The Cryptochrome CryA Regulates Lipid Droplet Accumulation, Conidiation, and Trap Formation via Responses to Light in Arthrobotrys oligospora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Bioinformatic Analysis
2.3. The AocryA Knockout
2.4. Determination of Mycelial Growth, Spore Yield, and Germination Rate
2.5. Observation of Mycelial Septa, Nuclei, Lipid Droplets (LDs), and Endocytosis
2.6. Analysis of Trap Formation, Pathogenicity, and Proteolytic Activity
2.7. Stress Tolerance Analysis
2.8. RT-PCR Analysis
2.9. Secondary Metabolic Analysis
2.10. Data Analysis
3. Results
3.1. Bioinformatics Analysis of AoCryA and Verification of Deletion Strains
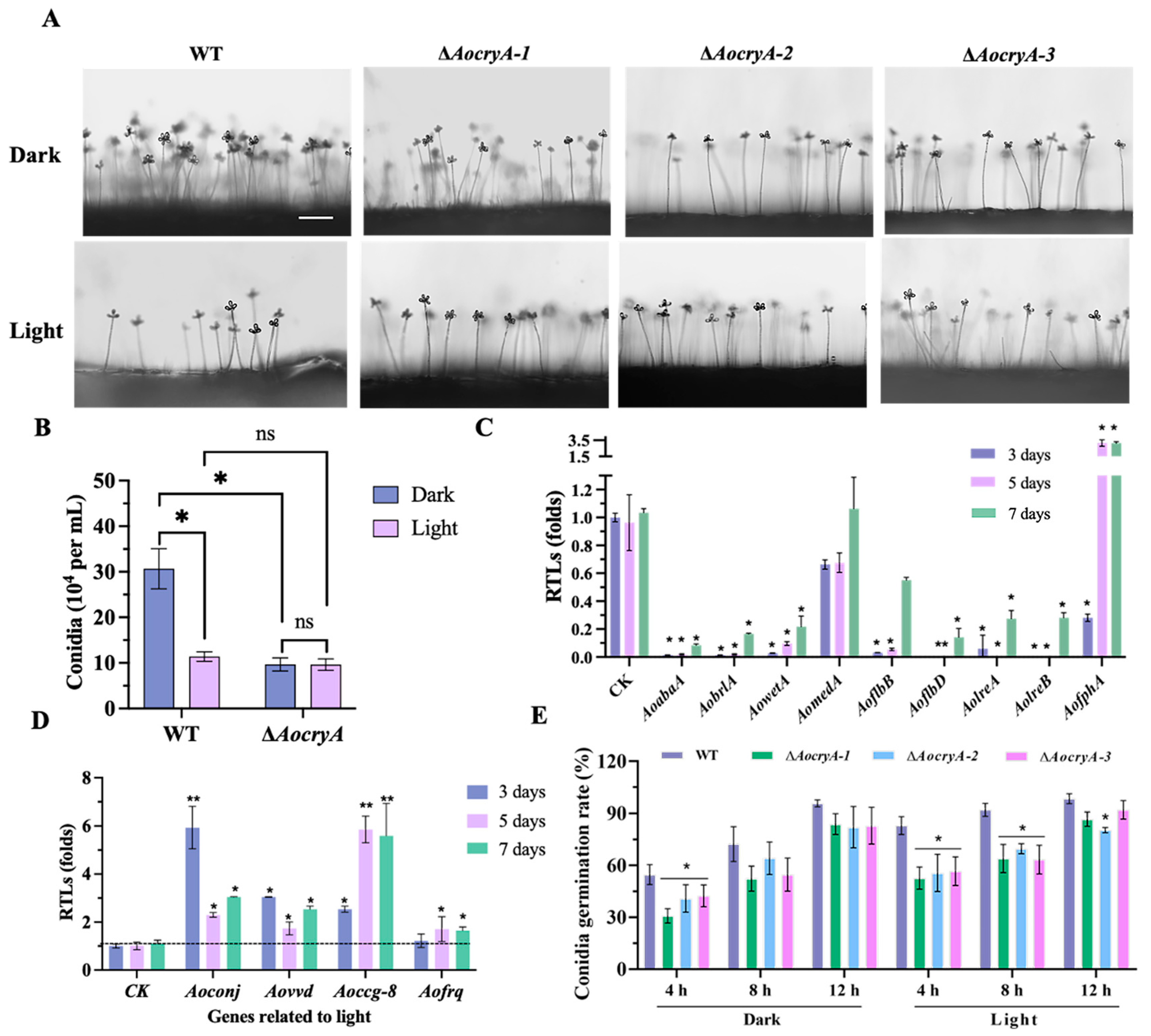
3.2. AoCryA Is Involved in Sporulation by Regulating the Response of Light
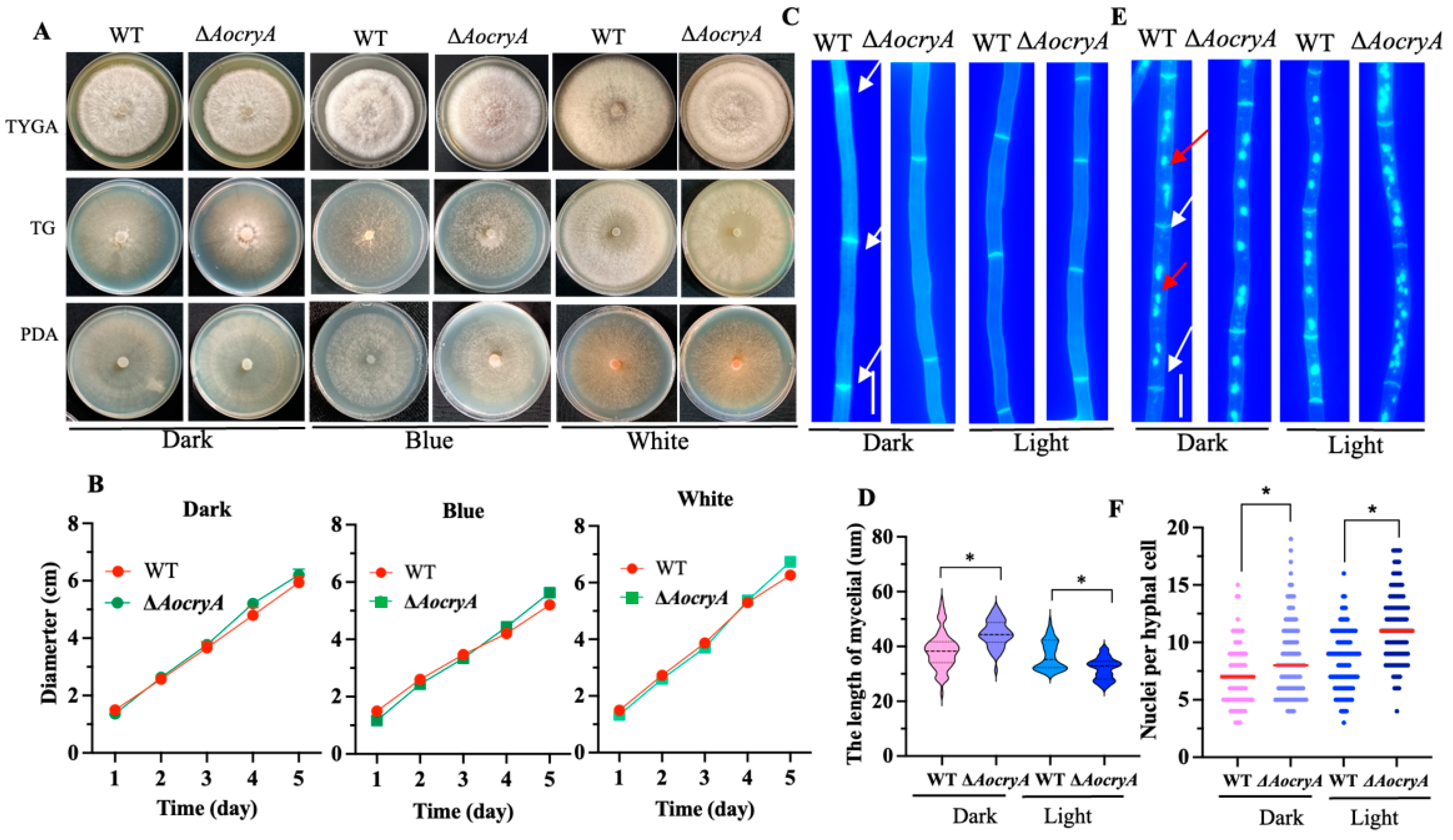
3.3. AoCryA Affects the Length of Hyphal Cells and the Number of Nuclei
3.4. AoCryA Negatively Regulates Trap Formation and Pathogenicity
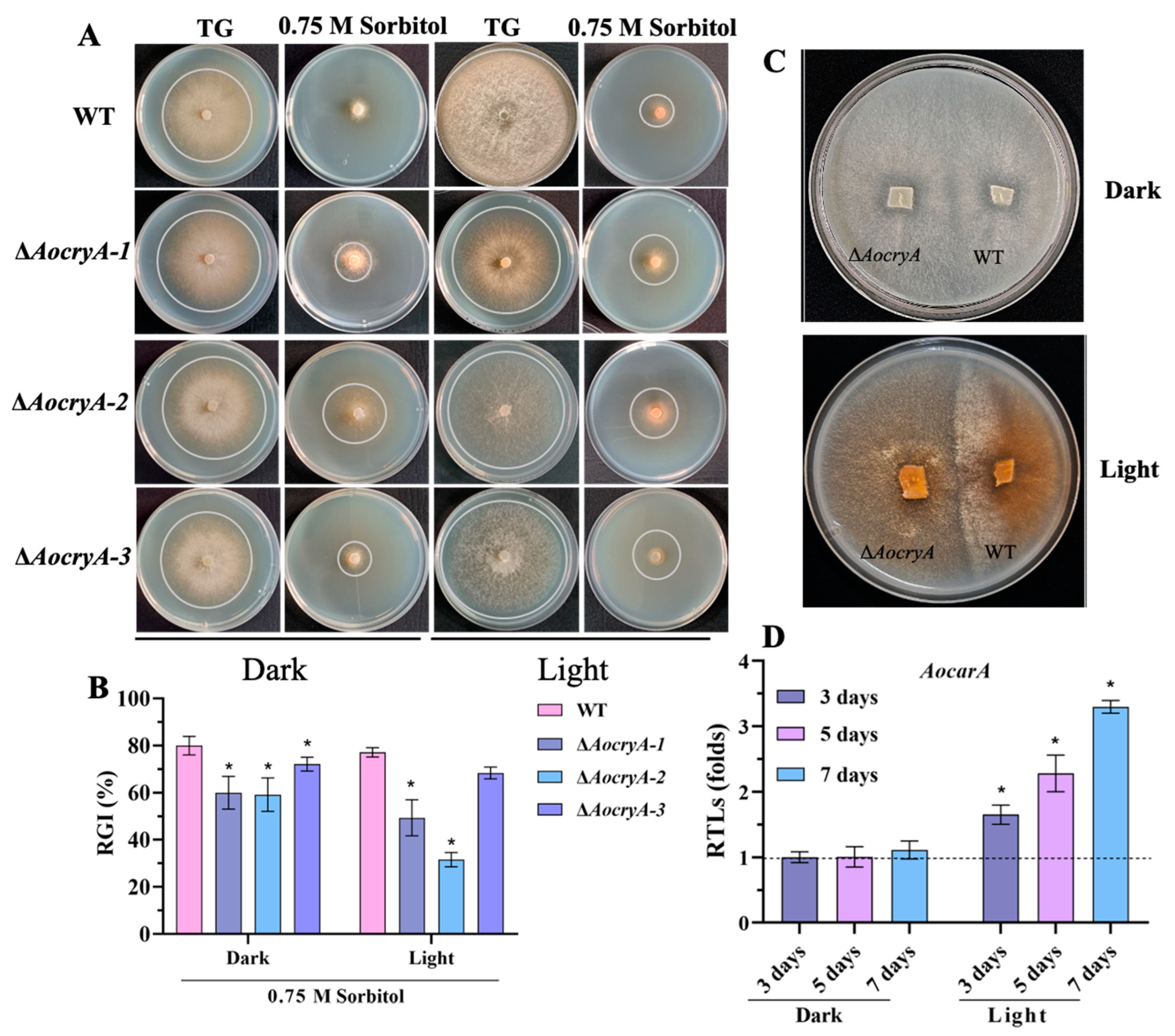
3.5. AoCryA Regulates Stress Response and Carotenoid Synthesis
3.6. AoCryA Regulates LD Accumulation and Endocytosis
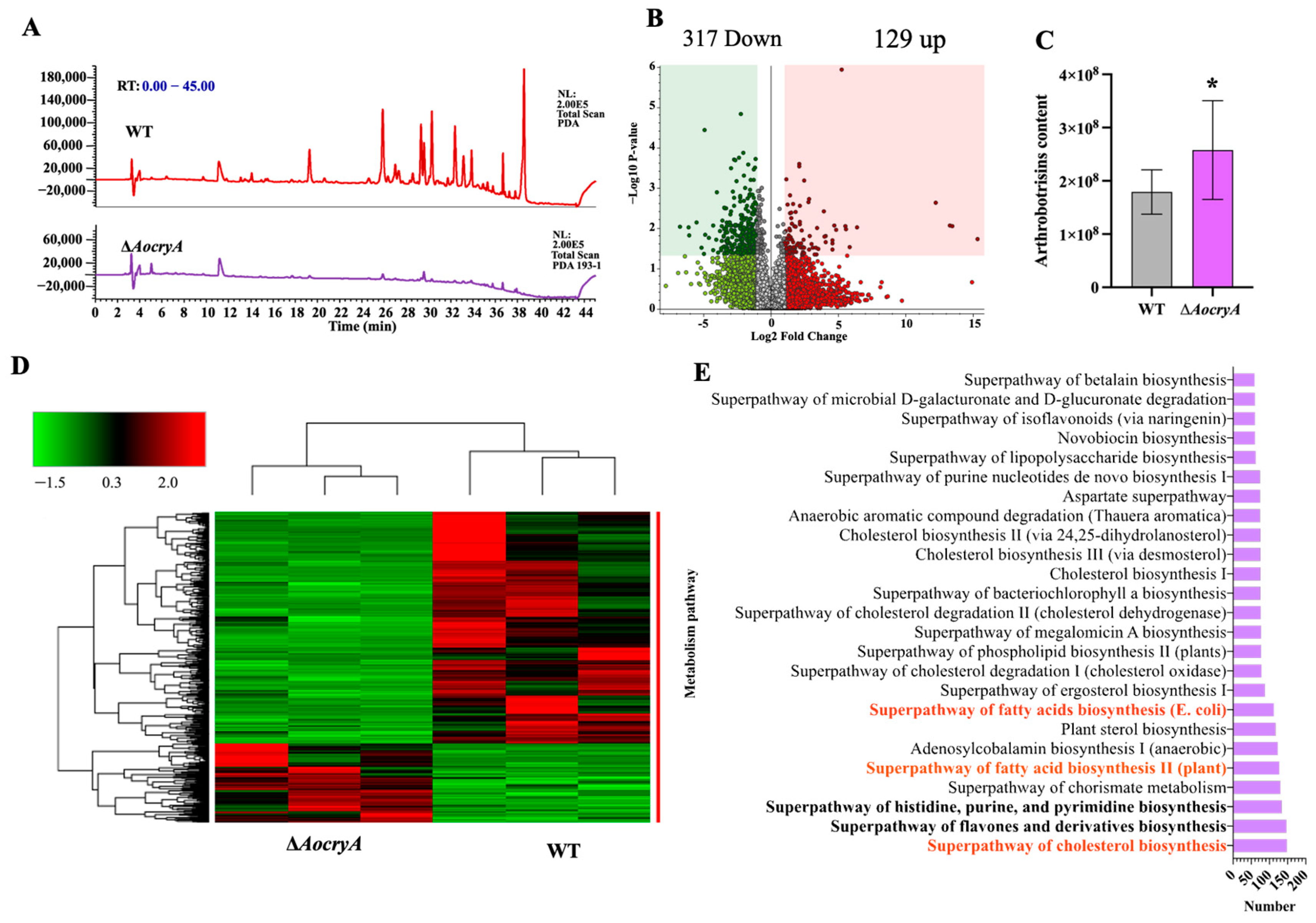
3.7. AoCryA Regulates Secondary Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, B.; Kim, S.; Lee, J. Microcyle conidiation in filamentous fungi. Mycobiology 2014, 42, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef]
- Stewart, J.I.P.; Fava, V.M.; Kerkaert, J.D.; Subramanian, A.S.; Gravelat, F.N.; Lehoux, M.; Howell, P.L.; Cramer, R.A.; Sheppard, D.C. Reducing Aspergillus fumigatus virulence through targeted dysregulation of the conidiation pathway. mBio 2020, 11, e03202-19. [Google Scholar] [CrossRef]
- McCormick, A.; Loeffler, J.; Ebel, F. Aspergillus fumigatus: Contours of an opportunistic human pathogen: Aspergillus fumigatus pathogenicity. Cell. Microbiol. 2010, 12, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Tillie-Leblond, I.; Tonnel, A.-B. Allergic bronchopulmonary Aspergillosis. Allergy 2005, 60, 1004–1013. [Google Scholar] [CrossRef]
- Stevens, D.A.; Moss, R.B.; Kurup, V.P.; Knutsen, A.P.; Greenberger, P.; Judson, M.A.; Denning, D.W.; Crameri, R.; Brody, A.S.; Light, M.; et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis—State of the art: Cystic fibrosis foundation consensus conference. Clin. Infect. Dis. 2003, 37, S225–S264. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.C.; Dagdas, Y.F.; Gupta, Y.K.; Mentlak, T.A.; Yi, M.; Martinez-Rocha, A.L.; Saitoh, H.; Terauchi, R.; Talbot, N.J.; Valent, B. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2013, 4, 1996. [Google Scholar] [CrossRef]
- Wilson, R.A. Magnaporthe oryzae. Trends Microbiol. 2021, 29, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Wernet, V.; Fischer, R. Establishment of Arthrobotrys flagrans as biocontrol agent against the root pathogenic nematode Xiphinema index. Environ. Microbiol. 2023, 25, 283–293. [Google Scholar] [CrossRef]
- Yu, X.; Hu, X.; Pop, M.; Wernet, N.; Kirschhöfer, F.; Brenner-Weiß, G.; Keller, J.; Bunzel, M.; Fischer, R. Fatal attraction of Caenorhabditis elegans to predatory fungi through 6-Methyl-Salicylic acid. Nat. Commun. 2021, 12, 5462. [Google Scholar] [CrossRef]
- Buzatti, A.; De Paula Santos, C.; Fernandes, M.A.M.; Yoshitani, U.Y.; Sprenger, L.K.; Dos Santos, C.D.; Molento, M.B. Duddingtonia flagrans in the control of gastrointestinal nematodes of horses. Exp. Parasitol. 2015, 159, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Han, K.H.; Yu, J.H. Upstream regulation of development and secondary metabolism in Aspergillus Species. Cells 2022, 12, 2. [Google Scholar] [CrossRef]
- Ni, M.; Yu, J.-H. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS ONE 2007, 2, e970. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Mah, J.H.; Seo, J.A. Growth and developmental control in the model and pathogenic aspergilli. Eukaryot. Cell 2006, 5, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Bayram, Ö.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.-J.; Keller, N.P.; Yu, J.-H.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-López, M.; Chen, W.; Eagle, C.E.; Gutiérrez, G.; Jia, W.L.; Swilaiman, S.S.; Huang, Z.; Park, H.-S.; Yu, J.-H.; Cánovas, D.; et al. Evolution of asexual and sexual reproduction in the Aspergilli. Stud. Mycol. 2018, 91, 37–59. [Google Scholar] [CrossRef]
- Son, Y.-E.; Yu, J.-H.; Park, H.-S. Regulators of the asexual life cycle of Aspergillus nidulans. Cells 2023, 12, 1544. [Google Scholar] [CrossRef]
- Cánovas, D.; Marcos, A.T.; Gacek, A.; Ramos, M.S.; Gutiérrez, G.; Reyes-Domínguez, Y.; Strauss, J. The histone acetyltransferase GcnE (GCN5) plays a central role in the regulation of Aspergillus asexual development. Genetics 2014, 197, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Urra, A.B.; Jiménez, C.; Nieto, M.I.; Rodríguez, J.; Hayashi, H.; Ugalde, U. Signaling the induction of sporulation involves the interaction of two secondary metabolites in Aspergillus nidulans. ACS Chem. Biol. 2012, 7, 599–606. [Google Scholar] [CrossRef]
- Park, H.-S.; Yu, J.-H. Developmental regulators in Aspergillus fumigatus. J. Microbiol. 2016, 54, 223–231. [Google Scholar] [CrossRef]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Bayram, Ö.; Braus, G.H. Coordination of secondary metabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol. Rev. 2012, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Romero, J.; Hedtke, M.; Kastner, C.; Müller, S.; Fischer, R. Fungi, Hidden in soil or up in the air: Light makes a difference. Annu. Rev. Microbiol. 2010, 64, 585–610. [Google Scholar] [CrossRef] [PubMed]
- Purschwitz, J.; Müller, S.; Kastner, C.; Schöser, M.; Haas, H.; Espeso, E.A.; Atoui, A.; Calvo, A.M.; Fischer, R. Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans. Curr. Biol. 2008, 18, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.L.; Loros, J.J.; Dunlap, J.C. The circadian clock of Neurospora crassa. FEMS Microbiol. Rev. 2012, 36, 95–110. [Google Scholar] [CrossRef]
- Brunner, M.; Simons, M.J.P.; Merrow, M. Lego clocks: Building a clock from parts. Genes. Dev. 2008, 22, 1422–1426. [Google Scholar] [CrossRef]
- Wang, B.; Kettenbach, A.N.; Gerber, S.A.; Loros, J.J.; Dunlap, J.C. Neurospora WC-1 recruits SWI/SNF to remodel frequency and initiate a circadian cycle. PLoS Genet. 2014, 10, e1004599. [Google Scholar] [CrossRef]
- Bayram, Ö.; Biesemann, C.; Krappmann, S.; Galland, P.; Braus, G.H. More than a repair enzyme: Aspergillus nidulans photolyase-like CryA is a regulator of sexual development. MBoC 2008, 19, 3254–3262. [Google Scholar] [CrossRef]
- Tagua, V.G.; Pausch, M.; Eckel, M.; Gutiérrez, G.; Miralles-Durán, A.; Sanz, C.; Eslava, A.P.; Pokorny, R.; Corrochano, L.M.; Batschauer, A. Fungal cryptochrome with DNA repair activity reveals an early stage in cryptochrome evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 15130–15135. [Google Scholar] [CrossRef] [PubMed]
- Veluchamy, S.; Rollins, J.A. A CRY-DASH-Type photolyase/cryptochrome from Sclerotinia sclerotiorum mediates minor UV-A-Specific effects on development. Fungal Genet. Biol. 2008, 45, 1265–1276. [Google Scholar] [CrossRef]
- Röhrig, J.; Kastner, C.; Fischer, R. Light inhibits spore germination through phytochrome in Aspergillus nidulans. Curr. Genet. 2013, 59, 55–62. [Google Scholar] [CrossRef]
- Chaves, I.; Pokorny, R.; Byrdin, M.; Hoang, N.; Ritz, T.; Brettel, K.; Essen, L.-O.; van der Horst, G.T.J.; Batschauer, A.; Ahmad, M. The cryptochromes: Blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 2011, 62, 335–364. [Google Scholar] [CrossRef]
- Dodson, C.A.; Hore, P.J.; Wallace, M.I. A Radical sense of direction: Signalling and mechanism in cryptochrome magnetoreception. Trends Biochem. Sci. 2013, 38, 435–446. [Google Scholar] [CrossRef]
- Esquivel-Naranjo, E.U.; García-Esquivel, M.; Medina-Castellanos, E.; Correa-Pérez, V.A.; Parra-Arriaga, J.L.; Landeros-Jaime, F.; Cervantes-Chávez, J.A.; Herrera-Estrella, A. A Trichoderma atroviride stress-activated MAPK pathway integrates stress and light signals. Mol. Microbiol. 2016, 100, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Armant, O.; Fischer, R. Fungi use the SakA (HogA) pathway for phytochrome-dependent light signalling. Nat. Microbiol. 2016, 1, 16019. [Google Scholar] [CrossRef] [PubMed]
- Lakin-Thomas, P.L.; Bell-Pedersen, D.; Brody, S. The genetics of circadian rhythms in Neurospora. Adv. Genet. 2011, 74, 55–103. [Google Scholar] [PubMed]
- Yu, Z.; Streng, C.; Seibeld, R.F.; Igbalajobi, O.A.; Leister, K.; Ingelfinger, J.; Fischer, R. Genome-wide analyses of light-regulated genes in Aspergillus nidulans reveal a complex interplay between different photoreceptors and novel photoreceptor functions. PLoS Genet. 2021, 17, e1009845. [Google Scholar] [CrossRef] [PubMed]
- Ruger-Herreros, C.; Rodríguez-Romero, J.; Fernández-Barranco, R.; Olmedo, M.; Fischer, R.; Corrochano, L.M.; Canovas, D. Regulation of conidiation by light in Aspergillus nidulans. Genetics 2011, 188, 809–822. [Google Scholar] [CrossRef]
- Froehlich, A.C.; Liu, Y.; Loros, J.J.; Dunlap, J.C. White collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 2002, 297, 815–819. [Google Scholar] [CrossRef]
- Hsueh, Y.-P.; Mahanti, P.; Schroeder, F.C.; Sternberg, P.W. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr. Biol. 2013, 23, 83–86. [Google Scholar] [CrossRef]
- Lin, H.C.; de Ulzurrun, G.V.; Chen, S.A.; Yang, C.T.; Tay, R.J.; Iizuka, T.; Huang, T.Y.; Kuo, C.Y.; Gonçalves, A.P.; Lin, S.Y.; et al. Key processes required for the different stages of fungal carnivory by a nematode-trapping fungus. PLoS Biol. 2023, 21, e3002400. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Tay, R.J.; Lin, H.C.; Juan, S.C.; Vidal-Diez de Ulzurrun, G.; Chang, Y.C.; Hoki, J.; Schroeder, F.C.; Hsueh, Y.P. The nematode-trapping fungus Arthrobotrys oligospora detects prey pheromones via G protein-coupled receptors. Nat. Microbiol. 2024, 9, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-C.; Li, X.-M.; Zhao, N.; Yang, L.; Zhang, K.-Q.; Yang, J.-K. Regulatory mechanism of trap formation in the nematode-trapping fungi. J. Fungi 2022, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hoffmann, D.S.; Wang, M.; Schuhmacher, L.; Stroe, M.C.; Schreckenberger, B.; Elstner, M.; Fischer, R. GprC of the nematode-trapping fungus Arthrobotrys flagrans activates mitochondria and reprograms fungal cells for nematode hunting. Nat. Microbiol. 2024, 9, 1752–1763. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, D.; Bai, N.; Liu, Q.; Zhao, N.; Yang, J. SNARE protein AoSec22 orchestrates mycelial growth, vacuole assembly, trap Formation, stress response, and secondary metabolism in Arthrobotrys oligospora. J. Fungi 2023, 9, 75. [Google Scholar] [CrossRef]
- Bai, N.; Xie, M.; Liu, Q.; Zhu, Y.; Yang, X.; Zhang, K.-Q.; Yang, J. AoMedA has a complex regulatory relationship with AoBrlA, AoAbaA, and AoWetA in conidiation, trap formation, and secondary metabolism in the nematode-trapping fungus Arthrobotrys oligospora. Appl. Environ. Microbiol. 2023, 89, e0098323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, M.; Wang, W.; Li, X.; Bai, N.; Xie, M.; Yang, J. AoMae1 regulates hyphal fusion, lipid droplet accumulation, conidiation, and trap formation in Arthrobotrys oligospora. J. Fungi 2023, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Bai, N.; Duan, S.; Shen, Y.; Zhu, L.; Yang, J. Characterizing the role of AosfgA and AofluG in mycelial and conidial development in Arthrobotrys oligospora and their role in secondary metabolism. Microorganisms 2024, 12, 615. [Google Scholar] [CrossRef]
- Mussi, M.A.; Gaddy, J.A.; Cabruja, M.; Arivett, B.A.; Viale, A.M.; Rasia, R.; Actis, L.A. The opportunistic human pathogen Acinetobacter baumannii senses and responds to light. J. Bacteriol. 2010, 192, 6336–6345. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Duan, S.; Bai, N.; Zhu, M.; Yang, J. Cellular communication and fusion regulate cell fusion, trap morphogenesis, conidiation, and secondary metabolism in Arthrobotrys oligospora. Microbiol. Res. 2024, 278, 127516. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Ma, Y.; Zhu, M.; Zhang, K.-Q.; Yang, J. The Arf-GAPs, AoAge1 and AoAge2, regulate diverse cellular processes, conidiation, trap formation, and secondary metabolism in Arthrobotrys oligospora. Microbiol. Res. 2024, 285, 127779. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, M.; Liu, Y.; Yang, L.; Yang, J. Aoatg11 and Aoatg33 are indispensable for mitophagy, and contribute to conidiation, the stress response, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Microbiol. Res. 2023, 266, 127252. [Google Scholar] [CrossRef]
- Xie, M.; Bai, N.; Yang, X.; Liu, Y.; Zhang, K.-Q.; Yang, J. Fus3 regulates asexual development and trap morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora. iScience 2023, 26, 107404. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Bai, N.; Liu, Q.; Yang, J. AoRab7A interacts with AoVps35 and AoVps41 to regulate vacuole assembly, trap formation, conidiation, and functions of proteasomes and ribosomes in Arthrobotrys oligospora. Microbiol. Res. 2024, 280, 127573. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, K.; Duan, S.; Zhao, N.; Shen, Y.; Zhu, L.; Zhang, K.-Q.; Yang, J. Identification of a transcription factor AoMsn2 of the Hog1 signaling pathway contributes to fungal growth, development and pathogenicity in Arthrobotrys oligospora. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Ruger-Herreros, C.; Corrochano, L.M. Conidiation in Neurospora crassa: Vegetative reproduction by a model fungus. Int. Microbiol. 2020, 23, 97–105. [Google Scholar] [CrossRef]
- He, Q.; Cheng, P.; Yang, Y.; Wang, L.; Gardner, K.H.; Liu, Y. White collar-1, a DNA binding transcription factor and a light sensor. Science 2002, 297, 840–843. [Google Scholar] [CrossRef]
- Jiang, K.-X.; Liu, Q.-Q.; Bai, N.; Zhu, M.-C.; Zhang, K.-Q.; Yang, J.-K. AoSsk1, a response regulator required for mycelial growth and development, stress responses, trap formation, and the secondary metabolism in Arthrobotrys oligospora. J. Fungi 2022, 8, 260. [Google Scholar] [CrossRef]
- Wang, F.; Sethiya, P.; Hu, X.; Guo, S.; Chen, Y.; Li, A.; Tan, K.; Wong, K.H. Transcription in fungal conidia before dormancy produces Phenotypically variable conidia that maximize survival in different environments. Nat. Microbiol. 2021, 6, 1066–1081. [Google Scholar] [CrossRef]
- Corrochano, L.M. Light in the fungal world: From photoreception to gene transcription and beyond. Annu. Rev. Genet. 2019, 53, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Igbalajobi, O.; Yu, Z.; Fischer, R. Red- and blue-light sensing in the plant pathogen Alternaria alternata depends on phytochrome and the white-collar protein LreA. mBio 2019, 10, e00371-19. [Google Scholar] [CrossRef]
- Gao, H.; Tang, Y.; Lv, R.; Jiang, W.; Jiang, Y.; Zhang, W.; Xin, F.; Jiang, M. Transcriptomic analysis reveals the potential mechanisms for improving carotenoid production in Rhodosporidium toruloides Z11 under light stress. J. Agric. Food Chem. 2024, 72, 3793–3799. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.D.; Shida, Y.; Miyata, A.; Takamizawa, T.; Suzuki, Y.; Ara, S.; Yamazaki, H.; Masaki, K.; Mori, K.; Aburatani, S.; et al. Effect of light on carotenoid and lipid production in the oleaginous yeast Rhodosporidium toruloides. Biosci. Biotechnol. Biochem. 2020, 84, 1501–1512. [Google Scholar] [CrossRef]
- Fuller, K.K.; Ringelberg, C.S.; Loros, J.J.; Dunlap, J.C. The fungal pathogen Aspergillus fumigatus regulates growth, metabolism, and stress resistance in response to light. mBio 2013, 4, e00142-13. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roldán, M.C.; Garre, V.; Guarro, J.; Mariné, M.; Roncero, M.I.G. Role of the white collar 1 photoreceptor in carotenogenesis, UV resistance, hydrophobicity, and virulence of Fusarium oxysporum. Eukaryot. Cell 2008, 7, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Tuttobene, M.R.; Pérez, J.F.; Pavesi, E.S.; Perez Mora, B.; Biancotti, D.; Cribb, P.; Altilio, M.; Müller, G.L.; Gramajo, H.; Tamagno, G.; et al. Light modulates important pathogenic determinants and virulence in ESKAPE pathogens Acinetobacter baumannii, Pseudomonas aeruginosa, and Staphylococcus aureus. J. Bacteriol. 2021, 203, e00566-20. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Larsen, L.N.; Granlund, L.; Holmeide, A.K.; Skattebøl, L.; Nebb, H.I.; Bremer, J. Sulfur-substituted and alpha-methylated fatty acids as peroxisome proliferator-activated receptor activators. Lipids 2005, 40, 49–57. [Google Scholar] [CrossRef]
- Wang, G.; Ran, H.; Fan, J.; Keller, N.P.; Liu, Z.; Wu, F.; Yin, W.-B. Fungal-fungal cocultivation leads to widespread secondary metabolite alteration requiring the partial loss-of-function VeA1 protein. Sci. Adv. 2022, 8, eabo6094. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Yang, X.; Zhu, M.; Duan, S.; Liu, Q.; Yang, J. The Cryptochrome CryA Regulates Lipid Droplet Accumulation, Conidiation, and Trap Formation via Responses to Light in Arthrobotrys oligospora. J. Fungi 2024, 10, 626. https://doi.org/10.3390/jof10090626
Shen Y, Yang X, Zhu M, Duan S, Liu Q, Yang J. The Cryptochrome CryA Regulates Lipid Droplet Accumulation, Conidiation, and Trap Formation via Responses to Light in Arthrobotrys oligospora. Journal of Fungi. 2024; 10(9):626. https://doi.org/10.3390/jof10090626
Chicago/Turabian StyleShen, Yanmei, Xuewei Yang, Meichen Zhu, Shipeng Duan, Qianqian Liu, and Jinkui Yang. 2024. "The Cryptochrome CryA Regulates Lipid Droplet Accumulation, Conidiation, and Trap Formation via Responses to Light in Arthrobotrys oligospora" Journal of Fungi 10, no. 9: 626. https://doi.org/10.3390/jof10090626




