Fungal Biocontrol Agents in the Management of Postharvest Losses of Fresh Produce—A Comprehensive Review
Abstract
1. Introduction
2. Characteristics of Ideal Fungal BCAs
3. Factors Influencing Fungal BCAs Effectiveness
3.1. Microbial Inoculum Pressure
3.2. Formulations of Fungal BCAs
3.2.1. Dry Formulation
3.2.2. Liquid Formulation
| Formulation of BCAs | References |
|---|---|
| Dry | |
| Freeze-drying | |
| Pichia anomala J121 | [43,50] |
| Candida sake CPA-1 | [41] |
| Rhodotorula glutinis, Cryptococcus laurentii | [44,45] |
| Spray-drying | |
| Candida sake CPA-1 | [46] |
| Fluidized bed-drying | |
| Pichia anomala J121 | [50] |
| Aureobasidium pullulans | [51] |
| Vacuum-drying | |
| Pichia anomala J121 | [43,50] |
| Liquid | |
| Rhodotorula minuta | [48] |
| Pichia anomala J121 | [38,50] |
| Candida sake CPA-1 | [47] |
| Cryptococcus laurentii, Pichia membranaefaciens | [49] |
3.3. Delivery Systems of Fungal BCAs
3.3.1. Preharvest Application of Fungal BCAs
3.3.2. Postharvest Application of Fungal BCAs
4. Fungal BCAs in Postharvest Diseases of Farm Produce
| Antagonist | Disease | Target | Produce | Mechanism of Activity | Reference |
|---|---|---|---|---|---|
| Cryptococcus laurentii C. albidus | Gray mold Brown rot | Monilinia fructicola Penicillium expansum Botrytis cinerea | Apple Tomato Orange Sweet cherry Peach Strawberry | - Induce host defense responses - Inducing accumulation of resistance related enzymes - ROS tolerance - Attachment and lytic enzyme secretion | [84,85,86,87,88] |
| Pichia guilliermondii P. menmbranaefaciencs P. guillermondii | Gray mold Alternaria rot Rhizopus rots Blue mold Green mold Anthracnose | Rhizopus stolonifer Botrytis cinerea Penicillium expansum Penicillium digitatum Collectrichum capsici Alternata alternata | Tomato Apple Citrus Peach Grape Chillie | - Induce host defense responses - Attachment and lytic enzyme secretion | [3,25,37,72,84] |
| Candida ciferii (283) C. sake C. saitoana (240) C. guilliermondii C. oleophila (1–182) | Botrytis rot Penicillium rot Penicillium rot Gray mold | Penicillium expansum Botrytis cinerea | Apple Orange Tomato | - Induce host defense responses - Adjustment of population density | [89,90] |
| Cystofilobasidium infirmominiatum | Botrytis rot | Botrytis cynerea | Lemon | - Iron depletion - ROS tolerance | [91] |
| Saccharomycess cereviciae (N.826 and N.831) | Penicillium rot | Penicillium expansum | Grape | - Morphology change | [92] |
| Metshnikowia fructicola (NRRL Y-27328) | Botrytis rot | Botrytis cynerea | Grape | - Iron depletion | [61] |
| Trichosporon pullulans | Alternaria rot Gray rot | Botrytis cynerea | Cherry | - Production of lytic enzymes | [84] |
| Pestalotiopsis neglecta | Anthracnose | Collectotrichum gloeosporoides | Apricot | - Production of lytic enzymes | [93] |
| Debaryomyces hansenii | Rhizopus rot Alternaria rot Gray mold | Botrytis cynerea Penicillium expansum | Tomato | - Induction of host resistance - Competition for nutrients and space | [3] |
| Rodotorula glutinis | Blue mold Gray mold Alternaria rot Green mold | Penicillium expansum Botrytis cinerea | Apple Orange Pear Strawberry Sweet cheery | - Competition for nutrients and space - Site exclusion | [3,67,87,94,95,96] |
| Trichordema harzianum | Anthracnose Gray mold | Collectrichum muse Collectotrichum gloeosporoides | Banana Strawberry Pear Kiwi Grape | - Production of antibiotics | [97,98] |
| Coprinellus micaceus | Not specified | Coryanebacterium xeroides | Not specified | - Natural bioactive compounds extracted from C. micaceus | [99] |
| Aureobasidium pullulans | Tomato late blight Blue mold Gray mold Rhizopus rot Botrytis rot Penicillium rot Monalinia rot | Phytophthora infestans Botrytis cinerea Penicillium expansum Penicillium spp. Monilinia laxa | Tomato seeds Tomato Peach Apples Cherries Grapes Bananas | - Not specified | [21,58,100,101] |
| Epicoccum nigram E. sorghinum | Late blight Fusarium wilt Esca disease | F. graminearum F. verticillioides F. oxysporum F. avenaceum, Colletotrichum falcatum Ceratocystis paradoxa Xanthomomas albilineans Pythium irregulare Phytophthora infestans Phaeomoniella chlamydospora Phaeoacremonium minimum | Tomato Peas | - Natural bioactive compounds extracted from Epicoccum spp. | [3,102,103,104] |
| Preussia africana | Fusarium wilt Alternaria rot Blast disease | F. solani C. albicans Pyricularia grisea | Tomato seeds Rice | - Not specified | [105,106] |
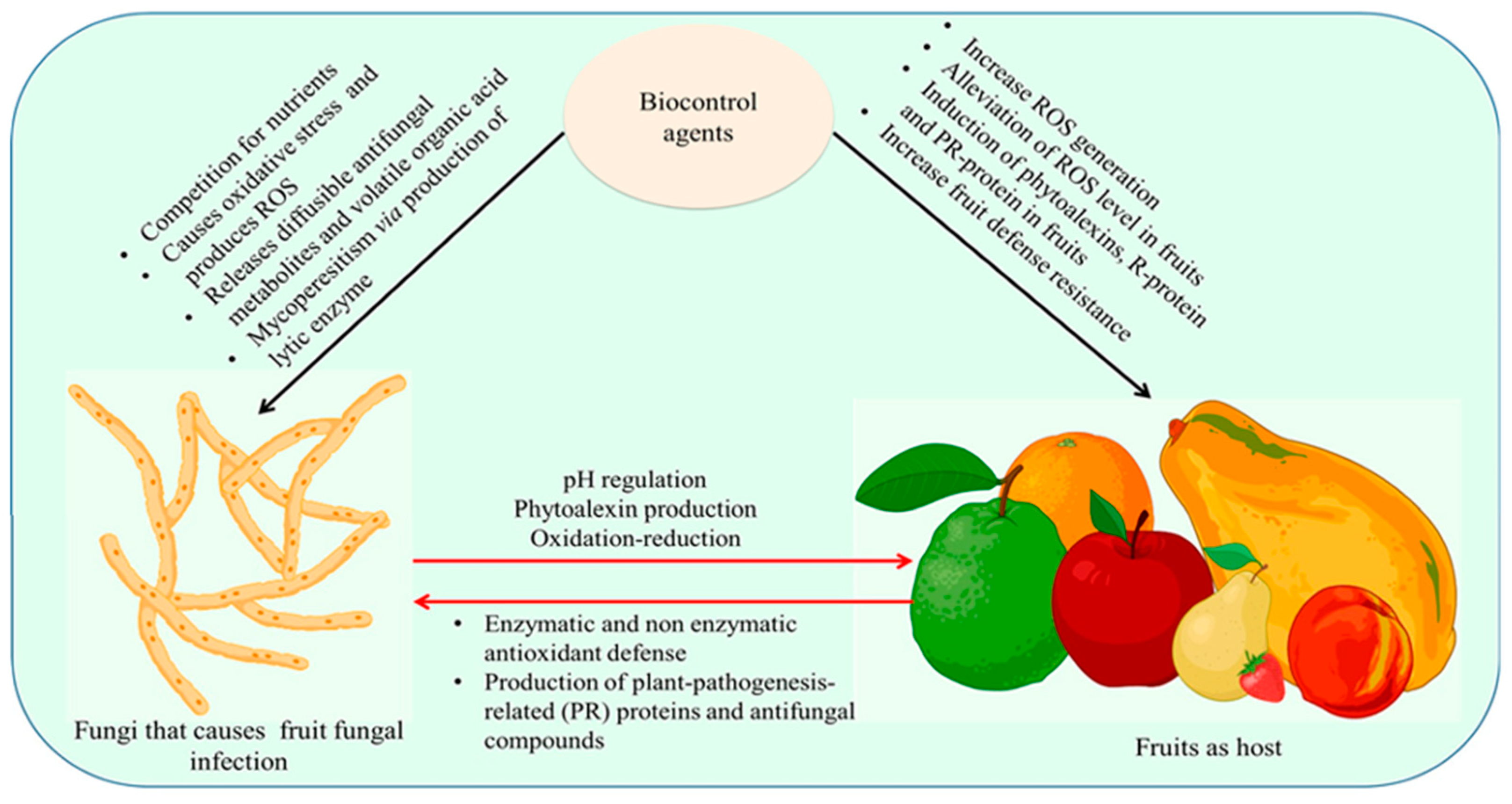
5. Mechanisms of Action of Fungal BCAs
5.1. Competition for Nutrients and Space
5.2. Production of Metabolites
5.3. Siderophores Production
5.4. Host Resistance
Tolerance to High Levels of Reactive Oxygen Species (ROS)
5.5. Direct Parasitism
6. Challenges and Difficulties in Establishing Beneficial Microbes as BCAs
7. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial interactions within multiple-strain biological control agents impact soil-borne plant disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, B. Review on postharvest handling to reduce loss of fruits and vegetables. Int. J. Hortic. Food. Sci. 2020, 2, 48–52. [Google Scholar] [CrossRef]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative stress associated with chilling injury in immature fruit: Postharvest technological and biotechnological solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef] [PubMed]
- Matityahu, I.; Marcianoa, P.; Holland, D.; Ben-Ariec, R.; Amira, R. Differential effects of regular and controlled atmosphere storage on the quality of three cultivars of pomegranate (Punica granatum L.). Post-Harvest. Biol. Technol. 2016, 115, 132–141. [Google Scholar] [CrossRef]
- Ferreira, E.M.S.; Malta, C.M.; Bicalho, J.O.; Pimenta, R.S. A safe method to control the anthracnose in papaya. Rev. Bras. Frutic. 2018, 40, 683. [Google Scholar] [CrossRef]
- Leng, J.; Dai, Y.; Qiu, D.; Zou, Y.; Wu, X. Utilization of the antagonistic yeast, Wickerhamomyces anamolus, combined with UV-C to manage postharevest rot of potato tubers caused by Alternaria tenuissima. Int. J. Food Microbiol. 2022, 377, 109782. [Google Scholar] [CrossRef]
- Wen, Y.; Li, M.; Yang, S.; Peng, L.; Fan, G.; Kang, H. Isolation of antagonistic endophytic fungi from postharvest chestnuts and their biocontrol on host fungal pathogens. J. Fungi 2024, 10, 573. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.; Zgârian, R.; Pandelea, G. Bio-Coatings for preservation of fresh fruits and vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Vischetti, C.; Feliziani, E.; Landi, L.; De Bernardi, A.; Marini, E.; Romanazzi, G. Effectiveness of four synthetic fungicides in the control of post-harvest gray mold of strawberry and analyses of residues on fruit. Agronomy 2024, 14, 65. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and table grapes: A review of the main physical, chemical, and bio-based control treatments in post-harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- Landi, L.; Peralta-Ruiz, Y.; Chaves-López, C.; Romanazzi, G. Chitosan coating enriched with Ruta graveolens L. essential oil reduces postharvest Anthracnose of papaya (carica papaya L.) and modulates defense-related gene expression. Front. Plant. Sci. 2021, 12, 765806. [Google Scholar] [CrossRef]
- Mamphogoro, T.P.; Babalola, O.O.; Aiyegoro, O.A. Exploitation of epiphytic bacterial antagonists for the management of post-harvest diseases of sweet pepper and other fresh produce—A viable option. Biocontrol Sci. Technol. 2020, 30, 741–761. [Google Scholar] [CrossRef]
- David, A.; Botías, C.; Abdul-Sada, A.; Nicholls, E.; Rotheray, E.L.; Hill, E.M.; Goulson, D. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Intern. 2016, 88, 169–178. [Google Scholar] [CrossRef]
- Gurel, F.B.; Miller, S.A.; Bledsoe, M. Post-harvest applications of disinfectants using a fog tunnel system to manage Botrytis gray mold in tomatoes. Acta Hortic. 2014, 1053, 237–244. [Google Scholar] [CrossRef]
- Prietto, L.; Moraes, P.S.; Kraus, R.B.; Meneghetti, V.; Fagundes, C.A.A.; Furlong, E.B. Post-harvest operations and aflatoxin levels in rice (Oryza sativa). Crop Prot. 2015, 78, 172–177. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, A.; Thakur, V.; Baswal, A.K.; Kaur, A.; Kumar, M.; Yadav, S.K. Exploring the role and potential of edible waxes in enhancing quality and shelf-life of vegetable crops: A comprehensive review. Ann. Phytomed. 2024, 13, 439–448. [Google Scholar] [CrossRef]
- Strano, M.C.; Altieri, G.; Allegra, M.; Di Renzo, G.C.; Paterna, G.; Matera, A.; Genovese, F. Postharvest technologies of fresh citrusf: Advances and recent developments for the loss reduction during handling and storage. Horticulturae 2022, 8, 612. [Google Scholar] [CrossRef]
- Wassermann, B.; Kusstatscher, P.; Berg, G. Microbiome response to hot water treatment and potential synergy with biological control on stored apples. Front. Microbiol. 2019, 10, 2502. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Linares-Morales, J.R.; Gutiérrez-Méndez, N.; Rivera-Chavira, B.E.; Pérez-Vega, S.B.; Nevárez-Moorillón, G.V. Biocontrol processes in fruits and fresh produce, the use of Lactic acid bacteria as a sustainable option. Front. Sustain. Food Syst. 2018, 2, 50. [Google Scholar] [CrossRef]
- Saxena, A.; Raghuwanshi, R.; Gupta, V.K.; Singh, H.B. Chilli anthracnose: The epidemiology and management. Front. Microbiol. 2016, 7, 1527. [Google Scholar] [CrossRef] [PubMed]
- Roca-Couso, R.; Flores-Félix, J.D.; Rivas, R. Mechanisms of action of microbial biocontrol agents against Botrytis cinerea. J. Fungi 2021, 7, 1045. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zeng, K.; Chen, Z. Postharvest disease management in fruits and vegetables: Recent advances and mechanisms. Front. Microbiol. 2023, 14, 1203010. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zheng, X.; Chen, W.; Ye, X.; Tu, P. Metabolomics reveals key resistant responses in tomato fruit induced by Cryptococcus laurentii. Food chemistry. Mol. Sci. 2022, 4, 100066. [Google Scholar] [CrossRef]
- Ansari, R.A.; Kumar, R.; Rizvi, R. Evaluation of effectiveness of phytoextracts and biological control agents on the mycelial growth of Fusarium oxysporum f. sp. Lycopersici. Int. J. Agric. Sci. 2023, 14, 82–88. [Google Scholar] [CrossRef]
- Ong, G.; Kasi, R.; Subramaniam, R. A review on plant extracts as natural additives in coating applications. Prog. Org. Coat. 2021, 151, 106091. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.d.J.; Hernández-Fuentes, A.D.; González-Lemus, U.; Zaldívar-Ortega, A.K.; González-Montiel, L.; Madariaga-Navarrete, A.; Hernández-Soto, I. Biofungicides based on plant extracts: On the road to organic farming. Int. J. Mol. Sci. 2024, 25, 6879. [Google Scholar] [CrossRef] [PubMed]
- Mesguida, O.; Haidar, R.; Yacoub, A.; Dreux-Zigha, A.; Berthon, J.-Y.; Guyoneaud, R.; Attard, E.; Rey, P. Microbial biological control of fungi associated with grapevine trunk diseases: A review of strain diversity, modes of action, and advantages and limits of current strategies. J. Fungi 2023, 9, 638. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Pereyra, M.M.; Díaz, M.A.; Soliz-Santander, F.F.; Poehlein, A.; Meinhardt, F.; Daniel, R.; Dib, J.R. Screening methods for isolation of biocontrol epiphytic yeasts against Penicillium digitatum in lemons. J. Fungi 2021, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, E.; James, S.A.; Breierova, E.; Antolak, H.; Kregiel, D. Biocontrol capability of local Metschnikowia sp. isolates. Antonie Van Leeuwenhoek 2019, 112, 1425–1445. [Google Scholar] [CrossRef]
- Horváth, E.; Dályai, L.; Szabó, E.; Barna, T.; Kalmár, L.; Posta, J.; Sipiczki, M.; Csoma, H.; Miklós, I. The antagonistic Metschnikowia andauensis produces extracellular enzymes and pulcherrimin, whose production can be promoted by the culture factors. Sci. Rep. 2021, 11, 10593. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challenges, and global perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef] [PubMed]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological control of plant diseases: An evolutionary and eco-economic consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Dwiastuti, M.E.; Soesanto, L.; Aji, T.G.; Devy, N.N.; Harddiyanto. Biological control strategy for postharvest diseases of citrus, apples, grapes and strawberries fruits and application in Indonesia. Egypt. J. Biol. Pest. Control 2021, 31, 141. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Llabot, J.M.; Cantoro, R.; Chiotta, M.L.; Allemandi, D.A.; Torres, A.M.; Chulze, S.N. Spray-drying process as a suitable tool for the formulation of Bacillus velezensis RC218, a proved biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation in wheat. Biocontrol Sci. Technol. 2019, 30, 329–338. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, W.; Sui, Y.; Ding, R.; Yi, W.; Hu, Y.; Liu, H.; Zhu, C. Sugar protectants improve the thermotolerance and biocontrol efficacy of the biocontrol yeast, Candida oleophila. Front. Microbiol. 2019, 10, 187. [Google Scholar] [CrossRef]
- Tan, D.T.; Poh, P.E.; Chin, S.K. Microorganism preservation by convective air-drying—A review. Dry Technol. 2017, 36, 764–779. [Google Scholar] [CrossRef]
- Lee, S.B.; Choi, W.S.; Jo, H.J.; Yeo, S.H.; Park, H.D. Optimization of air-blast drying process for manufacturing Saccharomyces cerevisiae and non-Saccharomyces yeast as industrial wine starters. AMB Express 2016, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Deng, L.; Zhou, Y.; Wang, W.; Yao, S.; Zeng, K. Optimization of a protective medium for freeze-dried Pichia membranifaciens and application of this biocontrol agent on citrus fruit. J. Appl. Microbiol. 2016, 121, 234–243. [Google Scholar] [CrossRef]
- Druvefors, U.A.; Passoth, V.; Schnürer, J. Nutrient effects on biocontrol of Penicillium roqueforti by Pichia anomala J121 during airtight storage of wheat. Appl. Environ. Microbiol. 2005, 71, 1865–1869. [Google Scholar] [CrossRef]
- Mahunu, G.K.; Abubakari, A.H.; Garti, H. Antagonistic yeasts and alternative chemical combinations as potential biocontrol agents in postharvest application. UDS Int. J. Develop. 2020, 7, 273–284. [Google Scholar]
- Li, B.Q.; Tian, S.P. Effect of intracellular trehalose in Cryptococcus laurentii and exogenous lyoprotectants on its viability and biocontrol efficacy on Penicillium expansum in apple fruit. Lett. Appl. Microbiol. 2007, 44, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Carbó, A.; Torres, R.; Usall, J.; Fons, E.; Teixidó, N. Dry formulations of the biocontrol agent Candida sake CPA-1 using fluidised bed drying to control the main postharvest diseases on fruits. J. Sci. Food Agric. 2017, 97, 3691–3698. [Google Scholar] [CrossRef]
- Teixidó, N.; Usall, J.; Torres, R. Insight into a successful development of biocontrol agents: Production, formulation, packaging, and shelf life as key aspects. Horticulturae 2022, 8, 305. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Sanphui, P.; Palanisamy, K.; Prakash, M.; Bansal, A.K. Design of ascorbic acid eutectic mixtures with sugars to inhibit oxidative degradation. Front. Chem. 2020, 10, 754269. [Google Scholar] [CrossRef] [PubMed]
- Gava, C.A.T.; de Castro, A.P.C.; Pereira, C.A.; Gonçalves, J.S.; Araújo, L.F.C.; da Paz, C.D. Photoprotector adjuvants to enhance UV tolerance of yeast strains for controlling mango decay using pre-harvest spraying. Biocontrol Sci. Technol. 2018, 28, 811–822. [Google Scholar] [CrossRef]
- Rueda-Mejia, M.P.; Nägeli, L.; Lutz, S.; Hayes, R.D.; Varadarajan, A.R.; Grigoriev, I.V.; Ahrens, C.H.; Freimoser, F.M. Genome, transcriptome and secretome analyses of the antagonistic, yeast-like fungus Aureobasidium pullulans to identify potential biocontrol genes. Microb. Cell. 2021, 8, 184–202. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Ranjbar, A. Advances in Postharvest diseases management of fruits and vegetables: A review. Horticulturae 2023, 9, 1099. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in analysis and detection of major mycotoxins in foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.N.; Fan, Z.Y.; Cai, Y.T.; Jin, L.F.; Yu, T. The influence of N-acetylglucosamine: Inducing Rhodosporidium paludigenum to enhance the inhibition of Penicillium expansum on pears. Postharvest Biol. Technol. 2021, 176, 111486. [Google Scholar] [CrossRef]
- Kheireddine, A.; Essghaier, B.; Hedi, A.; Dhieb, C.; Sadfi-Zouauin, N. New epiphytic yeasts able to reduce grey mold disease on apples. Plant Protect. Sci. 2018, 54, 248–257. [Google Scholar] [CrossRef]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef]
- Settier-Ramírez, L.; López-Carballo, G.; Hernández-Muñoz, P.; Fontana, A.; Strub, C.; Schorr-Galindo, S. New isolated Metschnikowia pulcherrima strains from apples for postharvest biocontrol of Penicillium expansum and Patulin Accumulation. Toxins 2021, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- Remolif, G.; Schiavon, G.; Garello, M.; Spadaro, D. Efficacy of Postharvest Application of Aureobasidium pullulans to control white haze on apples and effect on the fruit mycobiome. Horticulturae 2024, 10, 927. [Google Scholar] [CrossRef]
- Zhang, H.; Godana, E.A.; Sui, Y.; Yang, Q.; Zhang, X.; Zhao, L. Biological control as an alternative to synthetic fungicides for the management of grey and blue mould diseases of table grapes: A review. Crit. Rev. Microbiol. 2020, 46, 450–462. [Google Scholar] [CrossRef]
- Ayogu, P.; Martins, V.; Gerós, H. Grape berry native yeast microbiota: Advancing trends in the development of sustainable vineyard pathogen biocontrol strategies. OENO One 2024, 58, 1–18. [Google Scholar] [CrossRef]
- Macharia, J.M.; Gikungu, M.W.; Karanja, R.; Okoth, S. Managed bees as pollinators and vectors of bio control agent against grey mold disease in strawberry plantations. Afr. J. Agric. Res. 2020, 16, 1674–1680. [Google Scholar] [CrossRef]
- Bhatta, U.K. Alternative management approaches of citrus diseases caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Front. Plant Sci. 2022, 12, 833328. [Google Scholar] [CrossRef]
- Ullah, I.; Yuan, W.; Khalil, H.B.; Khan, M.R.; Lak, F.; Uzair, M.; Abbas, A.; Gohari, A.M.; Wu, H. Understanding Botrytis cinerea infection and gray mold management: A review paper on deciphering the rose’s thorn. Phytopathol. Res. 2024, 6, 42. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Kumar, A.; Fadiji, A.E.; Babalola, O.O.; Puopolo, G.; Santoyo, G. Agroecological management of the grey mould fungus Botrytis cinerea by plant growth-promoting bacteria. Plants 2023, 12, 637. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, H.P.; Bradshaw, M.; Jurick, W.M., II; Fonseca, J.M. The good, the bad, and the ugly: Mycotoxin production during postharvest decay and their influence on tritrophic host–pathogen–microbe interactions. Front. Microbiol. 2021, 12, 611881. [Google Scholar] [CrossRef]
- Ianiri, G.; Barone, G.; Palmieri, D.; Quiquero, M.; Gaeta, I.; De Curtis, F.; Castoria, R. Transcriptomic investigation of the interaction between a biocontrol yeast, Papiliotrema terrestris strain PT22AV, and the postharvest fungal pathogen Penicillium expansum on apple. Commun. Biol. 2024, 7, 359. [Google Scholar] [CrossRef]
- Obi, V.I.; Barriuso, J.J.; Gogorcena, Y. Peach Brown Rot: Still in search of an ideal management option. Agriculture 2018, 8, 125. [Google Scholar] [CrossRef]
- Hayat, U.; Li, W.; Bie, H.; Liu, S.; Guo, D.; Cao, K. An Overview on post-harvest technological advances and ripening techniques for increasing peach fruit quality and shelf life. Horticulturae 2024, 10, 4. [Google Scholar] [CrossRef]
- Sui, Y.; Wisniewski, M.; Droby, S.; Piombo, E.; Wu, X.; Yue, J. Genome sequence, assembly, and characterization of the antagonistic yeast Candida oleophila used as a biocontrol agent against post-harvest diseases. Front. Microbiol. 2020, 11, 295. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, I.M.; Sharma, M.; Sharma, M.; Sharma, K.; Sharma, A. Effectiveness of fungal, bacterial and yeast antagonists for management of mango anthracnose (Colletotrichum gloeosporioides). Egypt. J. Biol. Pest. Control. 2021, 31, 135. [Google Scholar] [CrossRef]
- Shi, J.F.; Sun, C.Q. Isolation, identification, and biocontrol of antagonistic bacterium against Botrytis cinerea after tomato harvest. Braz. J. Microbiol. 2017, 48, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Nujthet, Y.; Kaewkrajay, C.; Kijjoa, A.; Dethoup, T. Biocontrol efficacy of antagonists Trichoderma and Bacillus against post-harvest diseases in mangos. Eur. J. Plant. Pathol. 2024, 168, 315–327. [Google Scholar] [CrossRef]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Modelling the growth of pear postharvest fungal isolates at different temperatures. Food Microbiol. 2018, 76, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Khodaira, M.A.; Emam, A.E.; Goma, M.A.; Abdelaziz, A.M. Bioactive compounds and biomedical applications of endophytic fungi: A recent review. Microb. Cell. Fact. 2023, 22, 107. [Google Scholar] [CrossRef]
- Hammami, R.; Oueslati, M.; Smiri, M.; Nefzi, S.; Ruissi, M.; Comitini, F.; Romanazzi, G.; Cacciola, S.O.; Sadfi Zouaoui, N. Epiphytic yeasts and bacteria as candidate biocontrol agents of green and blue molds of citrus fruits. J. Fungi 2022, 8, 818. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Zhang, Z.; Chen, Y.; Tian, S. Antagonistic Yeasts: A Promising alternative to chemical fungicides for controlling postharvest decay of fruit. J. Fungi 2020, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Ackah, S.; Xue, S.; Osei, R.; Kweku-Amagloh, F.; Zong, Y.; Prusky, D.; Bi, Y. Chitosan treatment promotes wound healing of apple by eliciting phenylpropanoid pathway and enzymatic browning of wounds. Front. Microbiol. 2022, 13, 828914. [Google Scholar] [CrossRef]
- Fenta, L.; Mekonnen, H.; Kabtimer, N. The Exploitation of microbial antagonists against postharvest plant pathogens. Microorganisms 2023, 11, 1044. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef]
- Wang, W.; Liu, S.; Deng, L.; Ming, J.; Yao, S.; Zeng, K. Control of citrus post-harvest green molds, blue molds, and sour rot by the cecropin A-melittin hybrid peptide BP21. Front. Microbiol. 2018, 9, 2455. [Google Scholar] [CrossRef]
- Leszczuk, A.; Pieczywek, P.M.; Gryta, A.; Frac, M.; Zdunek, A. Immunocytochemical studies on the distribution of arabinogalactan proteins (AGPs) as a response to fungal infection in Malus x domestica fruit. Sci. Rep. 2019, 9, 17428. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Santos, M.; Moreno-Gavíra, A.; Marín-Rodulfo, T.; Gea, F.J.; Diánez, F. Biological control of fungal diseases by Trichoderma aggressivum f. europaeum and its compatibility with fungicides. J. Fungi 2021, 7, 598. [Google Scholar] [CrossRef]
- Di Canito, A.; Mateo-Vargas, M.A.; Mazzieri, M.; Cantoral, J.; Foschino, R.; Cordero-Bueso, G.; Vigentini, I. The role of yeasts as biocontrol agents for pathogenic fungi on postharvest grapes: A review. Foods 2021, 10, 1650. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Droby, S.; Preciado-Rangel, P.; Rivas-García, T.; González-Estrada, R.R.; Gutiérrez-Martínez, P.; Ávila-Quezada, G.D. A sustainable alternative for postharvest disease management and phytopathogens biocontrol in fruit: Antagonistic yeasts. Plants 2021, 10, 2641. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Fawole, O.A.; Makunga, N.P.; Masondo, N.A.; Moyo, M.; Buthelezi, N.M.D.; Amoo, S.O.; Spíchal, L.; Doležal, K. Applications of cytokinins in horticultural fruit crops: Trends and future prospects. Biomolecules 2020, 10, 1222. [Google Scholar] [CrossRef]
- Trandafirescu, M.; Văsîi, B. Identification and biological control of postharvest diseases of peach. Acta Hortic. 2021, 1304, 361–366. [Google Scholar] [CrossRef]
- Talibi, I.H.; Boubaker, H.; Boudyach, E.H.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef]
- Jindo, K.; Goron, T.L.; Pizarro-Tobías, P.; Sánchez-Monedero, M.A.; Audette, Y.; Deolu-Ajayi, A.O.; van der Werf, A.; Teklu, M.G.; Shenker, M.; Pombo Sudre’, C.; et al. Application of biostimulant products and biological control agents in sustainable viticulture: A review. Front. Plant Sci. 2022, 13, 932311. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Xu, Y.; Wang, Q.; Wu, B.; Li, D. Biological control and other alternatives to chemical fungicides in controlling postharvest disease of fruits caused by Alternaria alternata and Botrytis cinerea. Food Innov. Adv. 2024, 3, 135–143. [Google Scholar] [CrossRef]
- Sepúlveda, X.; Vargas, M.; Vero, S.; Zapata, N. Indigenous yeasts for the biocontrol of Botrytis cinerea on table grapes in Chile. J. Fungi 2023, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Esteves, M.; Lage, P.; Sousa, J.; Centeno, F.; de Fátima Teixeira, M.; Tenreiro, R.; Mendes-Ferreira, A. Biocontrol potential of wine yeasts against four grape phytopathogenic fungi disclosed by time-course monitoring of inhibitory activities. Front. Microbiol. 2023, 14, 1146065. [Google Scholar] [CrossRef]
- Madhupani, Y.D.S.; Adikaram, N.K.B. Delayed incidence of stem-end rot and enhanced defences in Aureobasidium pullulans-treated avocado (Persea americana Mill.) fruit. J. Plant Dis. Prot. 2017, 124, 227–234. [Google Scholar] [CrossRef]
- Nabila, E.A.; Soufiyan, E.A. Microbial biocontrol of post-harvest fungal rot in apples: Current state of the science. J. Bot. Res. 2020, 2, 31–58. [Google Scholar] [CrossRef]
- Piombo, E.; Sela, N.; Wisniewski, M.; Hoffmann, M.; Gullino, M.L.; Allard, M.W.; Levin, E.; Spadaro, D.; Droby, S. Genome Sequence, assembly and characterization of two Metschnikowia fructicola strains used as biocontrol agents of postharvest diseases. Front. Microbiol. 2018, 9, 593. [Google Scholar] [CrossRef]
- Kowalska, J.; Krzymińska, J.; Matysiak, K.; Jakubowska, M. Screening for antagonistic yeasts to manage Alternaria spp. in organic farming. Agriculture 2022, 12, 1693. [Google Scholar] [CrossRef]
- Kumar, V.; Koul, B.; Taak, P.; Yadav, D.; Song, M. Journey of Trichoderma from pilot scale to mass production: A review. Agriculture 2023, 13, 2022. [Google Scholar] [CrossRef]
- Li, S.; Zhang, F.M.; Shang, X.J.; Hou, R. Control effect and mechanism of Trichoderma asperellum TM11 against blueberry root rot. Pol. J. Microbiol. 2023, 72, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Hola, B.; Murshed, R.; Jbour, M. Chemical composition and antioxidant activity of some Syrian wild mushroom (Agaricus spp.) strains. Sci. Rep. 2023, 13, 15896. [Google Scholar] [CrossRef]
- di Francesco, A.; Milella, F.; Mari, M.; Roberti, R.A. Preliminary investigation into Aureobasidium pullulans as a potential biocontrol agent against Phytophthora infestans of tomato. Biol. Cont. 2017, 114, 144–149. [Google Scholar] [CrossRef]
- Pereyra, M.M.; Díaz, M.A.; Vero, S.; Dib, J.R. Enhancing biological control of postharvest green mold in lemons: Synergistic efficacy of native yeasts with diverse mechanisms of action. PLoS ONE 2024, 19, e0301584. [Google Scholar] [CrossRef]
- Del Frari, G.; Cabral, A.; Nascimento, T.; Ferreira, R.B.; Oliveira, H. Epicoccum layuense a potential biological control agent of esca-associated fungi in grapevine. PLoS ONE 2019, 14, e0213273. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.D.; Knorr, K.; Nicolaisen, M. In vitro competition between Fusarium graminearum and Epicoccum nigrum on media and wheat grains. Europ. J. Plant Pathol. 2016, 146, 657–670. [Google Scholar] [CrossRef]
- Li, T.; Im, J.; Lee, J. Genetic Diversity of Epicoccum nigrum and its effects on Fusarium graminearum. Mycobiology 2022, 5, 457–466. [Google Scholar] [CrossRef]
- Matlagh, M.R.S.; Usefipoor, P. Identification of non-pathogenic fungi of rice and the evaluation of their effect on biological control of Pyricularia grisea, the causal agent of rice blast disease in vitro. Acta Sci. Pol. Hortorum Cultus. 2016, 15, 69–86. [Google Scholar]
- Tane, C.; Barbu, L.; Sabina, S.R.; Cojanu, D.; Cosoveanu, A. One strain of endophytic Presussia, a potential biological partner of tomato seedlings against alternariosis. Biology 2019, 28, 15–19. [Google Scholar]
- Moraes Bazioli, J.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.d.M.; Kupper, K.C.; Augusto, F.; de Carvalho, J.E.; Fill, T.P. Biological control of citrus postharvest phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Verma, S.; Azevedo, L.C.B.; Pandey, J.; Khusharia, S.; Kumari, M.; Kumar, D.; Kaushalendra; Bhardwaj, N.; Teotia, P.; Kumar, A. Microbial intervention: An approach to combat the postharvest pathogens of Fruits. Plants 2022, 11, 3452. [Google Scholar] [CrossRef]
- Dutta, P.; Mahanta, M.; Singh, S.B.; Thakuria, D.; Deb, L.; Kumari, A.; Upamanya, G.K.; Boruah, S.; Dey, U.; Mishra, A.K.; et al. Molecular interaction between plants and Trichoderma species against soil-borne plant pathogens. Front. Plant Sci. 2023, 14, 1145715. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pelayo, C.; Martínez-Quiñones, J.; Gil, J.; Durango, D. Coumarins from the peel of citrus grown in Colombia: Composition, elicitation and antifungal activity. Heliyon 2019, 5, e01937. [Google Scholar] [CrossRef]
- Santhanam, P.; Madina, M.H.; Albuini, F.M.; Labbé, C.; Fietto, L.G.; Bélanger, R.R. A unique effector secreted by Pseudozyma flocculosa mediates its biocontrol activity. BMC Biol. 2023, 21, 118. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef]
- Agirman, B.; Erten, H. Biocontrol ability and action mechanisms of Aureobasidium pullulans GE17 and Meyerozyma guilliermondii KL3 against Penicillium digitatum DSM2750 and Penicillium expansum DSM62841 causing postharvest diseases. Yeast 2020, 37, 437–448. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) Revisited: Outlining their role in biological macromolecules (DNA, Lipids and Proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between medical plant-derived bioactive compounds: Focus on antimicrobial combination effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wei, X.; Wan, C.; Zhao, W.; Song, R.; Xin, S.; Song, K. Exploring the biological pathways of siderophores and their multidisciplinary applications: A comprehensive review. Molecules 2024, 29, 2318. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Klebba, P.E.; Newton, S.M.C.; Six, D.A.; Kumar, A.; Yang, T.; Nairn, B.L.; Munger, C.; Chakravorty, S. Iron acquisition systems of gram-negative bacterial pathogens define tonb-dependent pathways to novel antibiotics. Chem. Rev. 2021, 121, 5193–5239. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-San Millan, A.; Gamir, J.; Farran, I.; Larraya, L.; Veramendi, J. Identification of new antifungal metabolites produced by the yeast Metschnikowia pulcherrima involved in the biocontrol of postharvest plant pathogenic fungi. Postharvest Biol. Technol. 2022, 192, 111995. [Google Scholar] [CrossRef]
- Taha, N.A.; Elsharkawy, M.M.; Shoughy, A.A.; El-Kazzaz, M.K.; Khedr, A.A. Biological control of postharvest tomato fruit rots using Bacillus spp. and Pseudomonas spp. Egypt. J. Biol. Pest. Control. 2023, 33, 106. [Google Scholar] [CrossRef]
- Diabankana, R.G.C.; Frolov, M.; Islamov, B.; Shulga, E.; Filimonova, M.N.; Afordoanyi, D.M.; Validov, S. Identification and aggressiveness of Fusarium species associated with onion bulb (Allium cepa L.) during storage. J. Fungi 2024, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial siderophores: Classification, biosynthesis, perspectives of use in agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
- Kahli, H.; Béven, L.; Grauby-Heywang, C.; Debez, N.; Gammoudi, I.; Moroté, F.; Sbartai, H.; Cohen-Bouhacina, T. Impact of growth Conditions on Pseudomonas fluorescens morphology characterized by atomic force microscopy. Int. J. Mol. Sci. 2022, 23, 9579. [Google Scholar] [CrossRef]
- Di Francesco, A.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Lai, J.; Cao, X.; Yu, T.; Wang, Q.; Zhang, Y.; Zheng, X.; Lu, H. Effect of Cryptococcus laurentii on inducing disease resistance in cherry tomato fruit with focus on the expression of defense-related genes. Food Chem. 2018, 254, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Tsalgatidou, P.C.; Boutsika, A.; Papageorgiou, A.G.; Dalianis, A.; Michaliou, M.; Chatzidimopoulos, M.; Delis, C.; Tsitsigiannis, D.I.; Paplomatas, E.; Zambounis, A. Global transcriptome analysis of the peach (Prunus persica) in the interaction system of fruit–Chitosan–Monilinia fructicola. Plants 2024, 13, 567. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, B. Induced resistance in peach fruit as treated by Pichia guilliermondii and their possible mechanism. Int. J. Food Prop. 2020, 23, 34–51. [Google Scholar] [CrossRef]
- Brown, A.J.P. Fungal resilience and host-pathogen interactions: Future perspectives and opportunities. Parasite Immunol. 2023, 45, e12946. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Li, B.; He, C.; Chen, Y.; Tian, S. Influence of oxidative stress on biocontrol activity of Cryptococcus laurentii against blue mold on peach fruit. Front. Microbiol. 2017, 8, 151. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Gupta, A.; Prusty, M.R.; Kumar, M. Elicitation of fruit fungi infection and its protective response to improve the postharvest quality of fruits. Stresses 2023, 3, 231–255. [Google Scholar] [CrossRef]
- Yang, Q.; Solairaj, D.; Apaliya, M.T.; Abdelhai, M.; Zhu, M.; Yan, Y.; Zhang, H. Protein expression profile and transcriptome characterization of Penicillium expansum Induced by Meyerozyma guilliermondii. J. Food Qual. 2020, 2020, 8056767. [Google Scholar] [CrossRef]
- Sui, Y.; Wisniewski, M.; Droby, S.; Liu, J. Responses of yeast biocontrol agents to environmental stress. Appl. Environ. Microbiol. 2015, 81, 2968–2975. [Google Scholar] [CrossRef]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring therapeutic potential of catalase: Strategies in disease prevention and management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chi, M.; Li, G.; Chen, H.; Sui, Y.; Sun, H.; Wisniwski, M.; Liu, Y.; Liu, J. Heat shock improves stress tolerance and biocontrol performance of Rhodotorula mucilaginosa. Biol. Control 2016, 95, 49–56. [Google Scholar] [CrossRef]
- Yaakoub, H.; Mina, S.; Calenda, A.; Bouchara, J.P.; Papon, N. Oxidative stress response pathways in fungi. Cell. Mol. Life Sci. 2022, 79, 333. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Yang, Q.; Komla, M.G.; Zhang, X.; Zhu, S. Ascorbic acid enhances oxidative stress tolerance and biological control efficacy of Pichia caribbica against post-harvest blue mold decay of apples. J. Agric. Food Chem. 2014, 62, 7612–7621. [Google Scholar] [CrossRef]
- Masumoto, N.; Suzuki, Y.; Cui, S.; Wakazaki, M.; Sato, M.; Kumaishi, K.; Shibata, A.; Furuta, K.M.; Ichihashi, Y.; Shirasu, K.; et al. Three-dimensional reconstructions of haustoria in two parasitic plant species in the Orobanchaceae. Plant Physiol. 2021, 185, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Mapuranga, J.; Zhang, L.; Zhang, N.; Yang, W. The haustorium: The root of biotrophic fungal pathogens. Front. Plant Sci. 2022, 13, 963705. [Google Scholar] [CrossRef]
- Marques, J.P.R.; Ho, Y.J.W.; Appezzato-da-Glória, B.; Viveros, A.F.G.; Vieira, M.L.C.; Baisakh, N. Sugarcane cell wall-associated defense responses to infection by Sporisorium scitamineum. Front. Plant Sci. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Mamphogoro, T.P.; Babalola, O.O.; Aiyegoro, O.A. Sustainable management strategies for bacterial wilt of sweet peppers (Capsicum annuum) and other Solanaceous crops. J. Appl. Microbiol. 2020, 129, 496508. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Srivastava, A.; Singh, A.; Pandey, G.C.; Srivastava, G. An overview of symbiotic and pathogenic interactions at the fungi-plant interface under environmental constraints. Front. Fungal Biol. 2024, 5, 1363460. [Google Scholar] [CrossRef] [PubMed]
- Bisht, N.; Mishra, S.K.; Chauhan, P.S. Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. Int. J. Biol. Macromol. 2019, 143, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Ramudingana, P.; Mamphogoro, T.P.; Kamutando, C.N.; Maboko, M.M.; Modika, K.Y.; Moloto, K.W.; Thantsha, M.S. Antagonistic potential of endophytic fungal isolates of tomato (Solanum lycopersicum L.) fruits against post-harvest disease-causing pathogens of tomatoes: An in vitro investigation. Fungal Biol. 2024, 128, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.E.; Jousset, A.; Elphinstone, J.G.; Friman, V.P. Combining in vitro and in vivo screening to identify efficient Pseudomonas biocontrol strains against the phytopathogenic bacterium Ralstonia solanacearum. Microbiologyopen 2022, 11, e1283. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Grzywacz, D.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Tyagi, A.; Lama Tamang, T.; Kashtoh, H.; Mir, R.A.; Mir, Z.A.; Manzoor, S.; Manzar, N.; Gani, G.; Vishwakarma, S.K.; Almalki, M.A.; et al. A Review on biocontrol agents as sustainable approach for crop disease management: Applications, production, and future perspectives. Horticulturae 2024, 10, 805. [Google Scholar] [CrossRef]
- Falahzadah, M.H.; Karimi, J.; Gaugler, R. Biological control chance and limitation within integrated pest management program in Afghanistan. Egypt. J. Biol. Pest Control 2020, 30, 86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramudingana, P.; Makhado, N.; Kamutando, C.N.; Thantsha, M.S.; Mamphogoro, T.P. Fungal Biocontrol Agents in the Management of Postharvest Losses of Fresh Produce—A Comprehensive Review. J. Fungi 2025, 11, 82. https://doi.org/10.3390/jof11010082
Ramudingana P, Makhado N, Kamutando CN, Thantsha MS, Mamphogoro TP. Fungal Biocontrol Agents in the Management of Postharvest Losses of Fresh Produce—A Comprehensive Review. Journal of Fungi. 2025; 11(1):82. https://doi.org/10.3390/jof11010082
Chicago/Turabian StyleRamudingana, Phathutshedzo, Ndivhuho Makhado, Casper Nyaradzai Kamutando, Mapitsi Silvester Thantsha, and Tshifhiwa Paris Mamphogoro. 2025. "Fungal Biocontrol Agents in the Management of Postharvest Losses of Fresh Produce—A Comprehensive Review" Journal of Fungi 11, no. 1: 82. https://doi.org/10.3390/jof11010082
APA StyleRamudingana, P., Makhado, N., Kamutando, C. N., Thantsha, M. S., & Mamphogoro, T. P. (2025). Fungal Biocontrol Agents in the Management of Postharvest Losses of Fresh Produce—A Comprehensive Review. Journal of Fungi, 11(1), 82. https://doi.org/10.3390/jof11010082






