3% D-glucose, LBM 1–3% D-arabitol, LBM 1–3% D-ribitol, LBM 1–3% D-mannitol | 20 °C, Photoperiod: 14 h/10 h, Light intensity: 20 µmol/m2s, pH 6 | [76] + |
| Ascospore | BBM | MY, LBM 4% glucose | DNR | [69] | ||||
| Ascospore | BBM | LBM 4% glucose | 18–20 °C, in dark | [86] | ||||
| Ascospore | Agar | MY, P. D. Crittenden & E. Oliver medium | 18 °C in dark | [54] | ||||
| Ascospore Thallus fragment | MBB, BBM, LB, Agar | DNR | 14, 22, 28 °C in dark | [53] | ||||
| 110 | Xanthoria polycarpa(Hoffm.) Rieber | Lecanoromycetes/Teloschistales | Teloschistaceae | Ascospore | Agar, MY, Bark extract, lichen exract | DNR | 10, 15, 20 °C, pH 5, 6 | [56] |
References
- Honegger, R. Functional aspects of the lichen symbiosis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 553–578. [Google Scholar] [CrossRef]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist 2017, 119, 361–416. [Google Scholar] [CrossRef]
- Ranković, B. Bioactive properties and pharmaceutical potential. In Lichen Secondary Metabolites, 2nd ed.; Ranković, B., Ed.; Springer Nature Switzerland AG: Cham, Switzerland; Kragujevac, Serbia, 2019; pp. 155–174. [Google Scholar] [CrossRef]
- Elix, J.A.; Stocker-Worgotter, E. Biochemistry and Secondary Metabolites. In Lichen Biology; Nash, T.H., Ed.; Cambridge University Press: Cambridge, MA, USA, 2008; pp. 104–133. [Google Scholar]
- Stocker-Wörgötter, E. Metabolic diversity of lichen-forming ascomycetous fungi: Culturing polyketide and shikimate metabolite production and PKS genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Deep, P.; Singh, S.; Nayak, B. Lichen Secondary Metabolites and Its Biological Activity. Am. J. Pharm. Tech. Res. 2016, 6, 2249–3387. [Google Scholar]
- Rundel, P.W. The ecological role of secondary lichen substances. Biochem. Syst. Ecol. 1978, 6, 157–170. [Google Scholar] [CrossRef]
- Lawrey, J.D. Biological role of lichen substances. Bryologist 1986, 89, 111–122. [Google Scholar] [CrossRef]
- Ahad, A.M.; Goto, Y.; Kiuchi, F.; Tsuda, Y.; Kondo, K.; Sato, T. Nematocidal principles in “oakmoss absolute” and nematocidal activity of 2,4-dihydroxybenzoates. Chem. Pharm. Bull. 1991, 39, 1043–1046. [Google Scholar] [CrossRef]
- Gauslaa, Y. Lichen palatability depends on investments in herbivore defence. Oecologia 2005, 143, 94–105. [Google Scholar] [CrossRef]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites. Z. Naturforsch. C 2010, 65, 157–173. [Google Scholar] [CrossRef]
- Reyes-Tur, B.; González-Guillén, A.; Rosabal, D.; Capote-Danet, A. Associations between tree snails and corticolous lichens in a secondary forest in eastern Cuba. Poeyana 2020, 510, 18–26. Available online: https://www.revistasgeotech.com/index.php/poey/article/view/348/165 (accessed on 15 May 2022).
- Simko, P.; Kiskova, T. Uncovering the Anticancer Potential of Lichen Secondary Metabolites. J. Anal. Oncol. 2022, 11, 70–78. [Google Scholar] [CrossRef]
- Pyatt, F.B. Studies of the periodicity of spore discharge and germination in lichens. Bryologist 1969, 72, 48–53. [Google Scholar] [CrossRef]
- Marante, F.J.T.; Castellano, A.G.; Rosas, F.E.; Aguiar, J.Q.; Barrera, J.B. Identification and quantitation of allelochemicals from the lichen Lethariella canariensis: Phytotoxicity and antioxidative activity. J. Chem. Ecol. 2003, 29, 2049–2071. [Google Scholar] [CrossRef] [PubMed]
- Macias, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, J.C.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Welch, A.R. Competition in lichen communities. Symbiosis 2007, 43, 1–12. [Google Scholar]
- Galloway, D.J. Global environmental change: Lichens and chemistry. Bibl. Lichenol. 1993, 53, 87–95. [Google Scholar]
- Solhaug, K.A.; Gauslaa, Y. Parietin, a photoprotective secondary product of the lichen Xanthoria parietina. Oecologia 1996, 108, 412–418. [Google Scholar] [CrossRef]
- Sarret, G.; Manceau, A.; Cuny, D.; van Haluwyn, C.; Déruelle, S.; Hazemann, J.L.; Soldo, Y.; Eybert- Bérard, L.; Menthonnex, J.J. Molecular mechanisms of Pb and Zn hyperaccumulation and tolerance in lichens. Environ. Sci. Technol. 1998, 32, 3325–3330. [Google Scholar] [CrossRef]
- Białonska, D.; Dayan, F.E. Chemistry of the lichen Hypogymnia physodes transplanted to an industrial region. J. Chem. Ecol. 2005, 31, 2975–2991. [Google Scholar] [CrossRef]
- Hauck, M.; Huneck, S. Lichen substances affect metal adsorption in Hypogymnia physodes. J. Chem. Ecol. 2007, 33, 219–223. [Google Scholar] [CrossRef]
- Jayanthi, S.; Priya, P.; Monica Devi, D.; Benila Smily, J.M. Lichens: Origin, types, secondary metabolites and applications. J. Acad. Indus. Res. 2012, 1, 45–49. [Google Scholar]
- Osyczka, P.; Latkowska, E.; Rola, K. Metabolic processes involved with sugar alcohol and secondary metabolite production in the hyperaccumulator lichen Diploschistes muscorum reveal its complex adaptation strategy against heavy-metal stress. Fungal Biol. 2021, 125, 999–1008. [Google Scholar] [CrossRef]
- Norouzi, H.; Sohrabi, M.; Yousefi, M.; Boustie, J. Tridepsides as potential bioactives: A review on their chemistry and the global distribution of their lichenic and non-lichenic natural sources. Front. Fungal Biol. 2023, 4, 1088966. [Google Scholar] [CrossRef]
- Vaillant Flores, D.; Romeu Carballo, C.; Gómez Peralta, M.; Ramírez Ochoa, R.; Porras González, A. Actividad antifúngica de extractos de tres especies de líquenes en Cuba. Agron. Mesoam. 2015, 26, 345–350. [Google Scholar] [CrossRef]
- Ordoñez, J.; Estefanía, C. Actividad antifúngica y antibacteriana in vitro del extracto etanólico de Usnea laevis frente a Candida albicans, Staphylococcus aureus y Pseudomonas aeruginosa. Rev. Med. Hered. 2020, 31, 169–174. [Google Scholar] [CrossRef]
- Karagöz, A.; Aslan, A. Antiviral and cytotoxic activity of some lichen extracts. Biol. Bratisl. 2005, 60, 281–286. Available online: https://www.researchgate.net/publication/287835454_Antiviral_and_cytotoxic_activity_of_some_lichen_extracts (accessed on 15 May 2022).
- Omarsdottir, S.; Óladóttir, A.K.; Árnadóttir, T.; Ingólfsdóttir, K. Antiviral compounds from Icelandic lichens. Planta Med. 2006, 72. [Google Scholar] [CrossRef]
- Odimegwu, D.C. Low-dose Sekikaic acid modulates host immunity and protects cells from respiratory syncytial virus infection. Biotechnol. J. Int. 2018, 21, 1–10. [Google Scholar] [CrossRef]
- Okuyama, E.; Umeyama, K.; Yamazaki, M.; Kinoshita, Y.; Yamamoto, Y. Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med. 1995, 61, 113–115. [Google Scholar] [CrossRef]
- Vijayakumar, C.S.; Viswanathan, S.; Reddy, M.K.; Parvanthavarthini, S.; Kundu, A.B.; Sukumar, E. Anti-inflammatory activity of (+) usnic acid. Fitoterapia 2000, 71, 564–566. [Google Scholar] [CrossRef]
- Fernández Moriano, C.; Gómez Serranillo, M.P.; Crespo, A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef]
- Dwarakanath, P.R.; Abinaya, K.; Nagasathya, K.; Meenakumari, S.; Gopinath, S.; Raman, P. Profiling Secondary Metabolites from Lichens “Parmotrema perlatum (Huds.) M. Choisy” and Antibacterial and Antioxidant Potentials. Biomass Conversion and Biorefinery. Biomass Conv. Bioref. 2024, 14, 16461–16471. [Google Scholar] [CrossRef]
- Upreti, K.D.; Divakar, P.; Shukla, V.; Bajpai, R. Recent Advances in Lichenology Modern Methods and Approaches in Systematic and Culture Techniques; Springer: New Delhi, India, 2015. [Google Scholar]
- Ulus, G. Antiangiogenic properties of lichen secondary metabolites. Phytother. Res. 2021, 35, 3046–3058. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Recent Advances in Research for Potential Utilization of Unexplored Lichen Metabolites. Biotechnol. Adv. 2023, 62, 108072. [Google Scholar] [CrossRef]
- Ren, M.; Jiang, S.; Wang, Y.; Pan, X.; Pan, F.; Wei, X. Discovery and excavation of lichen bioactive natural products. Front. Microbiol. 2023, 14, 1177123. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, D.; Divakar, P.; Grewe, F.; Crespo, A.; Dal Grande, F.; Lumbsch, T. Genome-wide analysis of biosynthetic gene cluster reveals correlated gene loss with absence of usnic acid in lichen-forming fungi. Genome Biol. Evol. 2020, 12, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Jeong, M.H.; Yun, S.H.; Hur, J.S. Transcriptome analysis identifies a gene cluster for the biosynthesis of Biruloquinone, a rare phenanthraquinone, in a lichen-forming fungus Cladonia macilenta. J. Fungi 2021, 7, 398. [Google Scholar] [CrossRef]
- Kim, W.; Liu, R.; Woo, S.; Kang, K.B.; Park, H.; Yu, Y.H.; Ha, H.H.; Oh, S.Y.; Yang, J.H.; Kim, H.; et al. Linking a Gene Cluster to Atranorin, a Major Cortical Substance of Lichens, through Genetic Dereplication and Heterologous Expression. mBio 2021, 12, e0111121. [Google Scholar] [CrossRef]
- Singh, G.; Armaleo, D.; Dal Grande, F.; Schmitt, I. Depside and Depsidone Synthesis in Lichenized Fungi Comes into Focus through a Genome-Wide Comparison of the Olivetoric Acid and Physodic Acid Chemotypes of Pseudevernia furfuracea. Biomolecules 2021, 11, 1445. [Google Scholar] [CrossRef] [PubMed]
- Singh, G. Linking lichen metabolites to genes: Emerging concepts and lessons from molecular biology and metagenomics. J. Fungi 2023, 9, 160. [Google Scholar] [CrossRef]
- Llewellyn, T.; Nowell, R.W.; Aptroot, A.; Temina, M.; Prescott, T.A.; Barraclough, T.G.; Gaya, E. Metagenomics shines light on the evolution of “sunscreen” pigment metabolism in the Teloschistales (Lichen-forming Ascomycota). Genome Biol. Evol. 2023, 15, evad002. [Google Scholar] [CrossRef] [PubMed]
- Muggia, L.; Kopun, T.; Grube, M. Effects of Growth Media on the Diversity of Culturable Fungi from Lichens. Molecules 2017, 22, 824. [Google Scholar] [CrossRef]
- Pedišius, V. UV-B absorbing and bioactive secondary compounds in lichens Xanthoria elegans and Xanthoria parietina: A review. Czech Polar Rep. 2020, 10, 252–262. [Google Scholar] [CrossRef]
- Ndhlovu, N.T.; Minibayeva, F.; Richard, F.; Beckett, R.P. Lichen substances are more important for photoprotection in sun than shade collections of lichens from the same species. Bryologist 2023, 26, 180–190. [Google Scholar] [CrossRef]
- Oberwinkler, F. 16 Basidiolichens. In Fungal Associations; Oberwinkler, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 341–362. [Google Scholar]
- Ahmadjian, V. The Lichen Symbiosis. Nord. J. Bot. 1993, 14, 588. [Google Scholar] [CrossRef]
- Yoshimura, I.; Yamamoto, Y.; Nakano, T.; Finnie, J. Isolation and culture of lichen photobionts and mycobionts. In Methods in lichenology; Kranner, I., Becknett, R., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 3–33. [Google Scholar]
- Yamamoto, Y.; Mizuguchi, R.; Yamada, Y. Tissue culture of Usnea rubescens and Ramalina yasudae and production of usnic acid in their cultures. Agric. Biol. Chem. 1985, 49, 3347–3348. [Google Scholar] [CrossRef]
- Stocker-Wörgotter, E. Experimental Studies of the Lichen Symbiosis: DNA-Analyses, Differentiation and Secondary Chemistry of Selected Mycobionts, Artificial Resynthesis of Two- and Tripartite Symbioses. Symbiosis 2001, 30, 207–227. [Google Scholar]
- Zakeri, Z.; Junne, S.; Jäger, F.; Dostert, M.; Otte, V.; Neubauer, P. Lichen cell factories: Methods for the isolation of photobiont and mycobiont partners for defined pure and co-cultivation. Microb. Cell Factories 2022, 21, 80. [Google Scholar] [CrossRef]
- Crittenden, P.D.; David, J.C.; Hawksworth, D.L.; Campbell, F.S. Attempted isolation and success in the culturing of a broad spectrum of lichen-forming and lichenicolous fungi. New Phytol. 1995, 130, 267–297. [Google Scholar] [CrossRef]
- Černajová, I.; Škaloud, P. Lessons from culturing lichen soredia. Symbiosis 2020, 82, 109–122. [Google Scholar] [CrossRef]
- Ostrofsky, A.; Denison, W.C. Ascospore discharge and germination in Xanthoria polycarpa. Mycologia 1980, 72, 1171–1179. [Google Scholar] [CrossRef]
- Clayden, S.R. Seasonal variation in ascospore discharge by Rhizocarpon lecanorinum. Lichenologist 1997, 29, 495–499. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kinoshita, Y.; Takahagi, T.; Kroken, S.; Kurokawa, T.; Yoshimura, I. Factors affecting discharge and germination of lichen ascospores. J. Hattori Bot. Lab. 1998, 85, 267–278. [Google Scholar]
- Denison, W. Apothecia and ascospores of Lobaria oregana and Lobaria pulmonaria investigated. Mycologia 2003, 953, 513–518. [Google Scholar] [CrossRef]
- Sangvichien, E.K.; Hawksworth, D.L.; Whalley, A.J.S. Ascospore discharge, germination and culture of fungal partners of tropical lichens, including the use of a novel culture technique. IMA Fungus 2011, 2, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.C.; Divakar, P.; González, N. Success in the isolation and axenic culture of Anaptychia ciliaris (Physciaceae, Lecanoromycetes) mycobiont. Mycoscience 2015, 56, 351–358. [Google Scholar] [CrossRef]
- Belandria, G.; Asta, J.; Nurit, F. Effects of sulphur dioxide and fluoride on ascospore germination of several lichens. Lichenologist 1989, 21, 79–86. [Google Scholar] [CrossRef]
- Cardós, J.L.H.; Prieto, M.; Jylhä, M.; Aragón, G.; Molina, M.C.; Martínez, I.; Rikkinen, J. A case study on the re-establishment of the cyanolichen symbiosis: Where do the compatible photobionts come from? Ann. Bot. 2019, 124, 379–388. [Google Scholar] [CrossRef]
- Ahmadjian, V. Coevolution in Lichens. Ann. N. Y. Acad. Sci. 1987, 503, 307–315. [Google Scholar] [CrossRef]
- Behera, B.C.; Verma, N.; Sonone, A.; Makhija, U. Experimental studies on the growth and usnic acid production in lichen Usnea ghattensis in vitro. Microbiol. Res. 2006, 161, 232–237. [Google Scholar] [CrossRef]
- Deduke, C.; Piercey-Normore, M.D. Substratum preference of two species of Xanthoparmelia. Fungal Biol. 2015, 119, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Valarmathi, R.; Hariharan, G.N. Soredial culture of Dirinaria applanata (Fee) Awasthi: Observations on developmental stages and compound production. Symbiosis 2007, 43, 137–142. [Google Scholar]
- Cordeiro, L.; Iacomini, M.; Stocker-Wörgotter, E. Culture studies and secondary compounds of six Ramalina species. Mycol. Res. 2004, 108, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.C.; Stocker-Wörgötter, E.; Türk, R.; Vicente, C. Axenic culture of the mycobiont of Xanthoma parietina in different nutritive media: Effect of carbon source in spore germination. Endocytobiosis Cell Res. 1997, 12, 103–109. [Google Scholar]
- Verma, N.; Behera, B. In Vitro Culture of Lichen Partners: Need and Implications. In Recent Advances in Lichenology Modern Methods and Approaches in Systematic and Culture Techniques; Upreti, K.D., Divakar, P., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; pp. 148–157. [Google Scholar]
- Brunauer, G.; Stocker Worgotter, E. Culture of lichen fungi for future production of biologically active compounds. Symbiosis 2003, 38, 187–201. [Google Scholar]
- Stocker-Wörgötter, E.; Elix, J.A.; Grube, M. Secondary Chemistry of Lichen-forming Fungi: Chemosyndromic Variation and DNA-analyses of Cultures and Chemotypes in the Ramalina farinacea Complex. Bryologist 2004, 107, 152–162. [Google Scholar] [CrossRef]
- Behera, B.C.; Verma, N.; Sonone, A.; Makhija, U. Optimization of culture conditions for lichen Usnea ghattensis G. Awasthi to increase biomass and antioxidant metabolite production. Food Technol. Biotechnol. 2009, 47, 7–12. [Google Scholar]
- Kim, J.A.; Hong, S.G.; Cheong, Y.H.; Koh, Y.J.; Hur, J. A new reducing polyketide synthase gene from the lichen-forming fungus Cladonia metacorallifera. Mycologia 2012, 104, 362–370. [Google Scholar] [CrossRef]
- Timsina, B.A.; Sorensen, J.L.; Weihrauch, D.; Piercey-Normore, M.D. Effect of aposymbiotic conditions on colony growth and secondary metabolite production in the lichen-forming fungus Ramalina dilacerata. Fungal Biol. 2013, 117, 731–743. [Google Scholar] [CrossRef]
- Pichler, G.; Carniel, F.C.; Muggia, L.; Holzinger, A.; Tretiach, M.; Kranner, I. Enhanced culturing techniques for the mycobiont isolated from the lichen Xanthoria parietina. Mycol. Prog. 2021, 20, 797–808. [Google Scholar] [CrossRef]
- Díaz, E.M.; Zamora, J.C.; Ruibal, C.; Divakar, P.K.; González-Benítez, N.; LeDevehat, F.; Chollet, M.; Ferron, S.; Sauvager, A.; Boustie, J.; et al. Axenic culture and biosynthesis of secondary compounds in lichen symbiotic fungi, the Parmeliaceae. Symbiosis 2020, 82, 79–93. [Google Scholar] [CrossRef]
- Kon, Y.; Kashiwadani, H.; Wardlaw, J.H.; Elix, J.A. Effects of Culture Conditions on Dibenzofuran Production by Cultured Mycobionts of Lichens. Symbiosis 1997, 23, 97–106. [Google Scholar]
- Stocker-Wörgötter, E.; Elix, J.A. Secondary chemistry of cultured mycobionts: Formation of a complete chemosyndrome by the lichen fungus of Lobaria spathulata. Lichenologist 2002, 34, 351–359. [Google Scholar] [CrossRef]
- Shanmugam, K.; Srinivasan, M.; Hariharan, G.N. Developmental stages and secondary compound biosynthesis of mycobiont and whole thallus cultures of Buellia subsororioides. Mycol. Prog. 2016, 15, 1–13. [Google Scholar] [CrossRef]
- Hamada, N.; Tanahashi, T.; Miyagawa, H.; Miyawaki, H. Characteristics of Secondary Metabolites from Isolated Lichen Mycobionts. Symbiosis 2001, 31, 23–33. [Google Scholar]
- Molina, M.C.; Vicente, C.; Elix, J.A. Differences in the composition of phenolics and fatty acids of cultured mycobiont and thallus of Physconia distorta. Plant Physiol. Biochem. 2003, 41, 175–180. [Google Scholar] [CrossRef]
- McDonald, T.; Gaya, E.; Lutzoni, F. Twenty-five cultures of lichenizing fungi available for experimental studies on symbiotic systems. Symbiosis 2013, 59, 165–171. [Google Scholar] [CrossRef]
- Hamada, N. Induction of the production of lichen substances by non-metabolites. Bryologist 1996, 99, 68–70. [Google Scholar] [CrossRef]
- Athukorala, S.; Huebner, E.; Piercey-Normore, M.D. Identification and comparison of the 3 early stages of resynthesis for the lichen Cladonia rangiferina. Can. J. Microbiol. 2014, 60, 41–52. [Google Scholar] [CrossRef]
- Molina, M.C.; Crespo, A. Comparison of development of axenic cultures of five species of lichen-forming fungi. Mycol. Res. 2000, 104, 595–602. [Google Scholar] [CrossRef]
- Bertoni, M.D.; Adler, M.; Maier, M.S. Cultivo “in vitro” de micobiontes de Parmotrema eciliatum y Flavoparmelia exornata (Parmeliaceae, Ascomycota liquenizados) con producción de triglicéridos. Boletín Soc. Argent. Botánica 2000, 34, 179–183. [Google Scholar]
- Hager, A.; Brunauer, G.; Türk, R.; Stocker-Wörgötter, E. Production and bioactivity of common lichen metabolites as exemplified by Heterodea muelleri (Hampe) Nyl. J. Chem. Ecol. 2008, 34, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Alors, D.; Cendon-Florez, Y.; Divakar, P.K.; Crespo, A.; Benítez, N.G.; Molina, M.C. Differences in the sexual aposymbiotic phase of the reproductive cycles of Parmelina carporrhizans and P. quercina. Possible implications for their reproductive biology. Lichenologist 2019, 51, 175–186. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E.; Turk, R. Culture of the cyanobacterial lichen Peltigera didactyla from soredia under laboratory conditions. Lichenologist 1988, 20, 369–375. [Google Scholar] [CrossRef]
- Fazio, A.T.; Adler, M.T.; Bertoni, M.D.; Sepúlveda, C.S.; Damonte, E.B.; Maier, M.S. Lichen secondary metabolites from the cultured lichen mycobionts of Teloschistes chrysophthalmus and Ramalina celastri and their antiviral activities. Z. Naturforsch. 2007, 62, 543–549. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosabal, D.; Pino-Bodas, R. A Review of Laboratory Requirements to Culture Lichen Mycobiont Species. J. Fungi 2024, 10, 621. https://doi.org/10.3390/jof10090621
Rosabal D, Pino-Bodas R. A Review of Laboratory Requirements to Culture Lichen Mycobiont Species. Journal of Fungi. 2024; 10(9):621. https://doi.org/10.3390/jof10090621
Chicago/Turabian StyleRosabal, Dania, and Raquel Pino-Bodas. 2024. "A Review of Laboratory Requirements to Culture Lichen Mycobiont Species" Journal of Fungi 10, no. 9: 621. https://doi.org/10.3390/jof10090621







