Integrated Multi-Omics Analysis to Investigate the Molecular Mechanisms Underlying the Response of Auricularia heimuer to High-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi Materials and Treatment
2.2. Physiological Analysis
2.3. Transcriptome Analysis
2.4. Real-Time–Quantitative PCR (RT–qPCR) Analysis
2.5. Metabolome Analysis
3. Results
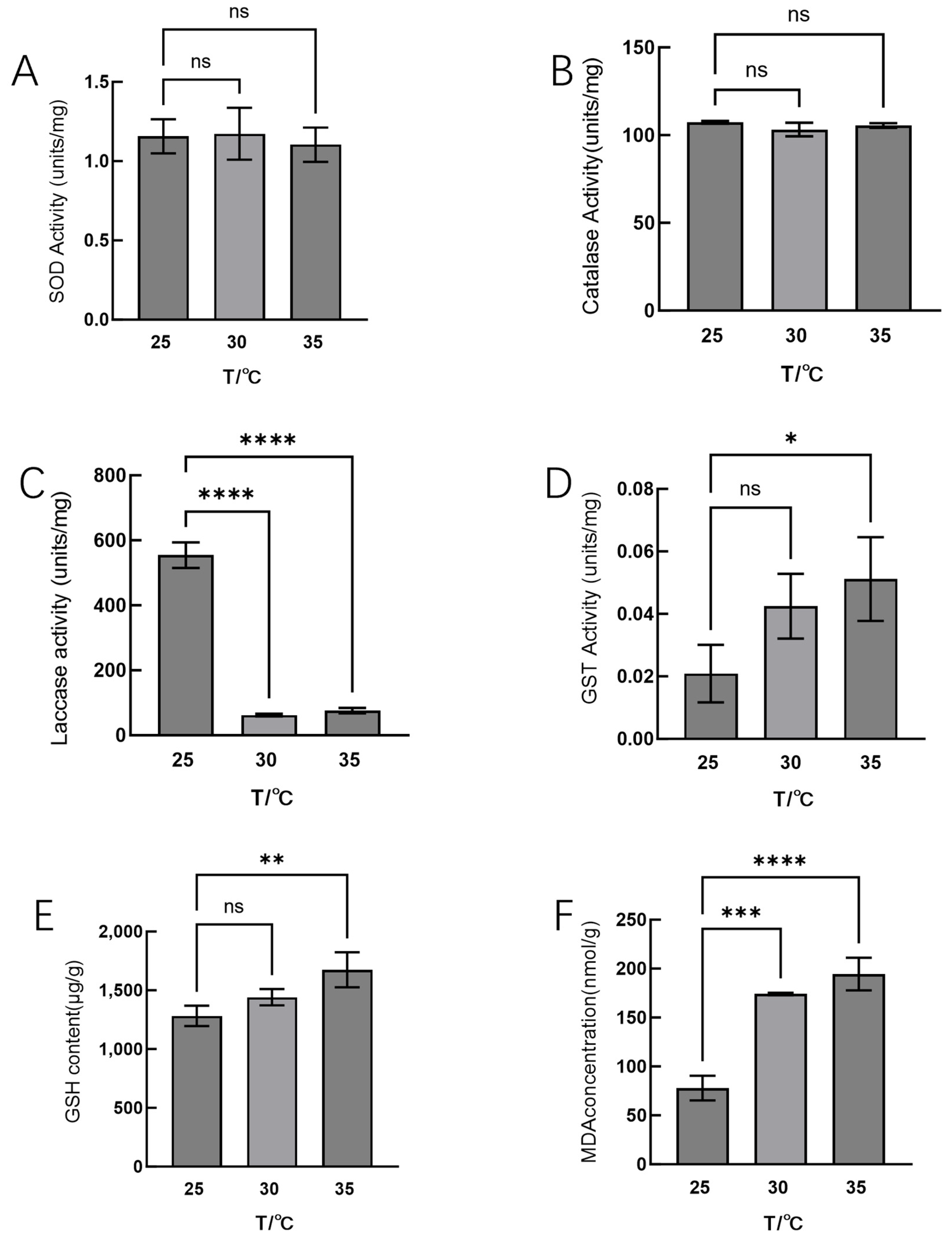
3.1. Effects of High-Temperature Stress on Physiological Phenotypes of Hei29
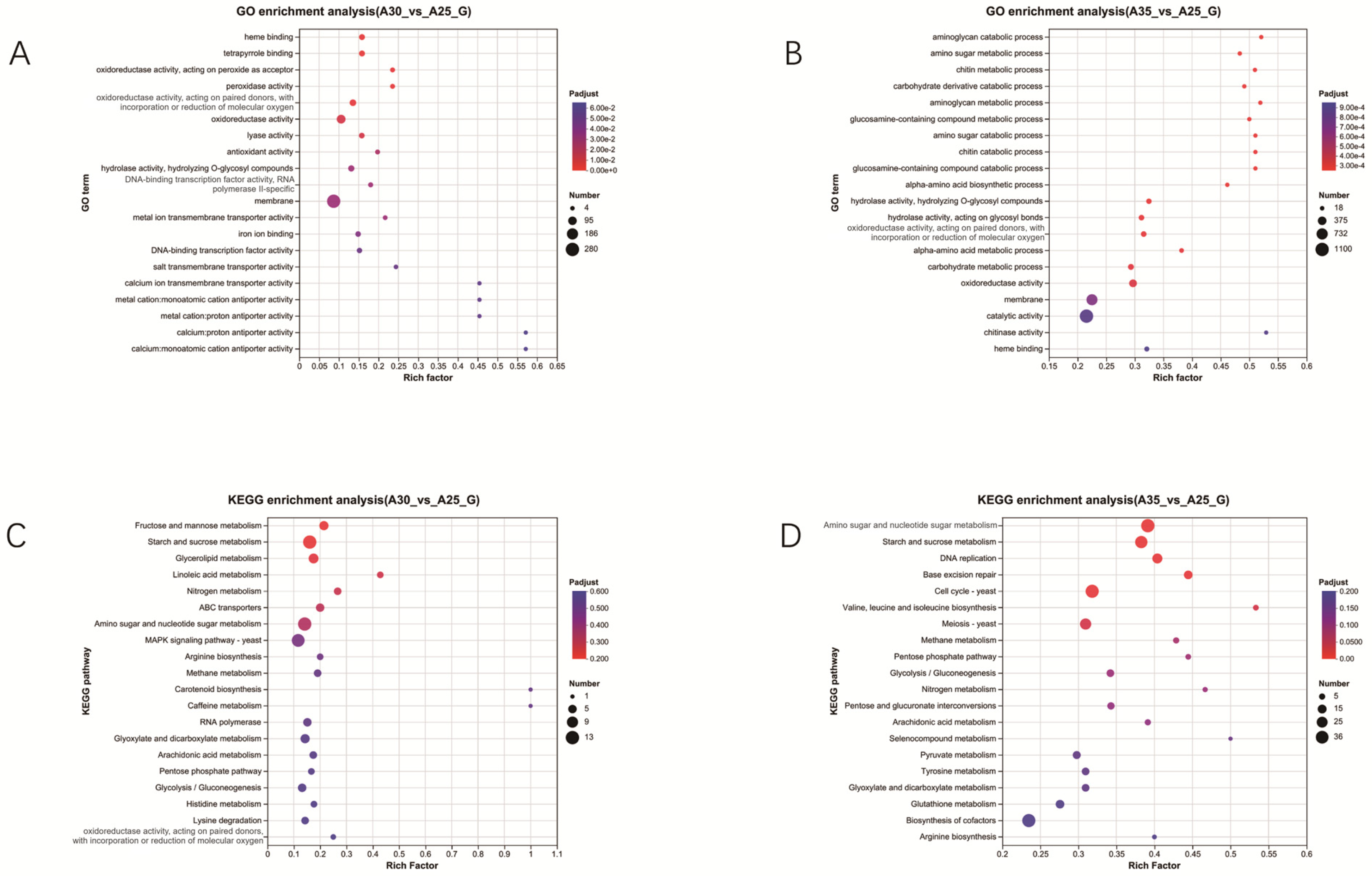
3.2. Transcriptomic Analysis Under High-Temperature Stress
3.3. RT–PCR Verification of DEGs
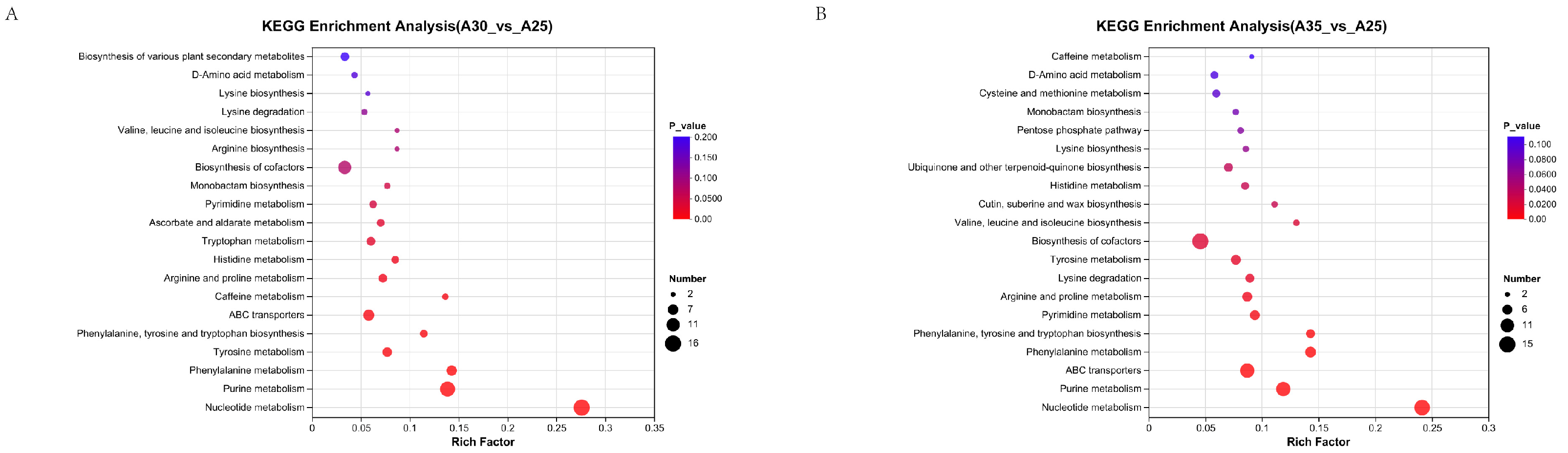
3.4. Metabolomic Analysis of Auricularia Under High-Temperature Stress
4. Discussion
4.1. Physiological Changes in Hei29 Under High-Temperature Stress
4.2. Transcriptomic Analysis Under Different Temperature Treatments
4.3. Metabolomic Analysis Under Different Temperature Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, L.; Zhang, S.; Wu, J.; Sun, X.; Ma, A. Heat Stress in Macrofungi: Effects and Response Mechanisms. Appl. Microbiol. Biotechnol. 2021, 105, 7567–7576. [Google Scholar] [CrossRef]
- Kumar, C.M.S.; Chugh, K.; Dutta, A.; Mahamkali, V.; Bose, T.; Mande, S.S.; Mande, S.C.; Lund, P.A. Chaperonin Abundance Enhances Bacterial Fitness. Front. Mol. Biosci. 2021, 8, 669996. [Google Scholar] [CrossRef]
- Li, W.; Zou, G.; Bao, D.; Wu, Y. Current Advances in the Functional Genes of Edible and Medicinal Fungi: Research Techniques, Functional Analysis, and Prospects. J. Fungi 2024, 10, 311. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Q.; Fan, Y.; Song, S.; Yan, D.; Zhao, J.; Chen, Y.; Liu, Y.; Wang, S. Two Strains of Lentinula Edodes Differ in Their Transcriptional and Metabolic Patterns and Respond Differently to Thermostress. J. Fungi 2023, 9, 179. [Google Scholar] [CrossRef]
- Ma, C.; Wang, G.; Zhou, S.; Luo, Y.; Gong, Y.; Bian, Y. Functional Analyses of Anthranilate Synthase Gene LetrpE in Lentinula Edodes by RNAi Mediated Gene Knockdown. Mycosystema 2018, 37, 576–583. [Google Scholar] [CrossRef]
- Hu, Y.-R.; Wang, Y.; Chen, Y.-J.; Chai, Q.-Q.; Dong, H.-Z.; Shen, J.-W.; Qi, Y.-C.; Wang, F.-Q.; Wen, Q. Salicylic Acid Enhances Heat Stress Resistance of Pleurotus ostreatus (Jacq.) P. Kumm through Metabolic Rearrangement. Antioxidants 2022, 11, 968. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, S.; Luo, Y.; Ma, C.; Gong, Y.; Zhou, Y.; Gao, S.; Huang, Z.; Yan, L.; Hu, Y.; et al. The Heat Shock Protein 40 LeDnaJ Regulates Stress Resistance and Indole-3-Acetic Acid Biosynthesis in Lentinula Edodes. Fungal Genet. Biol. FG B 2018, 118, 37–44. [Google Scholar] [CrossRef]
- Yang, H.; Chang, T.; Zhao, Y.; You, H.; Zha, L.; Li, Z.; Tong, Z.; Chen, M. Expression of Catalase Gene (VvCAT1) from Volvariella Volvacea in Escherichia coli and Its Temperature Tolerance. Microbiol. China 2021, 48, 4054–4060. [Google Scholar] [CrossRef]
- Xie, H.; Wan, L.; Han, J.; Huang, C.; Li, J.; Yao, Q.; Yang, P.; Zhang, Y.; Gong, Z.; Yu, H. TMT-Based Proteomic and Transcriptomic Analysis Reveal New Insights into Heat Stress Responsive Mechanism in Edible Mushroom Grifola frondosa. Sci. Hortic. 2024, 323, 112542. [Google Scholar] [CrossRef]
- Wu, F.; Dai, Y. Notes on the Nomenclature of the Auricularia Auricula-Judae Complex. Mycosystema 2015, 34, 604–611. [Google Scholar] [CrossRef]
- Dai, Y.; Li, X.; Song, B.; Sun, L.; Yang, C.; Zhang, X.; Wang, Y.; Zhang, Z.; Fu, Y.; Li, Y. Genomic Analyses Provide Insights Into the Evolutionary History and Genetic Diversity of Auricularia Species. Front. Microbiol. 2019, 10, 2255. [Google Scholar] [CrossRef]
- Wu, F.; Tohtirjap, A.; Fan, L.-F.; Zhou, L.-W.; Alvarenga, R.L.M.; Gibertoni, T.B.; Dai, Y.-C. Global Diversity and Updated Phylogeny of Auricularia (Auriculariales, Basidiomycota). J. Fungi 2021, 7, 933. [Google Scholar] [CrossRef]
- Fang, M.; Yao, F.; Lu, L.; Zhang, Y.; Wang, P.; Lu, J.; Wang, W.; Chen, X. Complete Mitochondrial Sequence of Auricularia Heimuer, One of the Most Popular Edible Fungus in China. Mitochondrial DNA Part B Resour. 2019, 4, 4029–4030. [Google Scholar] [CrossRef]
- Sun, X.; Yang, C.; Ma, Y.; Zhang, J.; Wang, L. Research Progress of Auricularia heimuer on Cultivation Physiology and Molecular Biology. Front. Microbiol. 2022, 13, 1048249. [Google Scholar] [CrossRef]
- Wang, F.; Han, C.; Zhang, J.; Zhang, P.; Zhang, X.; Yue, X.; Zhao, Y.; Dai, X. Comparative Genomic Analysis of Two Monokaryons of Auricularia heimuer Hei29. J. Fungi 2025, 11, 122. [Google Scholar] [CrossRef]
- He, M.; Wang, T.; Tang, C.; Xiao, M.; Pu, X.; Qi, J.; Li, Y.; Li, X. Metabolomics and Transcriptomics Reveal the Effects of Different Fermentation Times on Antioxidant Activities of Ophiocordyceps sinensis. J. Fungi 2025, 11, 51. [Google Scholar] [CrossRef]
- Tang, B.; Li, Y.; Xu, X.; Du, G.; Wang, H. Electroacupuncture Ameliorates Neuronal Injury by NLRP3/ASC/Caspase-1 Mediated Pyroptosis in Cerebral Ischemia-Reperfusion. Mol. Neurobiol. 2024, 61, 2357–2366. [Google Scholar] [CrossRef]
- Gao, M.; He, Y.; Yin, X.; Zhong, X.; Yan, B.; Wu, Y.; Chen, J.; Li, X.; Zhai, K.; Huang, Y.; et al. Ca2+ Sensor-Mediated ROS Scavenging Suppresses Rice Immunity and Is Exploited by a Fungal Effector. Cell 2021, 184, 5391–5404.e17. [Google Scholar] [CrossRef]
- Alpert, A.J.; Gilbert, H.F. Detection of Oxidized and Reduced Glutathione with a Recycling Postcolumn Reaction. Anal. Biochem. 1985, 144, 553–562. [Google Scholar] [CrossRef]
- Du, S.; Zhou, N.; Xie, G.; Chen, Y.; Suo, H.; Xu, J.; Tao, J.; Zhang, L.; Zhu, J. Surface-Engineered Triboelectric Nanogenerator Patches with Drug Loading and Electrical Stimulation Capabilities: Toward Promoting Infected Wounds Healing. Nano Energy 2021, 85, 106004. [Google Scholar] [CrossRef]
- Xu, J.; Xu, J.; Shi, T.; Zhang, Y.; Chen, F.; Yang, C.; Guo, X.; Liu, G.; Shao, D.; Leong, K.W.; et al. Probiotic-Inspired Nano-medicine Restores Intestinal Homeostasis in Colitis by Regulating Redox Balance, Immune Responses, and the Gut Microbi-ome. Adv. Mater. 2022, 35, 2207890. [Google Scholar] [CrossRef]
- Brunton, N.P.; Gormley, T.R.; Murray, B. Use of the Alditol Acetate Derivatisation for the Analysis of Reducing Sugars in Potato Tubers. Food Chem. 2007, 104, 398–402. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Chen, S.; Min, Y.; Tang, Y.; Ma, X.; Li, H.; Li, J.; Liu, Z. Spraying Chitosan on Cassava Roots Reduces Postharvest Deterioration by Promoting Wound Healing and Inducing Disease Resistance. Carbohydr. Polym. 2023, 318, 121133. [Google Scholar] [CrossRef]
- Wu, C.-D.; Zhang, M.; He, M.-T.; Gu, M.-F.; Lin, M.; Zhang, G. Selection of Solvent for Extraction of Antioxidant Components from Cynanchum Auriculatum, Cynanchum Bungei, and Cynanchum Wilfordii Roots. Food Sci. Nutr. 2019, 7, 1337–1343. [Google Scholar] [CrossRef]
- Weiner, J., III; Zimmerman, C.U.; Göhlmann, H.W.H.; Herrmann, R. Transcription Profiles of the Bacterium Mycoplasma Pneumoniae Grown at Different Temperatures. Nucleic Acids Res. 2003, 31, 6306–6320. [Google Scholar] [CrossRef]
- Kamitani, M.; Kashima, M.; Tezuka, A.; Nagano, A.J. Lasy-Seq: A High-Throughput Library Preparation Method for RNA-Seq and Its Application in the Analysis of Plant Responses to Fluctuating Temperatures. Sci. Rep. 2019, 9, 7091. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, F.J.; Sun, W.J.; Fang, M.; Wu, C.S. Screening of Reference Genes for Real-Time Quantitative PCR in Auricularia auricula-judae. Mycosystema 2020, 39, 1510–1519. [Google Scholar]
- Zeki, Ö.C.; Eylem, C.C.; Reçber, T.; Kır, S.; Nemutlu, E. Integration of GC–MS and LC–MS for Untargeted Metabolomics Profiling. J. Pharm. Biomed. Anal. 2020, 190, 113509. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Arslan, N.P.; Dawar, P.; Albayrak, S.; Doymus, M.; Azad, F.; Esim, N.; Taskin, M. Fungi-Derived Natural Antioxidants. Crit. Rev. Food Sci. Nutr. 2023, 2023, 1–24. [Google Scholar] [CrossRef]
- Yurina, N.P. Heat Shock Proteins in Plant Protection from Oxidative Stress. Mol. Biol. 2023, 57, 951–964. [Google Scholar] [CrossRef]
- Jan, R.; Kim, N.; Lee, S.-H.; Khan, M.A.; Asaf, S.; Lubna; Park, J.-R.; Asif, S.; Lee, I.-J.; Kim, K.-M. Enhanced Flavonoid Accumulation Reduces Combined Salt and Heat Stress Through Regulation of Transcriptional and Hormonal Mechanisms. Front. Plant Sci. 2021, 12, 796956. [Google Scholar] [CrossRef]
- Hu, Y.; Xue, F.; Chen, Y.; Qi, Y.; Zhu, W.; Wang, F.; Wen, Q.; Shen, J. Effects and Mechanism of the Mycelial Culture Temperature on the Growth and Development of Pleurotus ostreatus (Jacq.) P. Kumm. Horticulturae 2023, 9, 95. [Google Scholar] [CrossRef]
- Shanmugaraj, C.; Saranraj, K.; Biswas, M. Optimizing Growth Conditions for Shiitake Mushroom Cultivation in Birbhum, West Bengal: A Study on Media, Temperature, and Ph Variations. Int. J. Adv. Biochem. Res. 2024, 8, 219–223. [Google Scholar] [CrossRef]
- Taratima, W.; Chuanchumkan, C.; Maneerattanarungroj, P.; Trunjaruen, A.; Theerakulpisut, P.; Dongsansuk, A. Effect of Heat Stress on Some Physiological and Anatomical Characteristics of Rice (Oryza sativa L.) Cv. KDML105 Callus and Seedling. Biology 2022, 11, 1587. [Google Scholar] [CrossRef]
- Fang, M.; Sun, X.; Yao, F.; Lu, L.; Ma, X.; Shao, K.; Kaimoyo, E. A Combination of Transcriptome and Enzyme Activity Analysis Unveils Key Genes and Patterns of Corncob Lignocellulose Degradation by Auricularia heimuer under Cultivation Conditions. J. Fungi 2024, 10, 545. [Google Scholar] [CrossRef]
- Dogan, H.; Coteli, E.; Karatas, F. Determination of Glutathione, Selenium, and Malondialdehyde in Different Edible Mushroom Species. Biol. Trace Elem. Res. 2016, 174, 459–463. [Google Scholar] [CrossRef]
- Li, H.; Chai, Q.; Zheng, X.; Wen, Q.; Liu, Q.; Qi, Y.C.; Wang, F.; Shen, J.; Hu, Y. SIRT2-Mediated Deacetylation of Glutathione Transferase Alleviates Oxidative Damage and Increases the Heat Tolerance of Pleurotus ostreatus. Environ. Res. 2024, 263, 120147. [Google Scholar] [CrossRef]
- Shabaev, A.V.; Savinova, O.S.; Moiseenko, K.V.; Glazunova, O.A.; Fedorova, T.V. Saprotrophic Wood Decay Ability and Plant Cell Wall Degrading Enzyme System of the White Rot Fungus Crucibulum Laeve: Secretome, Metabolome and Genome Investigations. J. Fungi 2024, 11, 21. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Y.; Wang, G.; Xu, R.; Zhou, Y.; Gong, Y.; Bian, Y. Functional Analyses of Tryptophan Synthase Gene LetrpB in Lentinula edodes by RNAi Method. Acta Edulis Fungi 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Chen, X.M.; Wu, H.B.; Xiang, Q.J.; Zeng, X.F.; Zhang, X.P.; Gu, Y.F. Response Characteristics of Laccase Activity and Transcriptional Expression in Lentinula edodes to Different Temperatures. J. Sichuan Univ. (Nat. Sci. Ed.) 2023, 56, 155–160. [Google Scholar]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, Z.; Tan, Q.; Zhu, J.; Sun, W.; Zhang, W.; Yun, J. Effects of Mannitol on Production Traits and ROS Scavenging Ability of Subcultured Strains of Volvariella volvacea. Sci. Agric. Sin. 2024, 57, 190–203. [Google Scholar]
- Liu, R.; Wang, Y.; Shu, B.; Xin, J.; Yu, B.; Gan, Y.; Liang, Y.; Qiu, Z.; Yan, S.; Cao, B. SmHSFA8 Enhances the Heat Tolerance of Eggplant by Regulating the SmEGY3-SmCSD1 Module and Promoting SmF3H-Mediated Flavonoid Biosynthesis. Plant Cell Environ. 2024; Early View. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, M.; Wu, X.; Zhang, J. Metabolic Response of Pleurotus ostreatus to Continuous Heat Stress. Front. Microbiol. 2020, 10, 3148. [Google Scholar] [CrossRef]
- Chen, Y.; Shertzer, H.G.; Schneider, S.N.; Nebert, D.W.; Dalton, T.P. Glutamate Cysteine Ligase Catalysis. J. Biol. Chem. 2005, 280, 33766–33774. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, N.; Dong, L.; Zhang, D.; Fan, S.; Jiang, L.; Wang, X.; Xu, P.; Zhang, S. Overexpression of Soybean Isoflavone Reductase (GmIFR) Enhances Resistance to Phytophthora Sojae in Soybean. Front. Plant Sci. 2015, 6, 1024. [Google Scholar] [CrossRef]
- Ming, J.H.; Xie, J.; Liu, B.; He, Y.; Zhou, Q.; Pan, L.; Yu, J.H.; Xu, P. Cloning, Sequence Analysis of HSP70 cDNA and Effects of Heat Stress on Its mRNA Expression in Megalobrama Amblycephala. J. Fish. Sci. China 2009, 16, 635–648. [Google Scholar]
- Wang, H.; Feng, M.; Jiang, Y.; Du, D.; Dong, C.; Zhang, Z.; Wang, W.; Liu, J.; Liu, X.; Li, S.; et al. Thermosensitive SUMOylation of TaHsfA1 Defines a Dynamic ON/OFF Molecular Switch for the Heat Stress Response in Wheat. Plant Cell 2023, 35, 3889–3910. [Google Scholar] [CrossRef]
- Wu, X.; Fan, Y.; Wang, R.; Zhao, Q.; Ali, Q.; Wu, H.; Gu, Q.; Borriss, R.; Xie, Y.; Gao, X. Bacillus Halotolerans KKD1 Induces Physiological, Metabolic and Molecular Reprogramming in Wheat under Saline Condition. Front. Plant Sci. 2022, 13, 978066. [Google Scholar] [CrossRef]





| Kit Name | Brand Name | Item No. | Region |
|---|---|---|---|
| Total SOD Activity Assay Kit (WST-8 Method) | Beyotime | S0101S | Shanghai, China |
| Peroxidase Assay Kit | Beyotime | S0051 | Shanghai, China |
| Reduced Glutathione (GSH) Content Assay Kit | Solarbio | BC1175 | Beijing, China |
| Plant Flavonoid Content Assay Kit | Solarbio | BC1330 | Beijing, China |
| Total Phenol (TP) Content Assay Kit | Solarbio | BC1340 | Beijing, China |
| Glutathione S-Transferase (GST) Activity Assay Kit | Solarbio | BC0350 | Beijing, China |
| Malondialdehyde (MDA) Content Assay Kit | Solarbio | BC0025 | Beijing, China |
| Laccase Activity Assay Kit | Solarbio | BC1630 | Beijing, China |
| Reducing Sugar Content Assay Kit | Solarbio | BC0235 | Beijing, China |
| Quick RNA Isolation Kit | Huayueyang | 0416-50GX | Beijing, China |
| SYBR Green QuantiTect RT–PCR kit | LABLEAD | R0202 | Beijing, China |
| FastKing gDNA Dispelling RT SuperMix kit | TAKARA BIO INC | RR092A | Kusatsu, Shiga, Japan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, F.; Sun, X.; Dai, X.; Zhang, P.; Ma, Y.; Xu, Y.; Wang, L.; Zhang, J. Integrated Multi-Omics Analysis to Investigate the Molecular Mechanisms Underlying the Response of Auricularia heimuer to High-Temperature Stress. J. Fungi 2025, 11, 167. https://doi.org/10.3390/jof11030167
Lu F, Sun X, Dai X, Zhang P, Ma Y, Xu Y, Wang L, Zhang J. Integrated Multi-Omics Analysis to Investigate the Molecular Mechanisms Underlying the Response of Auricularia heimuer to High-Temperature Stress. Journal of Fungi. 2025; 11(3):167. https://doi.org/10.3390/jof11030167
Chicago/Turabian StyleLu, Fang, Xin Sun, Xiaodong Dai, Piqi Zhang, Yinpeng Ma, Yafei Xu, Lei Wang, and Jiechi Zhang. 2025. "Integrated Multi-Omics Analysis to Investigate the Molecular Mechanisms Underlying the Response of Auricularia heimuer to High-Temperature Stress" Journal of Fungi 11, no. 3: 167. https://doi.org/10.3390/jof11030167
APA StyleLu, F., Sun, X., Dai, X., Zhang, P., Ma, Y., Xu, Y., Wang, L., & Zhang, J. (2025). Integrated Multi-Omics Analysis to Investigate the Molecular Mechanisms Underlying the Response of Auricularia heimuer to High-Temperature Stress. Journal of Fungi, 11(3), 167. https://doi.org/10.3390/jof11030167








