Diversity and Patulin Production of Penicillium spp. Associated with Apple Blue Mold in Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Fungal Isolation
2.2. Morphological Analysis
2.3. Molecular Identification and Phylogeny
2.4. Pathogenicity Assay
2.5. Assessment of the Putative Ability of Penicillium Isolates to Produce Patulin
2.6. Patulin Production of Penicillium Isolates
2.7. Statistical Analysis
2.8. Principal Component Analysis
3. Results
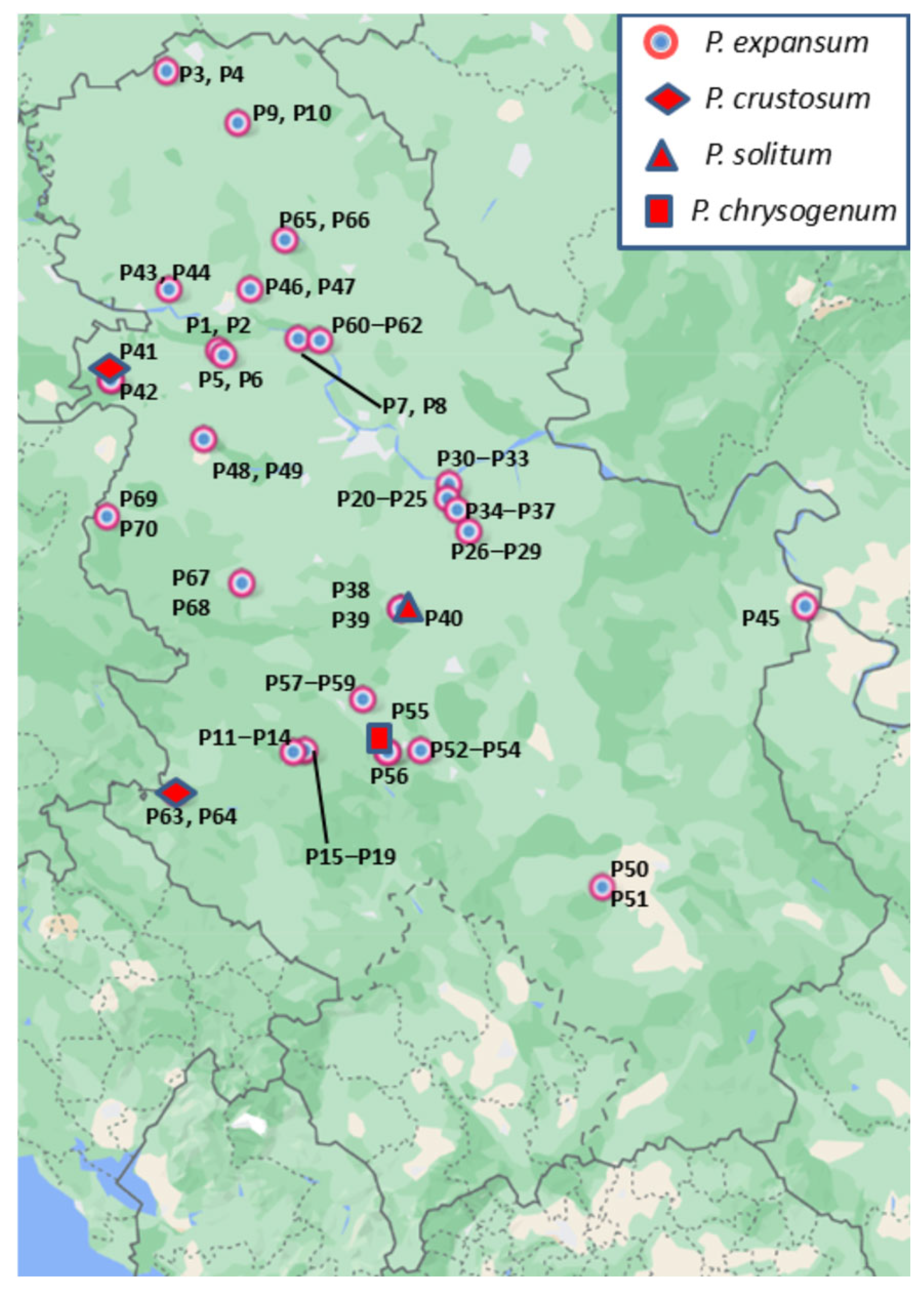
3.1. Sample Collection and Fungal Isolation
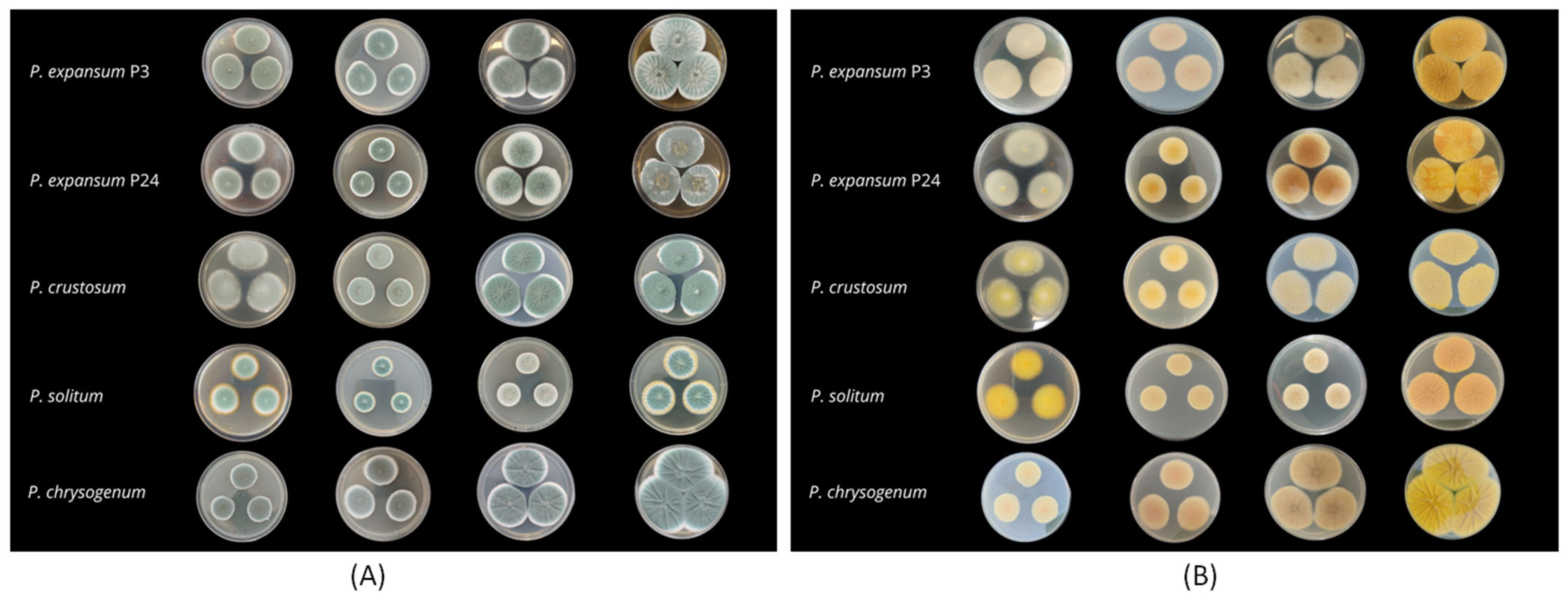
3.2. Morphological Analysis
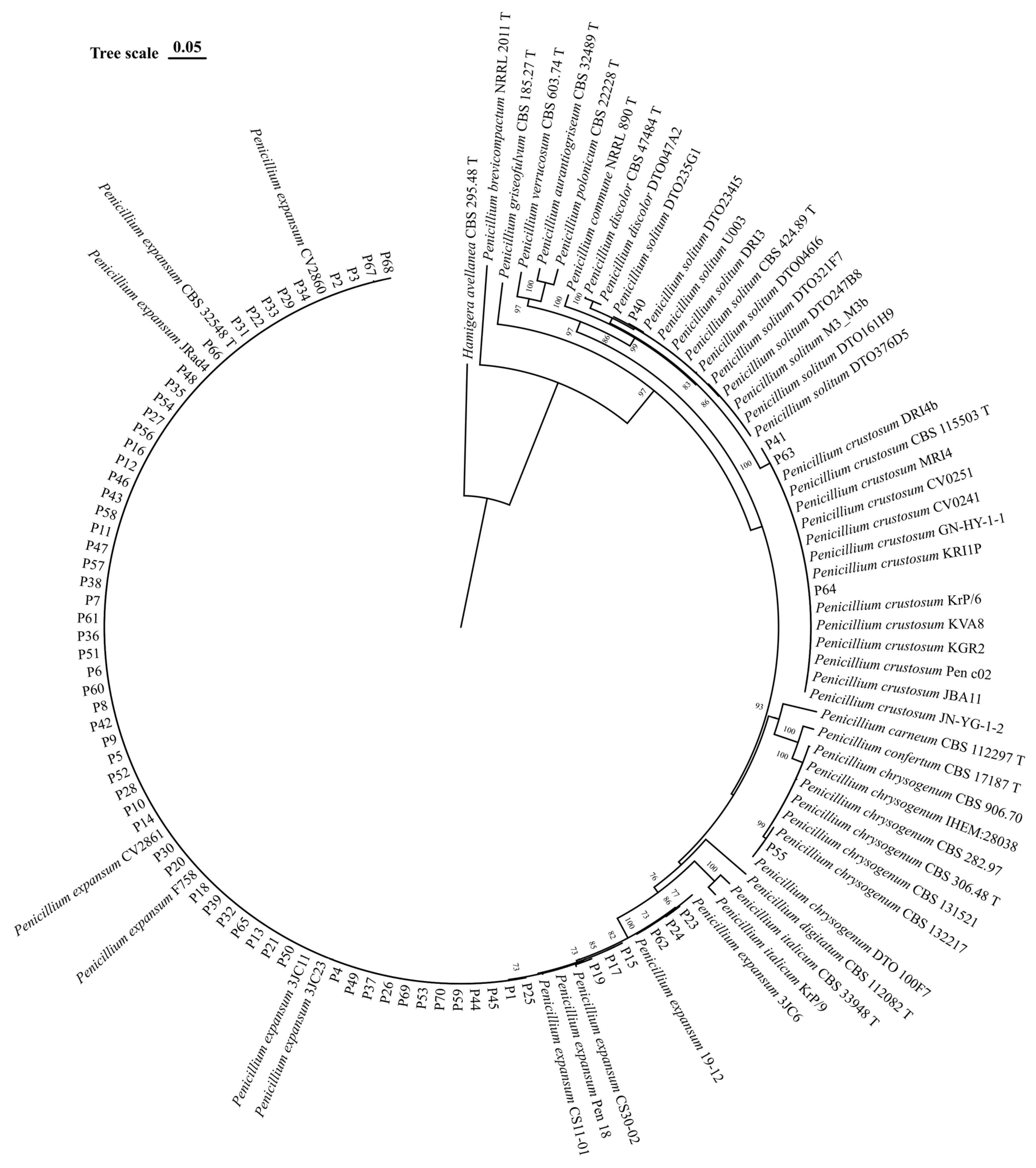
3.3. Molecular Identification and Phylogeny
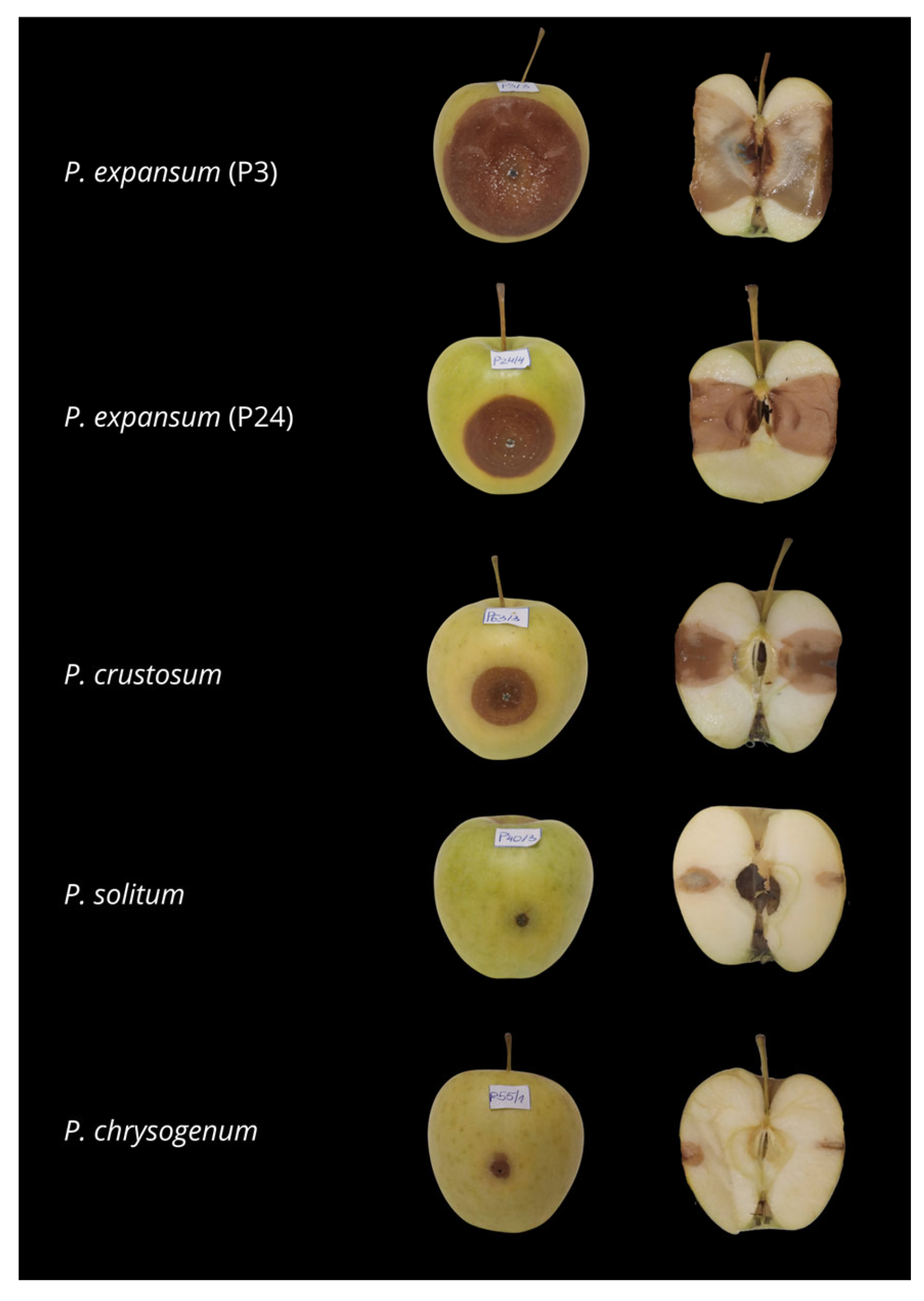
3.4. Pathogenicity Assay
3.5. Characterization of the Ability of Penicillium Isolates to Produce Patulin
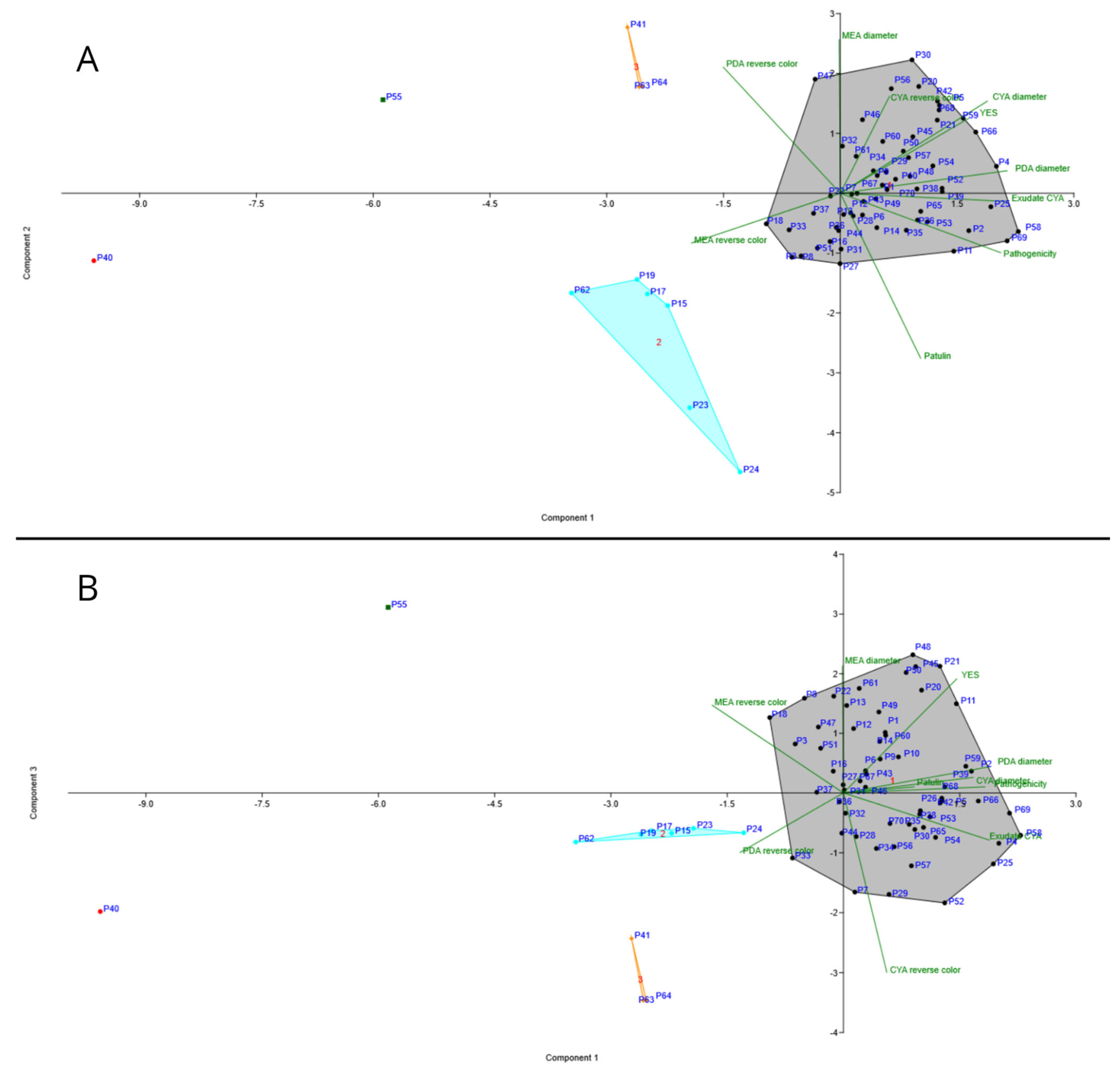
3.6. PCA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic review of phenolic compounds in apple fruits: Compositions, distribution, absorption, metabolism, and processing stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Šahinović, E.; Murtić, S. A comparison of antioxidant properties of two apple cultivars. Contemp. Agric. 2019, 68, 60–64. [Google Scholar] [CrossRef]
- Głos, H.; Bryk, H.; Michalecka, M.; Puławska, J. The recent occurrence of biotic postharvest diseases of apples in Poland. Agronomy 2022, 12, 399. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Patulin in apples and apple-based food products: The burdens and the mitigation strategies. Toxins 2018, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Bompeix, G. Diversity and population dynamics of Penicillium spp. on apples in pre-and postharvest environments: Consequences for decay development. Plant Pathol. 2005, 54, 74–81. [Google Scholar] [CrossRef]
- Louw, J.P.; Korsten, L. Pathogenic Penicillium spp. on apple and pear. Plant Dis. 2014, 98, 590–598. [Google Scholar] [CrossRef]
- van der Walt, L.; Spotts, R.A.; Visagie, C.M.; Jacobs, K.; Smit, F.J.; McLeod, A. Penicillium species associated with preharvest wet core rot in South Africa and their pathogenicity on apple. Plant Dis. 2010, 94, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, P.G.; Spotts, R.A. Postharvest decay of winter pear and apple fruit caused by species of Penicillium. Phytopathology 1995, 85, 103–110. [Google Scholar] [CrossRef]
- Sholberg, P.L.; Haag, P.D. Incidence of postharvest pathogens of stored apples in British Columbia. Can. J. Plant Pathol. 1996, 18, 81–85. [Google Scholar] [CrossRef]
- Pianzzola, M.J.; Moscatelli, M.; Vero, S. Characterization of Penicillium isolates associated with blue mold on apple in Uruguay. Plant Dis. 2004, 88, 23–28. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, W.; Ma, N.; Li, M.; Zhang, J.; Xu, G. Genetic identification, pathogenicity and patulin production of Penicillium species from apple blue mold in China. Food Qual. Saf. 2024, 8, fyad073. [Google Scholar] [CrossRef]
- Iwahashi, Y.; Hosoda, H.; Park, J.; Lee, J.; Suzuki, Y.; Kitagawa, E.; Murata, S.; Jwa, N.; Gu, M.; Iwahashi, H. Mechanisms of patulin toxicity under the condition that cause growth inhibition to yeast cells. J. Agric. Food Chem. 2006, 54, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission Regulation No. 1881/2006 of setting maximum levels of certain contaminants in foodstuffs. Off. J. Eur. Community 2006, L364, 7–16. [Google Scholar]
- Food and Agriculture Organization. FAOstat. World Agriculture Statistics Data Base. 2024. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 14 May 2024).
- Vico, I.; Gaskins, V.; Duduk, N.; Vasić, M.; Yu, J.J.; Peter, K.A.; Jurick, W.M. First report of Penicillium crustosum causing blue mold on stored apple fruit in Serbia. Plant Dis. 2014, 98, 1430. [Google Scholar] [CrossRef]
- Žebeljan, A.; Duduk, N.; Vučković, N.; Jurick, W.M.; Vico, I. Incidence, speciation, and morpho-genetic diversity of Penicillium spp. causing blue mold of stored pome fruits in Serbia. J. Fungi 2021, 7, 1019. [Google Scholar] [CrossRef]
- Torović, L.; Dimitrov, N.; Assunção, R.; Alvito, P. Risk assessment of patulin intake through apple-based food by infants and preschool children in Serbia. Food Addit. Contam. Part A 2017, 34, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Torović, L.; Dimitrov, N.; Lopes, A.; Martins, C.; Alvito, P.; Assunção, R. Patulin in fruit juices: Occurrence, bioaccessibility, and risk assessment for Serbian population. Food Addit. Contam. Part A 2018, 35, 985–995. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.-B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Somma, M. Extraction and purifcation of DNA. In The Analysis of Food Samples for the Presence of Genetically Modifed Organisms; Special Publication No. I.03.114, Session 4; Querci, M., Jermini, M., Van den Eede, G., Eds.; European Commission DG-JRC: Brussels, Belgium, 2004; pp. 13–17. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of pimer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Hong, S.-B.; Cho, H.-S.; Shin, H.-D.; Frisvad, J.C.; Samson, A. Novel Neosartorya species isolated from soil in Korea. Int. J. Syst. Evol. Microbiol. 2006, 56, 477–486. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGAX: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization By One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; Schena, L.; Nigro, F.; De Girolamo, A.; Ippolito, A. Effect of quercetin and umbelliferone on the transcript level of Penicillium expansum genes involved in patulin biosynthesis. Eur. J. Plant Pathol. 2009, 125, 223–233. [Google Scholar] [CrossRef]
- Sanzani, S.M.; De Girolamo, A.; Schena, L.; Solfrizzo, M.; Ippolito, A.; Visconti, A. Control of Penicillium expansum and patulin accumulation on apples by quercetin and umbelliferone. Eur. Food Res. Technol. 2009, 228, 381–389. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S.; Ullman, J.B. Using Multivariate Statistics; Pearson: Boston, MA, USA, 2013; Volume 6, pp. 497–516. [Google Scholar]
- Stevens, J. Applied Multivariate Statistics for the Social Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2002; Volume 4. [Google Scholar]
- Hammer, Ø.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Habib, W.; Masiello, M.; Chahine-Tsouvalakis, H.; Al Moussawi, Z.; Saab, C.; Tawk, S.T.; Piemontese, L.; Solfrizzo, M.; Logrieco, A.F.; Moretti, A.; et al. Occurrence and characterization of Penicillium species isolated from post-harvest apples in Lebanon. Toxins 2021, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Javaid, A. Molecular characterization of Penicillium expansum associated with blue mold disease of apple in Pakistan. Pak. J. Bot. 2021, 53, 2299–2303. [Google Scholar] [CrossRef]
- Morales, H.; Marín, S.; Obea, L.; Patiño, B.; Doménech, M.; Ramos, A.J.; Sanchis, V. Ecophysiological characterization of Penicillium expansum population in Lleida (Spain). Int. J. Food Microbiol. 2008, 122, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004, 49, 1–174. [Google Scholar]
- Barreto, M.C.; Houbraken, J.; Samson, R.A. Taxonomic studies of the Penicillium glabrum complex and the description of a new species P. subericola. Fungal Divers. 2011, 49, 23–33. [Google Scholar] [CrossRef]
- Duduk, N.; Lazarević, M.; Žebeljan, A.; Vasić, M.; Vico, I. Blue mold decay of stored onion bulbs caused by Penicillium polonicum, P. glabrum and P. expansum. J. Phytopathol. 2017, 165, 662–669. [Google Scholar] [CrossRef]
- Tannous, J.; El Khoury, R.; Snini, S.P.; Lippi, Y.; El Khoury, A.; Atoui, A.; Lteif, R.; Oswald, I.P.; Puel, O. Sequencing, physical organization and kinetic expression of the patulin biosynthetic gene cluster from Penicillium expansum. Int. J. Food Microbiol. 2014, 189, 51–60. [Google Scholar] [CrossRef]
- Ballester, A.R.; Marcet-Houben, M.; Levin, E.; Sela, N.; Selma-Lázaro, C.; Carmona, L.; Wisniewski, M.; Droby, S.; González-Candelas, L.; Gabaldón, T. Genome, transcriptome, and functional analyses of Penicillium expansum provide new insights into secondary metabolism and pathogenicity. Mol. Plant-Microbe Interact. 2015, 28, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zong, Y.; Du, Z.; Chen, Y.; Zhang, Z.; Qin, G.; Zhao, W.; Tian, S. Genomic characterization reveals insights into patulin biosynthesis and pathogenicity in Penicillium species. Mol. Plant-Microbe Interact. 2015, 28, 635–647. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zong, Y.; Shang, Y.; Zhang, Z.; Xu, X.; Wang, X.; Long, M.; Tian, S. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ. Microbiol. 2019, 21, 1124–1139. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C. A critical review of producers of small lactone mycotoxins: Patulin, penicillic acid and moniliformin. World Mycotoxin J. 2018, 11, 73–100. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Punelli, M.; Ippolito, A.; Fanelli, C. Study on the role of patulin on pathogenicity and virulence of Penicillium expansum. Int. J. Food Microbiol. 2012, 153, 323–331. [Google Scholar] [CrossRef]
- Barad, S.; Horowitz, S.B.; Kobiler, I.; Sherman, A.; Prusky, D. Accumulation of the mycotoxin patulin in the presence of gluconic acid contributes to pathogenicity of Penicillium expansum. Mol. Plant-Microbe Interact. 2014, 27, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Snini, S.P.; Tannous, J.; Heuillard, P.; Bailly, S.; Lippi, Y.; Zehraoui, E.; Barreau, C.; Oswald, I.P.; Puel, O. Patulin is a cultivar-dependent aggressiveness factor favouring the colonization of apples by Penicillium expansum. Mol. Plant Pathol. 2016, 17, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M. Penicillium expansum: Biology, omics, and management tools for a global postharvest pathogen causing blue mould of pome fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
- Stošić, S.; Ristić, D.; Savković, Ž.; Grbić, M.L.; Vukojević, J.; Živković, S. Penicillium and Talaromyces species as postharvest pathogens of pear fruit (Pyrus communis) in Serbia. Plant Dis. 2021, 105, 3510–3521. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Dijksterhuis, J.; Seifert, K.A.; Jacobs, K.; Samson, R.A. A taxonomic review of Penicillium species producing conidiophores with solitary phialides, classified in section Torulomyces. Pers. Mol. Phylogeny Evol. Fungi 2016, 36, 134–155. [Google Scholar] [CrossRef] [PubMed]
- Ghuffar, S.; Irshad, G.; Naz, F.; Khan, M.A. Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides. Green Process. Synth. 2021, 10, 21–36. [Google Scholar] [CrossRef]
- Strausbaugh, C.A. Incidence, distribution, and pathogenicity of fungi causing root rot in Idaho long-term sugar beet storage piles. Plant Dis. 2018, 102, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Zhang, Z.K.; Zhuang, W.Y. Species diversity of Penicillium in Southwest China with discovery of forty-three new species. J. Fungi 2023, 9, 1150. [Google Scholar] [CrossRef]
- Samson, R.A.; Seifert, K.A.; Kuijpers, A.F.; Houbraken, J.A.M.P.; Frisvad, J.C. Phylogenetic analysis of Penicillium subgenus Penicillium using partial β-tubulin sequences. Stud. Mycol. 2004, 49, 175–200. [Google Scholar]
- Yun, T.S.; Park, S.Y.; Yu, J.; Hwang, Y.; Hong, K.J. Isolation and identification of fungal species from the insect pest Tribolium castaneum in rice processing complexes in Korea. Plant Pathol. J. 2018, 34, 356. [Google Scholar] [CrossRef]
- Zadravec, M.; Lešić, T.; Brnić, D.; Pleadin, J.; Kraak, B.; Jakopović, Ž.; Perković, I.; Vahčić, N.; Tkalec, V.J.; Houbraken, J. Regional distribution and diversity of Aspergillus and Penicillium species on Croatian traditional meat products. Int. J. Food Microbiol. 2023, 406, 110404. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Yilmaz, N.; Vanderwolf, K.; Renaud, J.B.; Sumarah, M.W.; Houbraken, J.; Assebgui, R.; Seifert, K.A.; Malloch, D. Penicillium diversity in Canadian bat caves, including a new species, P. speluncae. Fungal Syst. Evol. 2020, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Agnolucci, M.; Daghio, M.; Mannelli, F.; Secci, G.; Cristani, C.; Palla, M.; Giannerini, F.; Giovannetti, M.; Buccioni, A. Use of chitosan and tannins as alternatives to antibiotics to control mold growth on PDO Pecorino Toscano cheese rind. Food Microbiol. 2020, 92, 103598. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Frisvad, J.C.; Seifert, K.A.; Overy, D.P.; Tuthill, D.M.; Valdez, J.G.; Samson, R.A. New penicillin-producing Penicillium species and an overview of section Chrysogena. Pers. Mol. Phylogeny Evol. Fungi 2012, 29, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus 2011, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.; van den Eynde, C.; Baert, F.; D’hooge, E.; De Pauw, R.; Normand, A.C.; Piarroux, R.; Stubbe, D. Remarkable fungal biodiversity on northern Belgium bats and hibernacula. Mycologia 2023, 115, 484–498. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.M.; Zhuang, W.Y. Penicillium macrosclerotiorum, a new species producing large sclerotia discovered in south China. Mycol. Res. 2007, 111, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Wang, L.; Lee, H.B.; Frisvad, J.C. New sections in Penicillium containing novel species producing patulin, pyripyropens or other bioactive compounds. Pers. Mol. Phylogeny Evol. Fungi 2016, 36, 299–314. [Google Scholar] [CrossRef]
- Peterson, S.W. Multilocus DNA sequence analysis shows that Penicillium biourgeianum is a distinct species closely related to P. brevicompactum and P. olsonii. Mycol. Res. 2004, 108 Pt 4, 434–440. [Google Scholar] [CrossRef] [PubMed]

| Sequence | Position | ||||
|---|---|---|---|---|---|
| BenA | 86 | 109 | 208 | 209 | 237 |
| I variant | A | A | G | T | C |
| II variant | G | A | G | T | C |
| III variant | A | G | G | G | T |
| IV variant | A | A | A | G | C |
| V variant | A | A | G | G | T |
| CaM | 68 | 143 | 345 | ||
| I variant | T | C | A | ||
| II variant | C | T | G | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudaš, T.; Cotugno, P.; Budakov, D.; Grahovac, M.; Stojšin, V.; Mihajlović, M.; Ippolito, A.; Sanzani, S.M. Diversity and Patulin Production of Penicillium spp. Associated with Apple Blue Mold in Serbia. J. Fungi 2025, 11, 175. https://doi.org/10.3390/jof11030175
Dudaš T, Cotugno P, Budakov D, Grahovac M, Stojšin V, Mihajlović M, Ippolito A, Sanzani SM. Diversity and Patulin Production of Penicillium spp. Associated with Apple Blue Mold in Serbia. Journal of Fungi. 2025; 11(3):175. https://doi.org/10.3390/jof11030175
Chicago/Turabian StyleDudaš, Tatjana, Pietro Cotugno, Dragana Budakov, Mila Grahovac, Vera Stojšin, Milica Mihajlović, Antonio Ippolito, and Simona Marianna Sanzani. 2025. "Diversity and Patulin Production of Penicillium spp. Associated with Apple Blue Mold in Serbia" Journal of Fungi 11, no. 3: 175. https://doi.org/10.3390/jof11030175
APA StyleDudaš, T., Cotugno, P., Budakov, D., Grahovac, M., Stojšin, V., Mihajlović, M., Ippolito, A., & Sanzani, S. M. (2025). Diversity and Patulin Production of Penicillium spp. Associated with Apple Blue Mold in Serbia. Journal of Fungi, 11(3), 175. https://doi.org/10.3390/jof11030175







