Trachemys scripta Eggs as Part of a Potential In Vivo Model for Studying Sea Turtle Egg Fusariosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
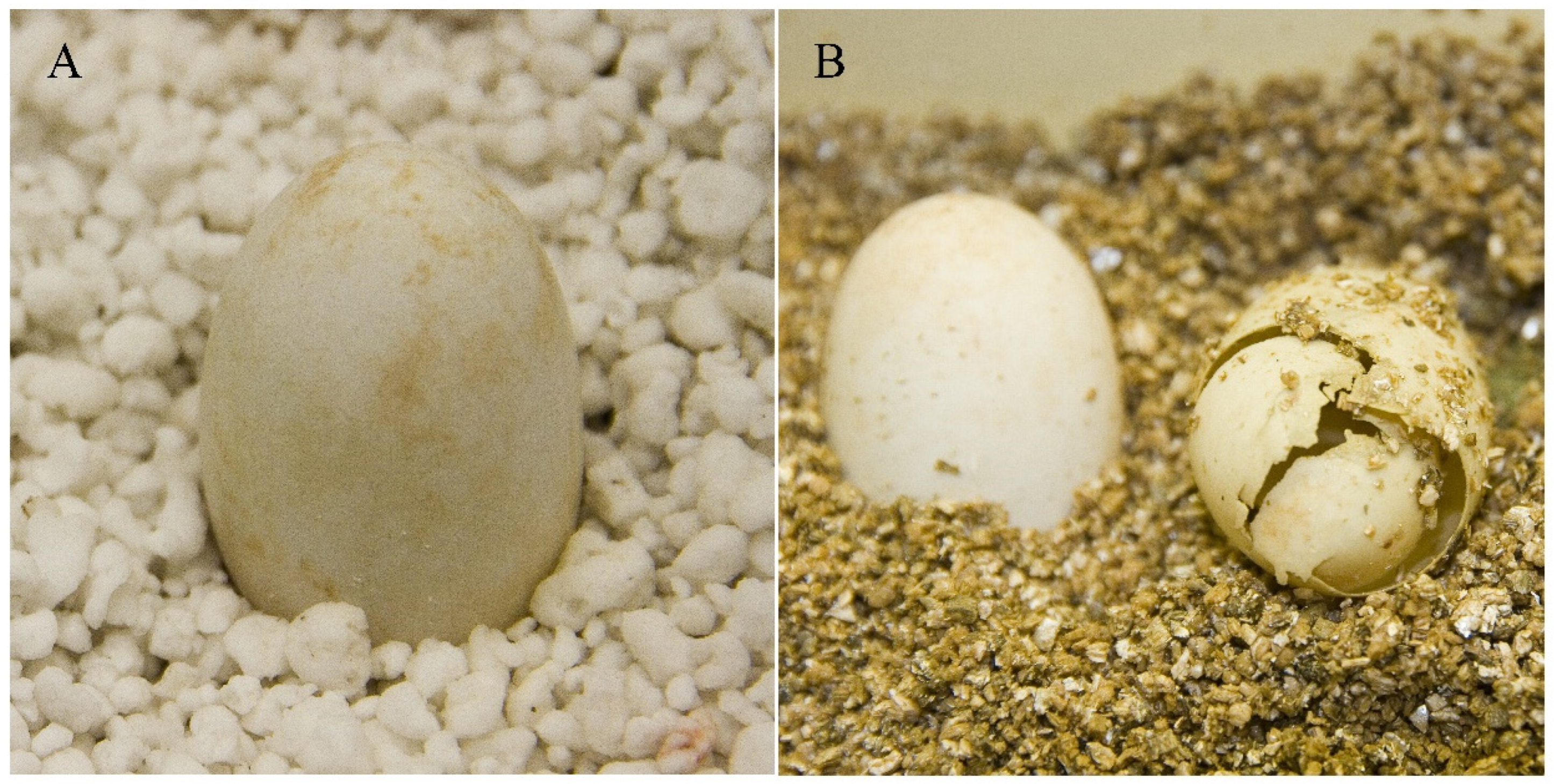
2.2. Inoculation of T. scripta Eggs with F. kertoplasticum
2.3. Disease Incidence, Disease Severity, and Hatching Rate
2.4. Reisolation of F. keratoplasticum from Diseased T. scripta Eggshell
2.5. Molecular Identification of F. keratoplasticum Isolates from Experimentally-Infected T. scripta Egg Shell
2.6. Physiological Characterization: pH Change
2.7. Statistical Analysis
3. Results
3.1. Inoculation Experiment Using Eggs of T. scripta: Disease Development
3.2. Disease Incidence, Disease Severity, and Hatching Rate in T. scripta Eggs
3.3. Koch’s Postulates: Fungal Isolation and Molecular Characterization
3.4. Physiological Characterization of F. keratoplasticum: Induced Changes in the pH
4. Discussion
5. Conclusions
- Trachemys scripta eggs can be used as an experimental in vivo host model organism for studying the biological properties and the infection process of the fungal pathogens responsible for STEF disease.
- Fusarium keratoplasticum is capable of modulating the host environment pH to allow egg colonization.
- Using this model organism, we can now envision further studies focused on improving our understanding of factors involved in the pathogenicity of these fungal species and the environmental conditions conducive to sea turtle egg fusariosis development.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. MBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Schertler, A.; Lenzner, B.; Dullinger, S.; Moser, D.; Bufford, J.L.; Ghelardini, L.; Santini, A.; Capinha, C.; Monteiro, M.; Reino, L.; et al. Biogeography and global flows of 100 major alien fungal and fungus-like oomycete pathogens. J. Biogeogr. 2024, 51, 599–617. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.A.; Daszak, P.; Wood, J.L.N. One Health, emerging infectious diseases and wildlife: Two decades of progress? Philos. Trans. R. Soc. B 2017, 372, 20160167. [Google Scholar] [CrossRef]
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusarium solani Species Complex That Cause Infections in Both Humans and Plants Are Common in the Environment. J. Clin. Microbiol. 2006, 44, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 2003, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; May, R. Superinfection and the evolution of parasite virulence. Proc. Roy. Soc. Lond. Ser. B Biol. Sci. 1994, 255, 81. [Google Scholar] [CrossRef]
- de Roode, J.C.; Pansini, R.; Cheesman, S.J.; Read, A.F. Virulence and competitive ability in genetically diverse malaria infections. Proc. Natl. Acad. Sci. USA 2005, 102, 7624–7628. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.B.; Bi, F.; Moral, J.; Barad, S. How Does Host Carbon Concentration Modulate the Lifestyle of Postharvest Pathogens during Colonization? Front. Plant Sci. 2016, 7, 1306. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017, 13, e1006149. [Google Scholar] [CrossRef] [PubMed]
- Diéguez-Uribeondo, J.; Förster, H.; Adaskaveg, J.E. Visualization of localized pathogen-induced pH modulation in almond tissues infected by Colletotrichum acutatum using confocal scanning laser microscopy. Phytopathology 2008, 98, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Brandao, R.L.; Castro, I.M.; Passos, J.B.; Nicoli, J.R.; Thevelein, J.M. Glucose-induced activation of the plasma membrane H(+)-ATPase in Fusarium oxysporum. J. Gen. Microbiol. 1992, 138, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.; Yakoby, N. Pathogenic fungi: Leading or led by ambient pH? Mol. Plant Pathol. 2003, 4, 509–516. [Google Scholar] [CrossRef]
- Hueffer, K.; O’Hara, T.; Follmann, E. Adaptation of mammalian host-pathogen interactions in a changing arctic environment. Acta Vet. Scand. 2011, 53, 17. [Google Scholar] [CrossRef]
- Smyth, C.W.; Sarmiento-Ramírez, J.M.; Short, P.G.; Diéguez-Uribeondo, J.; O’Donnell, K.; Geiser, D.M. Unraveling the ecology and epidemiology of an emerging fungal disease, sea turtle egg fusariosis (STEF). PLoS Pathog. 2019, 15, e1007682. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Ramírez, J.M.; Abella-Pérez, E.; Phillott, A.D.; Sim, P.; van West, P.; Martín, M.P.; Marco, A.; Diéguez-Uribeondo, J. Global Distribution of Two Fungal Pathogens Threatening Endangered Sea Turtles. PLoS ONE 2014, 9, e85853. [Google Scholar] [CrossRef]
- Summerbell, R.C.; Schroers, H.J. Analysis of Phylogenetic Relationship of Cylindrocarpon lichenicola and Acremonium falciforme to the Fusarium solani Species Complex and a Review of Similarities in the Spectrum of Opportunistic Infections Caused by These Fungi. J. Clin. Microbiol. 2002, 40, 2866–2875. [Google Scholar] [CrossRef]
- Short, D.P.G.; O’Donnell, K.; Thrane, U.; Nielsen, K.F.; Zhang, N.; Juba, J.H.; Geiser, D.M. Phylogenetic relationships among members of the Fusarium solani species complex in human infections and the descriptions of F. keratoplasticum sp. nov. and N. petroliphilum stat. nov. Fungal Genet. Biol. 2013, 53, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ríos, M.; Martín-Torrijos, L.; Diéguez-Uribeondo, J. The invasive alien red-eared slider turtle, Trachemys scripta, as a carrier of STEF-disease pathogens. Fungal Biol. 2022, 126, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Ramírez, J.M.; Abella, E.; Martín, M.P.; Tellería, M.T.; Lopez-Jurado, L.F.; Marco, A.; Diéguez-Uribeondo, J. Fusarium solani is responsible for mass mortalities in nests of loggerhead sea turtle, Caretta caretta, in Boavista, Cape Verde. FEMS Microbiol. Lett. 2010, 312, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Ramírez, J.M.; Sim, J.; Van West, P.; Diéguez-Uribeondo, J. Isolation of fungal pathogens from eggs of the endangered sea turtle species Chelonia mydas in Ascension Island. J. Mar. Biol. Assoc. UK 2016, 97, 661–667. [Google Scholar] [CrossRef]
- Brofft-Bailey, J.; Lamb, M.; Walker, M.; Weed, C.; Craven, K.S. Detection of potential fungal pathogens Fusarium falciforme and F. keratoplasticum in unhatched loggerhead eggs in Georgia, USA, using a molecular approach. Endanger. Species Res. 2018, 36, 111–119. [Google Scholar] [CrossRef]
- IUCN 2024. The IUCN Red List of Threatened Species. Version 2024-2. Available online: https://www.iucnredlist.org (accessed on 4 July 2024).
- Neely, M.N.; Pfeifer, J.D.; Caparon, M. Streptococcus-Zebrafish Model of Bacterial Pathogenesis. Infect. Immun. 2002, 70, 3904–3914. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Söderhäll, K.; Persson, M.; Ajaxon, R. The crayfish plague fungus, Aphanomyces astaci-diagnosis, isolation and pathobiology. Freshw. Crayfish 1987, 7, 131–144. [Google Scholar]
- Wuensch, A.; Trusch, F.; Iberahim, N.A.; van West, P. Galleria melonella as an experimental in vivo host model for the fish-pathogenic oomycete Saprolegnia parasitica. Fungal Biol. 2018, 122, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the world’s worst invasive alien species. In A Selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000; Volume 12, Available online: https://portals.iucn.org/library/sites/library/files/documents/2000-126.pdf (accessed on 19 June 2024).
- Schulz, M.; Della Vedova, B. Regulation (EU) 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. Off. J. Eur. Union 2014, 317, 35–55. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1417443504720&uri=CELEX:32014R1143 (accessed on 14 June 2024).
- Sancho, V.; Lacomba, I.; Bataller, J.V.; Carrasco, A.P. Manual para el control y erradicación de galápagos invasores. In Colección Manuales Técnicos de Biodiversidad, 6; Conselleria d’Agricultura, Medi Ambient, Canvi Climàtic i Desenvolupament Rural, Generalitat Valenciana: Valencia, Spain, 2015. [Google Scholar]
- Sancho, V.; Lacomba, J.I. Expansion of Trachemys scripta in the Valencian Community (Eastern Spain). Int. Symp. Fresh. Turt. Conserv. 2016, 1, 41–49. [Google Scholar]
- DOE (Diario Oficial de Extremadura). Especies exóticas invasoras. In Orden de 29 de abril de 2021 por la que se aprueba el protocolo para el control y/o erradicación del galápago de Florida (Trachemys scripta) en Extremadura; n.° 87-10-mayo-2021, 23059-23096; DOE (Diario Oficial de Extremadura): Madrid, Spain, 2021. [Google Scholar]
- Bataller, J.V.; Sancho, V.; Gil, J.M.; Lacomba, J. La Comunidad Valenciana lucha contra el galápago de Florida. Quercus 2008, 274, 28–34. [Google Scholar]
- Wyatt, T.T.; Wösten, H.A.; Dijksterhuis, J. Fungal spores for dispersion in space and time. Adv. Appl. Microbiol. 2013, 85, 43–91. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.J.; Vogt, R.C.; McCoy, C.J. Sex determining temperatures in turtles: A geographic comparison. Evolution 1982, 36, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Tenny, S.; Boktor, S.W. Incidence. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430746/ (accessed on 27 May 2024).
- Diéguez-Uribeondo, J.; Förster, H.; Adavaskaveg, J.E. Effect of wetness duration and temperature on the development of anthracnose on selected almond tissues and comparison of cultivar susceptibility. Phytopathology 2011, 101, 1013–1020. [Google Scholar] [CrossRef]
- Duhe, R.J. Koch’s Postulates. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1957–1959. [Google Scholar]
- Pérez-Santigosa, N. Ecología del Galápago Exótico, Trachemys scripta elegans, en la Península Ibérica. Efectos Sobre las Poblaciones de Mauremys leprosa y Emys orbicularis. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 2007. [Google Scholar]
- Martínez-Ríos, M.; Martín-Torrijos, L.; Diéguez-Uribeondo, J. Protocols for studying the crayfish plague pathogen, Aphanomyces astaci, and its host-pathogen interactions. J. Invertebr. Pathol. 2023, 201, 108018. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stone-Havas, S.; Cheng, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 30 April 2024).
- Alkan, N.; Espeso, E.A.; Prusky, D. Virulence regulation of phytopathogenic fungi by pH. ARS 2013, 19, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Shnaiderman, C.; Miyara, I.; Kobiler, I.; Sherman, A.; Prusky, D. Differential activation of ammonium transporters during the accumulation of ammonia by Colletotrichum gloeosporioides and its effect on appressoria formation and pathogenicity. Mol. Plant Microbe. Interact. 2013, 26, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Landraud, P.; Chuzeville, S.; Billon-Grande, G.; Poussereau, N.; Bruel, C. Adaptation to pH and role of PacC in the rice blast fungus Magnaporthe oryzae. PLoS ONE 2013, 8, e69236. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, A.; Pericolini, E.; Gabrielli, E.; Kenno, S.; Perito, S.; Cenci, E.; Monari, C. Elucidating the immunological function of the Cryptococcus neoformans capsule. Future Microbiol. 2013, 8, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, G.J.; Visser, J. Characterization of Aspergillus niger phosphoglucose isomerase. Use for quantitative determination of erythrose 4-phosphate. Biochimie 1999, 81, 267–272. [Google Scholar] [CrossRef]
- Cadi, A.; Joly, P. Competition for basking places between the endangered European pond turtle (Emys orbicularis galloitalica) and the introduced red-eared slider (Trachemys scripta elegans). Can. J. Zool. 2003, 81, 1392–1398. [Google Scholar] [CrossRef]
- Cadi, A.; Joly, P. Impact of the introduction of the red-eared slider (Trachemys scripta elegans) on survival rates of the European pond turtle (Emys orbicularis). Biodivers. Conserv. 2004, 13, 2511–2518. [Google Scholar] [CrossRef]
- GEIB (Grupo Especialista en Invasiones Biológicas). TOP 20: Las 20 Especies Exóticas Invasoras Más Dañinas Presentes en España; GEIB: Navatejera, Spain, 2006; p. 116. [Google Scholar]
- Polo-Cavia, N.; López, P.; Martín, J. Interspecific differences in chemosensory responses of freshwater turtles: Consequences for competition between native and invasive species. Biol. Invasions 2009, 11, 431–440. [Google Scholar] [CrossRef]
- Pleguezuelos, J.M.; Márquez, R.; Lizana, M. (Eds.) Atlas y Libro Rojo de los Anfibios y Reptiles de España; Dirección General de Conservación de la Naturaleza: Madrid, Spain, 2002; pp. 501–532. [Google Scholar]
- Resolucion del 01 de marzo de 2006 de la Directiva de Medio Ambiente de la Comunidad Valenciana. Available online: https://mediambient.gva.es/es/web/biodiversidad/accion-a.1-desarrollo-de-iniciativas-normativas (accessed on 5 September 2024).



| Treatment | Disease Incidence (%) a | Disease Severity b | Hatching Success c |
|---|---|---|---|
| Control 1 (non-treated eggs) | 33.3 | 0.87 ± 1.3 | 2/15 |
| Control 2 (washed eggs) | 53.3 | 1.6 ± 1.55 | 4/15 |
| Inoculated eggs with Fk | 93.3 * | 3.87 ± 1.73 * | 3/15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ríos, M.; Martín-Torrijos, L.; Diéguez-Uribeondo, J. Trachemys scripta Eggs as Part of a Potential In Vivo Model for Studying Sea Turtle Egg Fusariosis. J. Fungi 2025, 11, 23. https://doi.org/10.3390/jof11010023
Martínez-Ríos M, Martín-Torrijos L, Diéguez-Uribeondo J. Trachemys scripta Eggs as Part of a Potential In Vivo Model for Studying Sea Turtle Egg Fusariosis. Journal of Fungi. 2025; 11(1):23. https://doi.org/10.3390/jof11010023
Chicago/Turabian StyleMartínez-Ríos, María, Laura Martín-Torrijos, and Javier Diéguez-Uribeondo. 2025. "Trachemys scripta Eggs as Part of a Potential In Vivo Model for Studying Sea Turtle Egg Fusariosis" Journal of Fungi 11, no. 1: 23. https://doi.org/10.3390/jof11010023
APA StyleMartínez-Ríos, M., Martín-Torrijos, L., & Diéguez-Uribeondo, J. (2025). Trachemys scripta Eggs as Part of a Potential In Vivo Model for Studying Sea Turtle Egg Fusariosis. Journal of Fungi, 11(1), 23. https://doi.org/10.3390/jof11010023






