The Endoplasmic Reticulum Membrane Protein Complex Is Important for Deoxynivalenol Production and the Virulence of Fusarium graminearum
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Generation of EMC Mutants
2.3. Phylogenetic Evolutionary Tree Analysis
2.4. Assays for Asexual and Sexual Reproduction
2.5. Plant Infection Assays
2.6. DON Production Assays
2.7. Gene Expression Analysis
2.8. Microscopic Observation
2.9. Statistical Analyses
3. Results
3.1. Identification of F. graminearum EMC Subunits
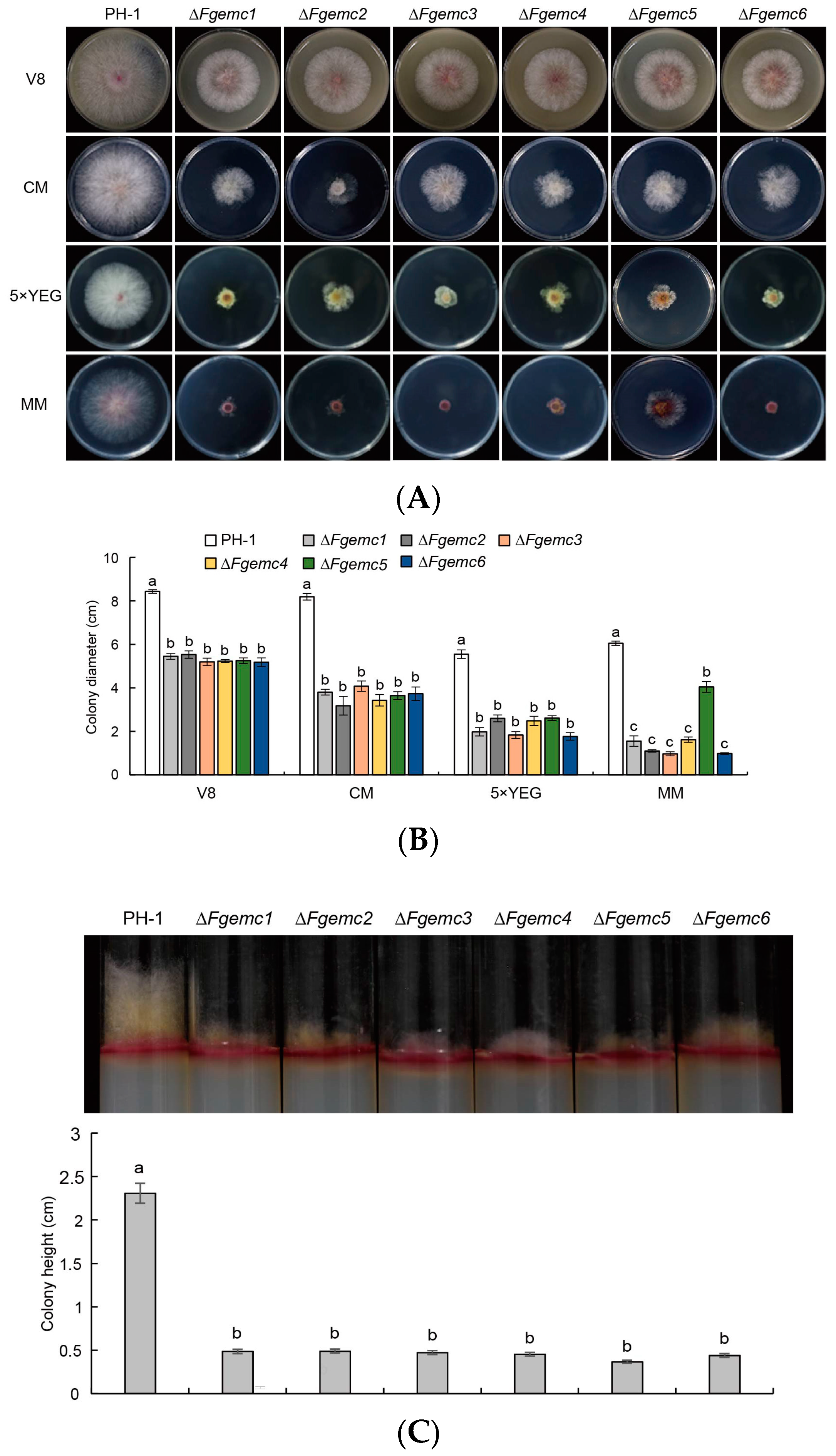
3.2. The EMC Is Involved in the Vegetative Growth of F. graminearum
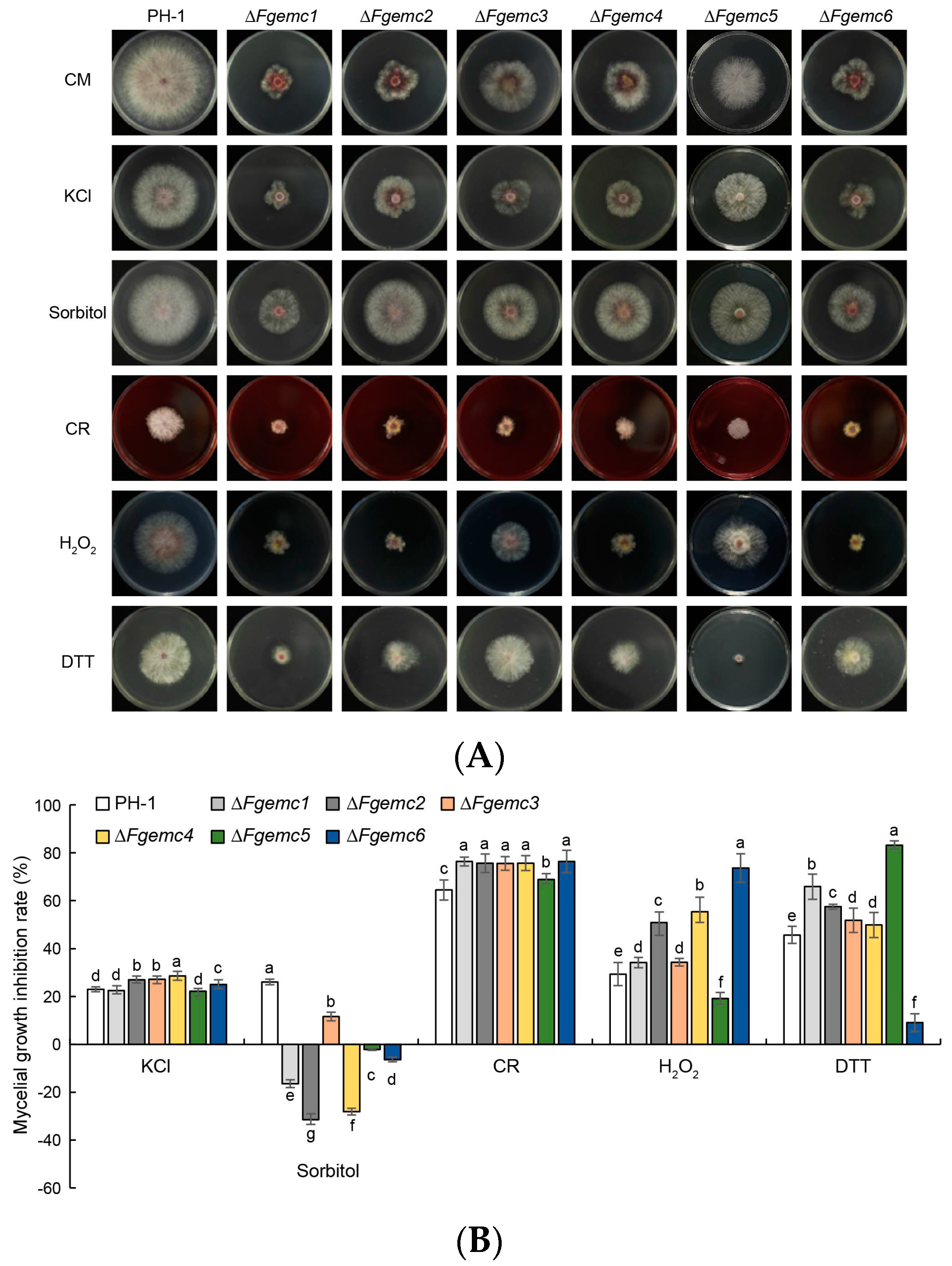
3.3. The EMC Is Essential in the Response of F. graminearum to Diverse Environmental Stressors
3.4. The EMC Is Critical for the Generation of Conidia, Perithecia, and Ascospores in F. graminearum
3.5. The EMC Plays a Crucial Role in Regulating F. graminearum Virulence
3.6. The EMC Is Required for DON Production and Pigmentation in F. graminearum
3.7. The EMC Is Involved in the Toxisome Formation of F. graminearum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Sempere, A.; Estiarte, N.; Marín, S.; Sanchis, V.; Ramos, A.J. Targeting Fusarium graminearum control via polyamine enzyme inhibitors and polyamine analogs. Food Microbiol. 2015, 49, 95–103. [Google Scholar] [CrossRef]
- He, X.; Dreisigacker, S.; Singh, R.P.; Singh, P.K. Genetics for low correlation between Fusarium head blight disease and deoxynivalenol (DON) content in a bread wheat mapping population. Theor. Appl. Genet. 2019, 132, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Beccari, G.; Colasante, V.; Tini, F.; Senatore, M.T.; Prodi, A.; Sulyok, M.; Covarelli, L. Causal agents of Fusarium head blight of durum wheat (Triticum durum Desf.) in central Italy and their in vitro biosynthesis of secondary metabolites. Food Microbiol. 2018, 70, 17–27. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Zhang, Y.; Jia, X.; Zhou, M. Hexokinase plays a critical role in deoxynivalenol (DON) production and fungal development in Fusarium graminearum. Mol. Plant Pathol. 2016, 17, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.H.; Desjardins, A.E.; Plattner, R.D. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2002, 153, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. The toxicity mechanisms of DON to humans and animals and potential biological treatment strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 790–812. [Google Scholar] [CrossRef]
- Beccari, G.; Arellano, C.; Covarelli, L.; Tini, F.; Sulyok, M.; Cowger, C. Effect of wheat infection timing on Fusarium head blight causal agents and secondary metabolites in grain. Int. J. Food Microbiol. 2019, 290, 214–225. [Google Scholar] [CrossRef]
- Wang, M.; Wu, N.; Wang, H.; Liu, C.; Chen, Q.; Xu, T.; Chen, Y.; Zhao, Y.; Ma, Z. Overproduction of mycotoxin biosynthetic enzymes triggers Fusarium toxisome-shaped structure formation via endoplasmic reticulum remodeling. PLoS Pathog. 2024, 20, e1011913. [Google Scholar]
- Boenisch, M.J.; Broz, K.L.; Purvine, S.O.; Chrisler, W.B.; Nicora, C.D.; Connolly, L.R.; Freitag, M.; Baker, S.E.; Kistler, H.C. Structural reorganization of the fungal endoplasmic reticulum upon induction of mycotoxin biosynthesis. Sci. Rep. 2017, 7, 44296. [Google Scholar] [CrossRef] [PubMed]
- Menke, J.; Weber, J.; Broz, K.; Kistler, H.C. Cellular development associated with induced mycotoxin synthesis in the filamentous fungus Fusarium graminearum. PLoS ONE 2013, 8, e63077. [Google Scholar] [CrossRef]
- Chong, X.; Wang, C.; Wang, Y.; Wang, Y.; Zhang, L.; Liang, Y.; Chen, L.; Zou, S.; Dong, H. The dynamin-like GTPase FgSey1 plays a critical role in fungal development and virulence in Fusarium graminearum. Appl. Environ. Microbiol. 2020, 86, e02720-19. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yun, Y.; Yin, Y.; Hahn, M.; Ma, Z.; Chen, Y. Lipid droplet biogenesis regulated by the FgNem1/Spo7-FgPah1 phosphatase cascade plays critical roles in fungal development and virulence in Fusarium graminearum. New. Phytol. 2019, 223, 412–429. [Google Scholar] [CrossRef] [PubMed]
- Burg, J.S.; Espenshade, P.J. Regulation of HMG-CoA reductase in mammals and yeast. Prog. Lipid Res. 2011, 50, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wideman, J.G. The ubiquitous and ancient ER membrane protein complex (EMC): Tether or not? F1000Research 2015, 4, 624. [Google Scholar] [CrossRef]
- Bagchi, P.; Inoue, T.; Tsai, B. EMC1-dependent stabilization drives membrane penetration of a partially destabilized non-enveloped virus. Elife 2016, 5, e21470. [Google Scholar] [CrossRef]
- Coelho, J.P.L.; Stahl, M.; Bloemeke, N.; Meighen-Berger, K.; Alvira, C.P.; Zhang, Z.R.; Sieber, S.A.; Feige, M.J. A network of chaperones prevents and detects failures in membrane protein lipid bilayer integration. Nat. Commun. 2019, 10, 672. [Google Scholar] [CrossRef]
- Shurtleff, M.J.; Itzhak, D.N.; Hussmann, J.A.; Schirle Oakdale, N.T.; Costa, E.A.; Jonikas, M.; Weibezahn, J.; Popova, K.D.; Jan, C.H.; Sinitcyn, P.; et al. The ER membrane protein complex interacts cotranslationally to enable biogenesis of multipass membrane proteins. Elife 2018, 7, e37018. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, S.; Hou, R.; Zhao, Z.; Zheng, Q.; Xu, Q.; Zheng, D.; Wang, G.; Liu, H.; Gao, X.; et al. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathog. 2011, 7, e1002460. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Zhang, L.; Yin, Z.; Liang, Y.; Chen, L.; Zou, S.; Dong, H. The Golgin protein RUD3 regulates Fusarium graminearum growth and virulence. Appl. Environ. Microbiol. 2021, 87, e02522-20. [Google Scholar] [CrossRef]
- Catlett, N.L.; Lee, B.-N.; Yoder, O.C.; Turgeon, B.G. Split-Marker Recombination for Efficient Targeted Deletion of Fungal Genes. Fungal Genet. Rep. 2003, 50, 9–11. [Google Scholar] [CrossRef]
- Frandsen, R.J.N.; Rasmussen, S.A.; Knudsen, P.B.; Uhlig, S.; Petersen, D.; Lysøe, E.; Gotfredsen, C.H.; Giese, H.; Larsen, T.O. Black perithecial pigmentation in Fusarium species is due to the accumulation of 5-deoxybostrycoidin-based melanin. Sci. Rep. 2016, 6, 26206. [Google Scholar] [CrossRef] [PubMed]
- Cavinder, B.; Sikhakolli, U.; Fellows, K.M.; Trail, F. Sexual development and ascospore discharge in Fusarium graminearum. J. Vis. Exp. 2012, 29, 3895. [Google Scholar]
- Boenisch, M.J.; Schäfer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Sun, F.; Lv, B.; Zhang, X.; Wang, C.; Zhang, L.; Chen, X.; Liang, Y.; Chen, L.; Zou, S.; Dong, H. The Endoplasmic reticulum cargo receptor FgErv14 regulates DON production, growth and virulence in Fusarium graminearum. Life 2022, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, R.; Li, T.; Zhang, L.; Chen, X.; Liang, Y.; Chen, L.; Zou, S.; Dong, H. Fusarium graminearum GGA protein is critical for fungal development, virulence and ascospore discharge through its involvement in vesicular trafficking. Environ. Microbiol. 2022, 24, 6290–6306. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, X.; Zhao, R.; Kou, R.; Zheng, X.; Zhang, H. The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in Fusarium graminearum. PLoS Pathog. 2019, 15, e1007754. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; He, F.; Ding, M.; Geng, C.; Chen, L.; Zou, S.; Liang, Y.; Yu, J.; Dong, H. Thioredoxin reductase is involved in development and pathogenicity in Fusarium graminearum. Front. Microbiol. 2019, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Alvira, S.; Corey, R.A.; Collinson, I.; Römisch, K. Membrane protein biogenesis by the EMC. EMBO J. 2021, 40, e107407. [Google Scholar] [CrossRef]
- Bai, L.; You, Q.; Feng, X.; Kovach, A.; Li, H. Structure of the ER membrane complex, a transmembrane-domain insertase. Nature 2020, 584, 475–478. [Google Scholar] [CrossRef]
- Volkmar, N.; Christianson, J.C. Squaring the EMC—How promoting membrane protein biogenesis impacts cellular functions and organismal homeostasis. J. Cell Sci. 2020, 133, jcs243519. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Huang, M.; Liang, X.; Cao, M.; Lun, Z.; Zhang, Y.; Yang, J.; Bhadauria, V.; Zhao, W.; Yan, J.; et al. Magnaporthe oryzae endoplasmic reticulum membrane complex regulates the biogenesis of membrane proteins for pathogenicity. New Phytol. 2023, 238, 1163–1181. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chi, H.; Li, W.; Zhang, L.; Zhang, L.; Chen, L.; Zou, S.; Liu, H.; Liang, Y.; Yu, J.; et al. FgPsd2, a phosphatidylserine decarboxylase of Fusarium graminearum, regulates development and virulence. Fungal Genet. Biol. 2021, 146, 103483. [Google Scholar] [CrossRef] [PubMed]
- Saharan, M.S. Current status of resistant source to Fusarium head blight disease of wheat: A review. Indian Phytopathol. 2020, 73, 3–9. [Google Scholar] [CrossRef]
- Mudge, A.M.; Dill-Macky, R.; Dong, Y.; Gardiner, D.M.; White, R.G.; Manners, J.M. A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum. Physiol. Mol. Plant Pathol. 2006, 69, 73–85. [Google Scholar] [CrossRef]
- Stephens, A.E.; Gardiner, D.M.; White, R.G.; Munn, A.L.; Manners, J.M. Phases of infection and gene expression of Fusarium graminearum during crown rot disease of wheat. Mol. Plant Microbe Interact. 2008, 21, 1571–1581. [Google Scholar] [CrossRef]
- Voigt, C.A.; Schäfer, W.; Salomon, S. A secreted lipase of Fusarium graminearum is a virulence factor required for infection of cereals. Plant J. 2005, 42, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.Y.; Pasquali, M.; Zhou, X.; Song, J.; Hilburn, K.; McCormick, S.; Dong, Y.; Xu, J.R.; Kistler, H.C. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol. Microbiol. 2009, 72, 354–367. [Google Scholar] [CrossRef]
- Tang, G.; Chen, Y.; Xu, J.R.; Kistler, H.C.; Ma, Z. The fungal myosin I is essential for Fusarium toxisome formation. PLoS Pathog. 2018, 14, e1006827. [Google Scholar] [CrossRef]
- Chitwood, P.J.; Hegde, R.S. The Role of EMC during Membrane Protein Biogenesis. Trends Cell Biol. 2019, 29, 371–384. [Google Scholar] [CrossRef]
- Lahiri, S.; Chao, J.T.; Tavassoli, S.; Wong, A.K.; Choudhary, V.; Young, B.P.; Loewen, C.J.; Prinz, W.A. A conserved endoplasmic reticulum membrane protein complex (EMC) facilitates phospholipid transfer from the ER to mitochondria. PLoS Biol. 2014, 12, e1001969. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Shen, X.; Liu, M.; Liu, X.; Yin, Z.; Li, X.; Feng, W.; Hu, J.; Zhang, H.; Zheng, X.; et al. The rice blast fungus MoRgs1 functioning in cAMP signaling and pathogenicity is regulated by casein kinase MoCk2 phosphorylation and modulated by membrane protein MoEmc2. PLOS Pathog. 2021, 17, e1009657. [Google Scholar] [CrossRef] [PubMed]
- Jonikas, M.C.; Collins, S.R.; Denic, V.; Oh, E.; Quan, E.M.; Schmid, V.; Weibezahn, J.; Schwappach, B.; Walter, P.; Weissman, J.S.; et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 2009, 323, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.C.; Olzmann, J.A.; Shaler, T.A.; Sowa, M.E.; Bennett, E.J.; Richter, C.M.; Tyler, R.E.; Greenblatt, E.J.; Harper, J.W.; Kopito, R.R. Defining human ERAD networks through an integrative mapping strategy. Nat. Cell Biol. 2011, 14, 93–105. [Google Scholar] [CrossRef]
- Hegde, R.S. The function, structure, and origins of the ER membrane protein complex. Annu. Rev. Biochem. 2022, 91, 651–678. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, C.J.; Vieira, L.C.; Santos, C.C.; Christianson, J.C.; Jakubec, D.; Strisovsky, K.; Adrain, C.; Domingos, P.M. EMC is required for biogenesis of Xport-A, an essential chaperone of Rhodopsin-1 and the TRP channel. EMBO Rep. 2022, 23, e53210. [Google Scholar] [CrossRef] [PubMed]
- Harel, T.; Yesil, G.; Bayram, Y.; Coban-Akdemir, Z.; Charng, W.L.; Karaca, E.; Al Asmari, A.; Eldomery, M.K.; Hunter, J.V.; Jhangiani, S.N.; et al. Monoallelic and biallelic variants in EMC1 identified in individuals with global developmental delay, Hypotonia, Scoliosis, and Cerebellar Atrophy. Am. J. Hum. Genet. 2016, 98, 562–570. [Google Scholar] [CrossRef]
- Sun, K.; Liu, L.; Jiang, X.; Wang, H.; Wang, L.; Yang, Y.; Liu, W.; Zhang, L.; Zhao, X.; Zhu, X. The endoplasmic reticulum membrane protein complex subunit Emc6 is essential for rhodopsin localization and photoreceptor cell survival. Genes Dis. 2024, 11, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Ballow, M.; Asiri, A.; Alyafee, Y.; Al Tuwaijri, A.; Alhamoudi, K.M.; Aloraini, T.; Abdelhakim, M.; Althagafi, A.T.; Kafkas, S.; et al. EMC10 homozygous variant identified in a family with global developmental delay, mild intellectual disability, and speech delay. Clin. Genet. 2020, 98, 555–561. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, F.; Zhang, M.; Xiong, Z.; Yin, Q.; Ru, Y.; Shi, H.; Li, J.; Mao, S.; Li, Y.; et al. EMC10 governs male fertility via maintaining sperm ion balance. J. Mol. Cell Biol. 2018, 10, 503–514. [Google Scholar] [CrossRef]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum trichothecene mycotoxins: Biosynthesis, regulation, and management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Liu, Y.; Wang, Y.; Zhang, Y.; Wang, S.; Zhang, L.; Lu, K.; Chen, X.; Dong, H.; Zou, S. The Endoplasmic Reticulum Membrane Protein Complex Is Important for Deoxynivalenol Production and the Virulence of Fusarium graminearum. J. Fungi 2025, 11, 108. https://doi.org/10.3390/jof11020108
Chen L, Liu Y, Wang Y, Zhang Y, Wang S, Zhang L, Lu K, Chen X, Dong H, Zou S. The Endoplasmic Reticulum Membrane Protein Complex Is Important for Deoxynivalenol Production and the Virulence of Fusarium graminearum. Journal of Fungi. 2025; 11(2):108. https://doi.org/10.3390/jof11020108
Chicago/Turabian StyleChen, Lei, Yaxian Liu, Yu Wang, Yaxin Zhang, Saisai Wang, Liyuan Zhang, Kai Lu, Xiaochen Chen, Hansong Dong, and Shenshen Zou. 2025. "The Endoplasmic Reticulum Membrane Protein Complex Is Important for Deoxynivalenol Production and the Virulence of Fusarium graminearum" Journal of Fungi 11, no. 2: 108. https://doi.org/10.3390/jof11020108
APA StyleChen, L., Liu, Y., Wang, Y., Zhang, Y., Wang, S., Zhang, L., Lu, K., Chen, X., Dong, H., & Zou, S. (2025). The Endoplasmic Reticulum Membrane Protein Complex Is Important for Deoxynivalenol Production and the Virulence of Fusarium graminearum. Journal of Fungi, 11(2), 108. https://doi.org/10.3390/jof11020108





